Abstract
Introduction
We have combined the minimally invasive single-port laparoscopic surgery and the transanal total mesorectal excision (TaTME) for rectal cancer with the goal to standardize the approach and improve the quality of rectal cancer resection.
Methods
By using two single-port platforms, selected patients were first operated by TaTME, and then a single-port laparoscopic surgery was introduced to assist and complete the abdominal portion. Short-term outcomes including perioperative outcome and pathologic results of these patients were evaluated.
Results
Between July 2014 and March 2015, six patients with low rectal cancer (five males and one female) at a median age of 68 years were successfully operated in a median time of 360 min (range 310–420). The median estimated blood loss was 150 ml (range 50–800). In one patient, the spleen was removed because of a lesion identified preoperatively. Their postoperative recovery was uneventful except one acute myocardial infarction on postoperative day 3. Pathologic specimens showed negative margins and a complete excision of the mesorectum in all cases. The median number of harvested lymph nodes was 11.5 (range 4–12). At a median follow-up of 4 months (range 3–9), after ileostomy closure, none of the patients suffered from fecal incontinence.
Conclusion
TaTME assisted by abdominal single-port may be safely achieved in selected rectal cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the last few decades, there has been a tremendous development in minimally invasive surgery, especially in the field of colorectal cancer. While conventional multi-ports laparoscopic surgery (MPLS) for rectal cancer is still under evaluation in randomized control trails [1, 2], new approaches such as single-port laparoscopic surgery (SPLS), i.e., single-incision laparoscopic surgery (SILS) for rectal cancer, have emerged [3–5]. The recently developed transanal total mesorectal excision (TaTME) surgery embodies the concept of natural orifice translumenal surgery (NOTES) [6], and may be a better approach to resect rectal cancer [5, 7]. This is because TaTME permits a clear and magnified filed to get access to the confined distal rectum (once called “no-man’s-land”) from below by employing a transanal platform—either the rigid transanal endoscopic platform (i.e., TEM device) [8] or the disposable transanal minimally invasive surgery (TAMIS) platform (more frequently used) [9]. Therefore, it can reduce the difficulty of the operation, avoiding some difficult situations encountered by conventional laparoscopic surgery such as the “multi-stapling” that increased the likelihoods of anastomotic leak and involved distal resection margin [10].
Currently most of TaTME cases have been performed in a hybrid approach—assisted by laparoscopy, among whom most are MPLS-assisted. Previous studies have reported a small number of cases using the SPLS–TaTME strategy [5, 11, 12]. Herein, we present our series of consecutive patients, primarily focusing on the technical details, short-term results, and oncologic safety of this technique.
Methods
Patient
Consecutive patients with biopsy-proven adenocarcinoma or high-grade dysplasia who were scheduled to undertake radical surgery were eligible. All lesions were located ≤6 cm from the anal verge. Patients who presented recurrence and unresectable distal metastasis, cT4 tumors, obstruction, synchronous colorectal cancer, fecal incontinence, history of inflammatory bowel disease (IBD), and familial adenomatous polyposis (FAP) were excluded. All patients had undergone full assessment preoperatively, such as thorough colonoscopy, pelvic MRI and/or endorectal ultrasonography, thoracoabdominal CT scan, and sphincter manometry. Patients whose T stage ≥3 or lymph node positive on preoperative evaluation were scheduled to undergo neoadjuvant therapy.
Approval of institutional review board (IRB) had been obtained. All patients had been given full explanations of the benefits and adverse risks of the procedure, and informed consent had been obtained from each patient.
Surgical technique
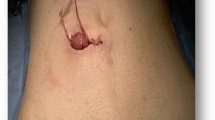
The key technical steps of SPLS–TaTME surgery can be summarized as follows: (1) The patient was placed in lithotomy position. An anal retractor was applied to fully expose the rectum after washout with antiseptic solution; (2) a purse string was placed to tightly occlude the rectal lumen, followed by a full-thickness circumferential dissection into the proper perirectal plane (for low tumor, an intersphincteric dissection was required); (3) proximal dissection progressed after introducing a transanal SILS port (Covidien, Mansfield, MA) and establishing a pneumo-pelvis (Fig. 1a), and a rubber tube (16F) was placed through the ischiorectal fossa as a mini-trocar for suction or countertraction by the assistant; (4) after fully mobilizing the extraperitoneal rectum, the peritoneal reflection was cut open in the anterior aspect, and the peritoneal cavity was thereby entered; (5) the second SILS port placed at the future ileostomy site was introduced by one team or simultaneously two teams if step 4 was done or the above steps could not be smoothly progressed (Fig. 1b); (6) after abdominal exploration, a medial-to-lateral approach was adopted: The inferior mesenteric vessels (IMV) were skeletonized, ligated and divided. Attachments of descending colon, splenic flexure (if necessary) and the upper rectum were dissected until the specimen was in free continuity with the previous transanal dissection. (7) The specimen was extracted through the anus (Fig. 1c). After extracting the specimen, a stapled end-to-end coloanal anastomosis (Fig. 1d) was fashioned, while a protective ileostomy was created. The rubber tube was left in place as a pelvic drain and would usually be removed in the postoperative days 3–5 (Fig. 1 white arrow), while abdominal drain was not regularly placed.
