Abstract
Introduction
The aim of the study was to determine the impact of positive surgical margins (PSM) after PN on very long-term recurrence in a contemporary cohort.
Methods
Patients who underwent PN for a localized renal tumour were included. Patients were stratified according to the presence of PSM. Data on patients’ characteristics, the tumour, the peri- and postoperative events were collected. Disease-free survival (DFS) and overall survival (OS) were assessed by the Kaplan–Meier method and compared by the log-rank test. Sensitivity analyses using weighted propensity score analysis was performed to account for potential selection biases arising from the nonrandom allocation of patients to different groups.
Results
A total of 1115 patients were included in the study. The incidence of PSM was 5.4% (n = 61). The median follow-up time was 51 months for the PSM group and 61 months for the NSM group (p = 0.31). Recurrence rates were significantly higher in the PSM group (13%, n = 8) compared to the NSM group (7%, n = 73) (p = 0.05). This resulted in a significant reduction in DFS in the PSM group (p = 0.004), particularly pronounced in patients with clear cell renal cell carcinoma. Additionally, OS was significantly lower in the PSM group (p < 0.01). Propensity score analysis confirmed a decrease in DFS for the PSM group (p = 0.05), while there was no significant difference in OS between the two groups (p = 0.49).
Conclusion
In this retrospective multicenter study, PSM impact on oncological outcomes, increasing recurrence, but no difference in OS was observed post-adjustment for biases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Partial nephrectomy (PN) is a preferred treatment for localized renal tumors, offering comparable oncological results and superior preservation of renal function compared to radical nephrectomy [1, 2].
The primary aim of nephron-sparing surgery is to secure adequate surgical margins while conserving as much healthy renal parenchyma as possible. Nevertheless, instances of positive surgical margins (PSM) have been reported, with their occurrence ranging from 0.1% to 15% [3]. The impact of surgical margin status on the survival of patients with renal cell carcinoma (RCC) after PN remains to be clearly defined. The data on survival outcomes are subject to debate, often constrained by the scope of single-institution cohort studies and limited follow-up durations. Some teams concluded that the survival of PSM patients was not worse [4, 5]. Conversely, other research indicates a higher risk of recurrence associated with PSM, particularly local recurrence attributed to aggressive tumor types, potentially leading to a more severe disease progression [6, 7].
Given the lack of consensus, a preference for surveillance is observed, with no endorsement of adjuvant treatment in such cases [8]. Recent discussions have been revitalized by the Keynote-564 trial results, which highlighted the benefits of adjuvant pembrolizumab over placebo for patients with RCC. However, the criteria used to identify at-risk patients in this trial are contentious [9], raising questions about the benefits of adjuvant treatment for a specific subgroup of patients with positive margins.
This study aimed to investigate the long-term effects of PSM on oncological outcomes following PN in a sizable, contemporary cohort.
Patients and methods
Study population
After institutional review board approval (CEERB Paris Nord, IRB n°00006477-15-073), a retrospective review was performed to identify all patients who underwent PN for a localized renal tumor between January 1, 2000 and December 31, 2020, at three tertiary academic institutions.
The choice of surgical approach, resection techniques, and clamping type was based on the surgeon’s preference. Excluded were patients with multiple renal tumors, benign final pathology, and those who underwent conversion to radical nephrectomy.
Evaluations of surgical margin status were according to each institution’s pathology report and were not standardized across each institution. PSM was defined by the presence of cancer cells at the edge of the tissue.
Covariates
Demographics, tumor, and pathological characteristics were collected in an electronic database. Demographics included age, gender, American Society of Anesthesiologists [ASA] classification, Charlson score (CCI), body mass index (BMI), anticoagulant/antiplatelet treatments, and surgical approach. Kidney function (creatinine and GFR according MDRD: modification of diet in renal disease) was evaluated before the surgery and at 1 month. Tumours’ characteristics included tumour size, tumour side, tumour position, RENAL nephrometry score [10]. Pathologic data included stage, Fuhrman grade, histologic subtype, microscopic vascular invasion, tumor necrosis and sarcomatoid features.
Perioperative outcomes
The following variables were collected: operative time (OT), warm ischemia time (WIT), estimated blood loss (EBL), overall complication rate, major complication rate, positive surgical margins, absolute change in eGFR and length of hospital stay (LOS). Postoperative complications were graded using the Clavien–Dindo classification [11]. Major complications were defined as a Clavien score of three or higher. All outcomes were recorded within 30 days after the procedure.
