Abstract
Background
Frameless stereotaxy for real-time, image-guided surgery has been most utilized for neurological and orthopedic surgery. Recently, our center has reported the application of real-time navigation for transanal total mesorectal excision.
Methods
During a 5-month period (June 2013–October 2013), three male patients underwent transanal minimally invasive surgery for total mesorectal excision with image-guided real-time navigation during the transanal portion of the operation. This was completed using a frameless stereotactic navigational system as shown in a demonstration video. Male patients with anterior, locally advanced rectal cancer were selected for enrollment into the pilot study.
Results
Three male patients (mean age 69) underwent transanal total mesorectal excision with stereotactic navigation during a 5-month study period. Mean operative time was 402 min, and there were no intra-operative complications recorded. The mean distance from anal verge of the tumor was 6.3 cm (range 4–8 cm). The navigational accuracy was computed to be ±3.69 mm (range ±3.20 to ±4.02 mm). The average navigation setup time was 47 min, not including scan time. The surgical specimens were found to have completely intact mesorectal envelopes (Quirke 3) in all cases. All margins, including radial and distal margins, were negative. Mean postoperative length of stay was 5 days. At a median of 18-month follow-up, there was no evidence of locoregional recurrence or distant metastatic disease.
Conclusion
This is the first pilot series to report the use of frameless stereotactic navigation for TAMIS-TME. Stereotactic navigation for transanal total mesorectal excision is shown to be feasible, and may aid in providing colorectal surgeons with the ability to better perform safe, high-quality surgery in select cases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Most of the advancements in modern surgery can be credited to the evolution of technology. As the operating theater becomes increasingly transformed to what is sometimes termed the ‘Digital OR,’ surgeons—in some fields—have been able to successfully apply 3D imaging and advanced de novo model construction to integrate with navigational software that can be used to determine the operative approach for planning (preoperative), and also for real-time surgical decision making. Navigation techniques in principle augment the surgeon’s understanding of (a) target organ location, (b) approach to the target anatomy, and (c) location of critical anatomic structures relative to the target organ. When navigational aides resolve these points, surgery has the potential of being safer with better outcomes [1].
Currently, neurosurgeons are the principle users of real-time navigation. The best evidence for the value of navigation is for glioma surgery, where neuronavigation has been shown to improve resection with improved patient outcomes [2]. Orthopedic navigation systems are also in use. They often employ a ‘model’-based map that is computer generated and thus does not require a radiographic scan for navigation. This system is used in joint replacement surgery to assure precise length and offset for accurate component alignment [1, 3].
While navigation is principally utilized by neurological and orthopedic surgeons, the modality of real-time imaging can be applied to any area where the target organ is fixed. Recently, our center has described the technique for performing transanal minimally invasive surgery (TAMIS) for total mesorectal excision (TME) using real-time navigation [4]. We propose that this technique could improve TME quality and may decrease the risk of urethral injury during transanal TME. Here we report the results of our pilot study of the first three patients who underwent TAMIS-TME with navigation.
Methods
During a 5-month period (June 2013–October 2013), three male patients underwent TAMIS-TME with image-guided real-time navigation. Navigation was used for the transanal portion of the operation only. Internal Review Board (IRB) approval was obtained (IRB No. 581811-2, Florida Hospital, Orlando, FL, USA), and a retrospective analysis of this pilot study was performed.
The planned operative resection was a laparoscopic resection with TAMIS-TME. This step-by-step approach to TAMIS-TME has been described by our group elsewhere [5], and this approach has also been described by other investigators [6]. The TAMIS-TME portion of the operation was to be completed using frameless stereotaxy, using the methodology described previously [4]. Special departmental approval was granted for this approach from our institution under the guidelines of a pilot study. Male patients with anterior rectal cancers were selected because it was hypothesized that stereotactic navigation could help achieve negative margins while preventing urethral injury—an important technique—specific morbidity of transanal [7, 8].
General operative approach
The abdominal portion of the TAMIS-TME was performed as the first step in each case, and this did not involve stereotactic navigation. All patients underwent a full mechanical bowel preparation with polyethylene glycol. Prior to surgery, parenteral antibiotics (1 g ertapenem) were administered. Bilateral ureteral lighted stents were placed in two of the three patients, and the abdomen and perineum were prepped and draped with the patient in modified lithotomy. Patients were kept in the modified lithotomy position for all parts of the operation, except during intra-operative imaging, where the patient was positioned supine. The abdominal portion of the operation was completed prior to proceeding with stereotactic TAMIS-TME. This included division of the inferior mesenteric vein and artery, as well as mobilization of the splenic flexure, and in each case a defunctioning loop ileostomy was created.