Demonstrations of surgical procedure. a Transanal dissection. The demonstration of the first transanal SILS port (Covidien, Mansfield, MA). b Single-port assisted laparoscopic dissection. The demonstration of the second SILS port in the future ileostomy site. c Specimen extracted transanally and d an end-to-end stapled anastomosis. White arrow indicates the rubber tube that was introduced through the ischiorectal fossa into the anorectum to act as a mini-trocar and a postoperative pelvic drain
Result
Totally six patients operated in the dual-mode SPLS–TaTME surgery between July 2014 and March 2015 were included in this study. The detailed demographic characteristics, operative outcomes and pathologic results of the patients are summarized in Table 1. Apart from one female patient, the other five patients were males. Their median age was 68 years (range 55–80). None of them were obese patients (BMI < 30 kg/m2). The biopsied proven adenocarcinomas were all located in low rectum with a median distance from anal verge of 4.0 cm (range 3–6). One patient with a history of prostatectomy for prostate cancer presented splenic mass (Fig. 2). Metastasis could not be excluded, and an extra splenectomy was planned. Due to the locally advanced stage of tumors, case 2, 3 and 6 underwent neoadjuvant therapy. In the case requiring splenectomy, the spleen was not morcellated and extracted transanally (Fig. 2b). Median operative time of these six patients was 360 min (range 310–420). The case requiring splenectomy was the most time-consuming with an estimated blood loss of 800 ml due to a massive hemorrhage when mobilizing the gastrosplenic ligament, which necessitated a transfusion postoperatively. The splenic tumor was not a metastasis but an inflammatory myofibroblastic tumor that was characterized by proliferation of spindle cells with variable inflammatory cells, according to the pathologic (Fig. 2c) and immunostaining results (Actin++, CD 21+, CD 23+, ALK+, Desmin−, not shown in figure). The mesorectal fascia was intact, and the distal and circumferential margins were uninvolved in all patients. The median number of the harvested lymph nodes was 11.5 (range 4–12). The pathologic TNM stage is listed in Table 1. In the fourth case, we did not fashion a protective ileostomy because the patient asked for no stoma and during operation his risk of leak was not estimated high. As for postoperative recovery, the median time of recovering to flatus was 3.5 days (range 3–4). All patients recovered uneventfully except the first case who experienced an acute myocardial infarction in the postoperative day 3 which was treated conservatively. Up till now, after a short-term follow-up (median 4 months, range 3–9 months), all ileostomies had been closed without complications, and all the six patients have been free of recurrence and are fully continent.
a Well-demarcated and non-enhanced mass (32 × 24 mm) located in the upper pole of spleen (white arrow) was shown in abdominal CT scan. b Gross specimen showing the tumor in the cut-opened spleen and c optical microscopy at low power showing the hematoxylin–eosin-stained lesion of the spleen. Spindle cell proliferated in a collagenous stroma, predominantly infiltrated by an admixture of inflammatory cells
Discussion
The quest of less surgical trauma has been an important direction of current abdominal surgery. So the progress of multiple-port laparoscopic surgery (MPLS) to single-port laparoscopic surgery (SPLS), to natural orifice transluminal endoscopic surgery (NOTES)—so called “no-scar” surgery, represents a logical and important developing route. Although the feasibility of SPLS for colon cancer has been well demonstrated [13], SPLS for rectal cancer, particularly for cancer lying in the distal rectum, is definitely more difficult and challenging [3, 14, 15]. NOTES in the field of rectal surgery was more of an ideal concept rather than a general practice before the advent of TaTME. The emerging of TaTME makes it possible to resect the diseased rectum through the anus without difficulty of opening the vagina or enlarging the stoma site [8]. The preferred TaTME platform is the disposable multi-channels single-port (TAMIS) [16]. However, TaTME performed totally in a transanal with the division of the inferior mesenteric vessels and mobilization of the proximal colon and splenic flexure can be challenging [17]. That is why in subsequent cadaveric studies, laparoscopic assistance through the abdomen was introduced (TAMIS-assisted) [18]. Many surgical teams still prefer to use the standard MPLS as assistance on human patients, that s probably because it is considered easier and more straightforward than SPLS assistance. In fact, some authors have employed a SPLS-assisted technique in which they used a single-port plus one or more extra trocars, which, strictly speaking, represents MPLS assistance [18, 19].
However, as shown in Table 2, some surgical teams have already shown the feasibility of the pure SPLS–TaTME technique. In 2011, Tuech et al. [12] reported the first case of a 45-year-old female using two endorec trocars (Aspide France). Recently, they reported a large case series of TaTME in a study (n = 56) with eight cases operated by SPLS–TaTME technique (no detailed information provided) [4]. In 2012, Dumont et al. [5] specifically enrolled four consecutive male patients with narrow pelvis in a small study and concluded that SPLS–TaTME approach might be easier and safer to operate than traditional approach. Similar to us, in 2013, Velthuis et al. [11] reported five cases using two SILS ports, which all achieved clear surgical margins and intact mesorectal fascia.
Given the fact that totally more than 10 cases of pure TaTME without laparoscopic assistance have been reported so far [20–22], pure TaTME is no longer regarded as a mission impossible. Therefore, it is rational to hypothesize that if the majority of operation including the most difficult part encountered by conventional laparoscopic surgery—the mobilization of the extraperitoneal rectum could be completed by the transanal approach, the abdominal assistance would become much easier and less important, even be neglected in selected patients. Thus, SPLS assistance might be adequate. A direct comparison between SPLS–TaTME and MPLS–TaTME is needed. In fact, previous studies have utilized both approaches, e.g., Tuech et al. [4], Velthuis et al. [23], Sourrouille et al. [7] and Chouillard et al. [20]. Unfortunately, none of them made such a subgroup comparison, which might be due to the limited sample size. Herein, we made a list of several theoretical advantages and disadvantages among SPLS–TaTME, MPLS–TaTME and conventional SPLS (Table 3).
Our results are comparable to the studies using SPLS–TaTME technique (Table 2). Furthermore, we presented the first case combined a splenectomy with SPLS–TaTME. The specimen was extracted through the anus, which represented a better embodiment of NOTES. This case was more time-consuming, but bleeding was managed without adding extra trocars or converting to open laparotomy. Given that the single-port placement is similar, liver resection may also be attempted with this method.
Despite SPLS–TaTME being more costly as shown in Table 3, it might be superior to the pure SPLS or pure TaTME technique with respect to the operative complexity and difficulty in low rectal cancer. As for considerations of economics and asepsis, we wonder whether it is feasible to complete the abdominal portion first, and then perform TaTME by transferring down to reuse the same port.
The present study has several limitations. First of all, the small sample size. Second, none of the patients in the present series were obese, which increases operative difficulty. Third, despite the fact that we suggested SPLS–TaTME is more minimally invasive, we could not make a direct comparison with MPLS–TaTME or conventional rectal surgery regarding postoperative pain, trauma-induced inflammatory response [24], or body image and scar scale [5]. Last but not least, due to the limited follow-up, we could not draw any conclusion about the oncologic and functional outcomes of this technique, particularly given the fact that the benefits of TaTME itself have not been adequately proven.
Conclusion
In conclusion, this study demonstrated that SPLS–TaTME technique is safe and feasible in low rectal cancer in selected patients. Further studies with larger sample size and long-term results including oncologic and functional outcomes are warranted in future.