Technique for navigation
The detailed approach to stereotaxy for TAMIS-TME has been described by our group previously [4], and is also demonstrated here in the multimedia video supplement [electronic supplementary material]. Briefly, stereotaxy requires 3D localization technology which utilizes a stereoscopic camera emitting infrared light (Fig. 1), a computer platform containing commercially available navigation software (Stryker Navigation, Kalamazoo, Michigan, USA), and two navigational ‘trackers.’ One tracker is assigned to the patient, while the other is assigned to a surgical instrument—such as a laparoscopic cautery device (Figs. 2, 3, 4). An MRI or CT scan was then used as a ‘map’ to allow for image-guided, real-time navigation. The imaging and navigation software (after a calibration process) allow the system to correlate any point on the scanned portion of the patient with the corresponding (actual) point on the patient. This is done by creating a reference array near the operative field (Fig. 5). Once calibration is completed, the operating surgeon can use real-time image-guided navigation for TAMIS-TME (Fig. 6a, b).
Modern stereotaxy for real-time intraoperative navigation relies on a stereoscopic infrared camera that is capable of tracking the 3D position of reflective ‘marker spheres’ (not shown). The camera is mounted from a fixed point, in this case the OR theater’s ceiling. The operating theater is also equipped with a CT (as shown) or MRI scanner that allows for intra-operative scanning which creates the image used by the navigational software
Tracking tool (bottom) is used to identify points of a reference array (shown in Fig. 5); this information is stored in the navigational software and is used to compute the tip of the tracking tool during surgery so that important anatomic structures may be properly identified. A tracking tool (with infrared reflective spheres) can be secured to most laparoscopic tools, and in this case has been secured to the cautery device which will be used to perform TAMIS-TME. Using a calibration tool (not shown), the position of the cautery tip can be computed in real time allowing the operating surgeon to know the anatomic plane of dissection with sub-centemeric accuracy
In vivo view of TAMIS-TME with intra-operative stereotactic navigation. While the dissector can be freely manipulated by the surgeon’s hand, the tracker of the device must always maintain line of sight with the ceiling-mounted camera, much as a global positioning system relies on direct line of sight with geostationary satellites. The marker spheres on the tracking device are clearly visible
Navigation requires a fixed point of reference. In this case, a bedside rail mounted tracker (called the patient tracker) does not move, and is shown mounted over the abdomen. It has a direct line of sight with the wall-mounted camera (shown in Fig. 1)
Reference array of skin-fixed, radiopaque markers are placed overlying the area of anatomic interest, in this case the pelvis. By registering each of these points using the pointer navigation tool as shown in Fig. 2, any point in the patient’s anatomy can be correlated to a point on an image—such as a CT or MRI scan allowing for real-time and accurate navigation
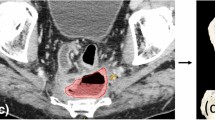
a Real-time navigation in progress during TAMIS-TME. The surgeon can utilize optical information and navigational data. The 3D position of the tip of the surgeon’s laparoscopic dissector shows as a green wand overlying the scan and it moves in real time following the actions of the surgeons’ hand. The navigational information is so precise that the surgeon is able to operate while focusing the majority of the time on the imaging rather than the optical feed, as shown. b The precise location of the dissection tip is determined by the navigational software in real time (shown as a green, virtual wand overlying MRI scan) and correlates with the actual location of the dissector as seen on the optical image from the laparoscopic camera
Results
Three male patients (mean age 69) with mean BMI 26 kg/m2 underwent TAMIS-TME with the aid of stereotactic navigation during a 5-month study period. All patients had received neoadjuvant long-course external beam radiotherapy with 5400 cGy and concomitant infusional 5-fluorouracil. Mean operative time was 402 min, and there were no intra-operative complications recorded. The mean distance from anal verge of the tumor was 6.3 cm (range 4–8 cm). In case 1, a CT scan was used as the navigation, while for cases 2 and 3, an MRI scan was used. The navigational accuracy was computed to be ±3.69 mm (range ±3.20 to ±4.02 mm). The average navigation setup time was 47 min, not including scan time.
Examination of the TME specimen was performed by an experienced GI pathologist and independently graded. Analysis included macroscopic evaluation of the mesorectal envelope and cross-sectional evaluation through the portion of rectum containing the tumor. Grading was performed according to the Quirke method. The specimens were found to have completely intact mesorectal envelopes (Quirke 3) in all cases (Fig. 7a, b). All margins, including radial and distal margins, were negative with the closest margin being 0.6 cm from the radial margin. Postoperative length of stay was 5 days. At a median of 18-month follow-up, there was no evidence of locoregional recurrence or distant metastatic disease. The mean time to ileostomy closure was 7.3 months. All three patients had a normal barium enema prior to ileostomy reversal, and there was no operative morbidity with closure.
Perioperative morbidity included two patients who developed pre-renal syndrome due to high output from the defunctioning loop ileostomy. Both instances required re-admission and fluid resuscitation, and both patients responded to treatment without further sequelae. Long-term morbidity included lifestyle-limiting fecal incontinence which was managed with dietary changes and pelvic floor rehabilitation, but which ultimately required placement of a sacral nerve stimulator 6 months after ileostomy closure. Characteristics and clinical outcomes for patients undergoing TAMIS-TME with real-time, image-guided navigation are delineated in Table 1.
Discussion
This pilot study applies an existing navigational system to a fixed pelvic target organ, and it is shown that the approach used allows for acceptable results with TAMIS-TME and precision to within ±3.69 mm deviation. While this navigational approach is theoretically capable of providing near ±1 mm accuracy, in this pilot study, it was not achieved. Potential factors causing loss of precision could be explained by fiducial placement. Multiple options were considered for positioning the fiducials; positioning on the thighs was considered; however, this was not feasible as the patient is positioned supine during the imaging acquisition (both CT and MRI). Placement of fiducials along the dermis of the lower aspect of the anterior abdominal wall (Fig. 5) is stationary, but there are small changes in fiducial position during positive pressure ventilation while the patient is anesthetized. It was also noted that there was some very small but measurable changes in the fiducial position (relative to the rendered scan) as the lower extremities were positioned into high dorsal lithotomy once the scan process has been completed.
The technique of navigation for surgery beyond neurological surgery and orthopedic surgery is quite limited, although feasibility has been established for adrenal gland surgery [9], otologic surgery [10], and on an experimental basis, liver surgery [11–13]. Navigation is most applicable to fixed organ targets—such as bone, brain, retroperitoneal organs such as the kidneys, adrenal glands, pancreas—as well as fixed pelvic viscera including the sub-peritoneal rectum. Because movement of the organ after imaging is obtained will greatly decrease accuracy, navigation for non-fixed abdominal viscera (such as the ileum and jejunum) is not practical. In this study, MRI was more useful than CT scan for maintaining the proper TME plane, because the mesorectal envelope, like all soft tissue, is best visualized using magnetic resonance and not X-rays. While 2D multi-planar rendering of both CT and MRI images was used during navigation, the most useful modality was 3D multi-planar rendering of rectal protocol, 3-Tesla MRI imaging. This provided the clearest image for the operating surgeon, and 3D multi-planar rendering—as computed by the navigation software—provided a more accurate reflection of the actual anatomy.
A limitation of navigation for TAMIS-TME is that it is not able to delineate the contiguous planes of the mesorectal envelope and the endopelvic fascia, which are fused by mesothelial and connective tissue layers [14]. These anatomically attached planes require precise separation by careful, sharp dissection in the Bill Heald plane. Because of the proximity of the autonomic pelvic nerve plexus (providing innervation to the internal anal sphincter), injury to nerves during TAMIS-TME can still occur even with stereotactic navigation. As observed in this pilot study, one patient developed lifestyle-limiting fecal incontinence, presumably secondary to nerve injury during TAMIS-TME. Thus, navigation aids provide a more general understanding of the surgeon’s plane of dissection, and currently navigation is not likely to result in improved nerve preservation as current imaging is not able to discern planes between contiguous fascia. Furthermore, the utility for TAMIS-TME is most appreciated for the distal two-thirds of the rectum. In this study, the proximal rectum was best approached with standard laparoscopic techniques as there was less complexity in performing a resection at the level of the upper rectum extending to the level of the peritoneal reflection. However, it is imperative that these planes are communicated at the correct level—because the rectum and sigmoid colon represent mesenteric organs with mesenteric contiguity [15]. This represents an important principle in oncologic resection that should be underscored.