References
Jeong SY, Park JW, Nam BH et al (2014) Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol 15:767–774
van der Pas MH, Haglind E, Cuesta MA et al (2013) Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 14:210–218
Hamabe A, Takemasa I, Uemura M et al (2014) Feasibility of single-port laparoscopic surgery for sigmoid colon and rectal cancers and preoperative assessment of operative difficulty. J Gastrointest Surg 18:977–985
Tuech JJ, Karoui M, Lelong B et al (2015) A step toward NOTES total mesorectal excision for rectal cancer: endoscopic transanal proctectomy. Ann Surg 261:228–233
Dumont F, Goere D, Honore C, Elias D (2012) Transanal endoscopic total mesorectal excision combined with single-port laparoscopy. Dis Colon Rectum 55:996–1001
Chen WH, Luo SL, Kang L (2015) Transanal total mesorectal excision: will it be a valid alternative in rectal cancer surgery? Ann Surg. doi:10.1097/SLA.0000000000001108
Sourrouille I, Dumont F, Goere D, Honore C, Elias D (2013) Resection of rectal cancer via an abdominal single-port access: short-term results and comparison with standard laparoscopy. Dis Colon Rectum 56:1203–1210
Sylla P, Rattner DW, Delgado S, Lacy AM (2010) NOTES transanal rectal cancer resection using transanal endoscopic microsurgery and laparoscopic assistance. Surg Endosc 24:1205–1210
Atallah S, Martin-Perez B, Albert M et al (2014) Transanal minimally invasive surgery for total mesorectal excision (TAMIS-TME): results and experience with the first 20 patients undergoing curative-intent rectal cancer surgery at a single institution. Tech Coloproctol 18:473–480
Heald RJ (2013) A new solution to some old problems: transanal TME. Tech Coloproctol 17:257–258
Velthuis S, van den Boezem PB, van der Peet DL, Cuesta MA, Sietses C (2013) Feasibility study of transanal total mesorectal excision. Br J Surg 100:828–831 (discussion 831)
Tuech JJ, Bridoux V, Kianifard B et al (2011) Natural orifice total mesorectal excision using transanal port and laparoscopic assistance. Eur J Surg 37:334–335
Makino T, Milsom JW, Lee SW (2012) Feasibility and safety of single-incision laparoscopic colectomy: a systematic review. Ann Surg 255:667–676
Jung KU, Yun SH, Cho YB, Kim HC, Lee WY, Chun HK (2014) Single incision and reduced port laparoscopic low anterior resection for rectal cancer: initial experience in 96 cases. ANZ J Surg. doi:10.1111/ans.12775
Sirikurnpiboon S, Jivapaisarnpong P (2013) Single-access laparoscopic rectal surgery is technically feasible. Minim Invasive Surg 2013:687134
Martin-Perez B, Andrade-Ribeiro GD, Hunter L, Atallah S (2014) A systematic review of transanal minimally invasive surgery (TAMIS) from 2010 to 2013. Tech Coloproctol 18:775–788
Whiteford MH, Denk PM, Swanstrom LL (2007) Feasibility of radical sigmoid colectomy performed as natural orifice translumenal endoscopic surgery (NOTES) using transanal endoscopic microsurgery. Surg Endosc 21:1870–1874
McLemore EC, Coker AM, Devaraj B et al (2013) TAMIS-assisted laparoscopic low anterior resection with total mesorectal excision in a cadaveric series. Surg Endosc 27:3478–3484
Chen CC, Lai YL, Jiang JK et al (2015) The evolving practice of hybrid natural orifice transluminal endoscopic surgery (NOTES) for rectal cancer. Surg Endosc 29:119–126
Chouillard E, Chahine E, Khoury G et al (2014) NOTES total mesorectal excision (TME) for patients with rectal neoplasia: a preliminary experience. Surg Endosc 28:3150–3157
Zhang H, Zhang YS, Jin XW, Li MZ, Fan JS, Yang ZH (2013) Transanal single-port laparoscopic total mesorectal excision in the treatment of rectal cancer. Tech Coloproctol 17:117–123
Leroy J, Barry BD, Melani A, Mutter D, Marescaux J (2013) No-scar transanal total mesorectal excision: the last step to pure NOTES for colorectal surgery. JAMA Surg 148:226–230 (discussion 231)
Velthuis S, Nieuwenhuis DH, Ruijter TE, Cuesta MA, Bonjer HJ, Sietses C (2014) Transanal versus traditional laparoscopic total mesorectal excision for rectal carcinoma. Surg Endosc 28:3494–3499
Bulut O, Aslak KK, Levic K et al (2015) A randomized pilot study on single-port versus conventional laparoscopic rectal surgery: effects on postoperative pain and the stress response to surgery. Tech Coloproctol 19:11–22
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and with the ethical standards of Helsinki declaration.
Informed consent
Informed consent was obtained from all individual participants included in the study. Patients also gave permission for publication of technique and results.
Additional information
W.-H. Chen and L. Kang contributed equally to this work and share the first author.
Rights and permissions
About this article
Cite this article
Chen, WH., Kang, L., Luo, SL. et al. Transanal total mesorectal excision assisted by single-port laparoscopic surgery for low rectal cancer. Tech Coloproctol 19, 527–534 (2015). https://doi.org/10.1007/s10151-015-1342-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10151-015-1342-1