Navigation requires careful planning and adds to the operative time. In this pilot series, the average increase in case time (navigation setup time) was 47 min. This did not include scan times; it was theorized that much of this was due to non-familiarity of the OR team and personal with the navigational techniques and due to the inherent learning curve with this new application of existing technology. The technique of navigation for TAMIS-TME was found to be useful in three areas: (a) maintaining an appropriate plane of dissection, (b) avoiding key anatomic structures, such as the male urethra, and (c) determining the progress of the dissection. As we unlock new integration pathways which include surgical navigation, the OR of tomorrow will provide the surgeon continuous and reliable anatomic orientation. This could render inherently complex operations safer with the potential for improving surgical outcomes as the surgeon’s understanding of anatomic planes is augmented.
Conclusion
This is the first pilot series to report the use of frameless stereotactic navigation for TAMIS-TME (taTME). Stereotactic navigation for transanal TME is shown to be feasible and accurate. It may aid in providing colorectal surgeons with the ability to perform a safe, high-quality rectal cancer operation for selected patients.
References
Mezger U, Jendrewski C, Bartels M (2013) Navigation in surgery. Langenbecks Arch Surg 398:501–514
Wirtz CR, Albert FK, Schwaderer M et al (2000) The benefit of neuronavigation for neurosurgery analyzed by its impact on glioblastoma surgery. Neurol Res 22:354–360
Wixson RL, MacDonald MA (2005) Total hip arthroplasty through a minimal posterior approach using imageless computer-assisted hip navigation. J Arthroplasty 20:51–56
Atallah S, Nassif G, Larach S (2015) Stereotactic navigation for TAMIS-TME: opening the gateway to frameless, image-guided abdominal and pelvic surgery. Surg Endosc 29:207–211
Atallah S, Albert M, DeBeche-Adams T, Nassif G, Polavarapu H, Larach S (2013) Transanal minimally invasive surgery for total mesorectal excision (TAMIS-TME): a stepwise description of the surgical technique with video demonstration. Tech Coloproctol 17:321–325
Araujo SE, Crawshaw B, Mendes CR, Delaney CP (2015) Transanal total mesorectal excision: a systematic review of the experimental and clinical evidence. Tech Coloproctol 19:69–82
Atallah S (2015) Transanal total mesorectal excision: full steam ahead. Tech Coloproctol 19:57–61
Rouanet P, Mourregot A, Azar CC et al (2013) Transanal endoscopic proctectomy: an innovative procedure for difficult resection of rectal tumors in men with narrow pelvis. Dis Colon Rectum 56:408–415
Mårvik R, Langø T, Tangen GA et al (2004) Laparoscopic navigation pointer for three-dimensional image-guided surgery. Surg Endosc 18:1242–1248
Kohan D, Jethanamest D (2012) Image-guided surgical navigation in otology. Laryngoscope 122:2291–2299
Herline A, Stefansic JD, Debelak J, Galloway RL, Chapman WC (2000) Technical advances toward interactive image-guided laparoscopic surgery. Surg Endosc 14:675–679
Herline AJ, Stefansic JD, Debelak JP et al (1999) Image-guided surgery: preliminary feasibility studies of frameless stereotactic liver surgery. Arch Surg 134:644–649 (discussion 649–650)
Lamadé W, Vetter M, Hassenpflug P, Thorn M, Meinzer HP, Herfarth C (2002) Navigation and image-guided HBP surgery: a review and preview. J Hepatobiliary Pancreat Surg 9:592–599
Culligan K, Walsh S, Dunne C et al (2014) The mesocolon: a histological and electron microscopic characterization of the mesenteric attachment of the colon prior to and after surgical mobilization. Ann Surg 260:1048–1056
Coffey JC, Sehgal R, Culligan K et al (2014) Terminology and nomenclature in colonic surgery: universal application of a rule-based approach derived from updates on mesenteric anatomy. Tech Coloproctol 18:789–794
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests. The authors received no funding for this study, and the research was not supported by any grants or other funding. Dr. S. Atallah is a paid consultant for Applied Medical, Inc. Dr. B. Martin-Perez and S. Larach have no disclosures.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (MOV 429000 kb)
Rights and permissions
About this article
Cite this article
Atallah, S., Martin-Perez, B. & Larach, S. Image-guided real-time navigation for transanal total mesorectal excision: a pilot study. Tech Coloproctol 19, 679–684 (2015). https://doi.org/10.1007/s10151-015-1329-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10151-015-1329-y