Follow-up protocol and outcomes
Postoperative follow-up was institution- and physician-dependent, but generally followed national and international guidelines. Typically, this involved an initial outpatient appointment 1 month after surgery, followed by semi-annual check-ups for the first 3 years and yearly examinations for at least another 3 years. The follow-up process included evaluating patient-specific medical history, conducting physical exams, and performing enhanced CT scans of the thorax, abdomen, and pelvis. Disease recurrence was defined as the appearance of a tumoral mass in the resection bed or kidney fossa (local recurrence), regional lymph nodes, or metastasis in distant organs. Overall survival (OS) was defined as the time from surgery to death from any cause. Patients alive were censored at the date of their last follow-up.
Statistical analysis
Continuous variables were summarized with medians and interquartile ranges (IQRs); categorical variables were summarized with frequency counts and percentages and groups were compared using the chi-squared and Fisher’s exact tests. Quantitative variables were compared using the Mann–Whitney U test. DFS and OS, as well as the respective percentages of patients who were alive and disease-free or alive at key time points, were estimated by means of the nonparametric Kaplan–Meier method. Hazard ratios and 95% confidence intervals were estimated with the use of a Cox proportional-hazard model and log-rank tests were used to compare the differences between groups. Finally, various sensitivity analyses were performed. First, an analysis was performed to test the hypothesis that the impact of PSM was different according to the histological subtype. Second, to account for potential selection biases arising from nonrandom allocation of patients to different groups, we performed a weighted propensity score analysis [12]. The propensity score was calculated using a multivariable logistic model that included the following variables: age, Charlson score, tumor size, T stage, nuclear grade, and histologic subtypes. Inverse probability of treatment weighting (IPTW)-adjusted Kaplan–Meier curves were used to estimate DFS and OS in each group (PSM vs. NSM). To test for equality of survival in the two groups, an IPTW- adjusted log-rank test was used [13]. Statistical analyses were performed using Stata 14.1 statistical software (Stata, College Station, TX, USA). All tests were two-sided, with a significance threshold set at p < 0.05.
Results
Patients’ operative and tumours’ characteristics
We identified 1115 patients who met the criteria for study inclusion. Patients’ characteristics are summarized in Table 1. The PSM rate was 5% (N = 61). The groups were similar regarding gender, anticoagulant/antiplatelet treatments, BMI, and operative technique. Tumors were larger (38 vs. 32 mm) in the PSM group (p = 0.03).
Table 2 presents the perioperative outcomes for each group. Most intraoperative outcomes, including OT, WIT, EBL, and early unclamping rate, were comparable between the two groups (all p > 0.05). The rates of overall complications and major complications were higher in the PSM group (41% vs 25%; p = 0.007 and 21% vs 11%; p = 0.01; respectively). There was no statistically significant difference in eGFR variation at 1 month (−10 vs −12 mL/min; p = 0.63).
Pathological findings (Table 3) revealed that clear cell RCC was the most common histological subtype of RCC in both groups (70% vs. 75%). There was no statistically significant difference between the two groups regarding pathologic stages (p = 0.10), Fuhrman grade (p = 0.21), tumor necrosis (0.37), microscopic vascular invasion (p = 0.77), or sarcomatoid features (p = 0.11).
Survival analysis
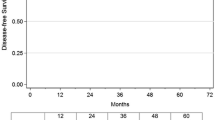
The median follow-up time was 51 months for the PSM group and 61 months for the NSM group (p = 0.31). There was a total of 81 recurrences: 8 (13%) in the PSM group and 73 (7%) in the NSM group. Figure 1 displays Kaplan–Meier curves for Disease-Free Survival, segmented by surgical margin status. The 10-year DFS rates were 76% for the PSM group and 80% for the NSM group, respectively. The risk of disease recurrence was found to be higher in the PSM group than in the NSM group (p = 0.044). Over the follow-up period, 67 patients died. The risk of death was found to be higher in the PSM group than in the NSM group (Fig. 2).
Sensitivity analysis
To mitigate selection bias and validate the robustness of our findings, we conducted a weighted propensity score analysis. The IPTW-adjusted Kaplan–Meier curves, as illustrated in Fig. 3A, B, demonstrate that patients with PSM experienced a significantly shorter median DFS compared to those with NSM (p = 0.05; Fig. 3A). However, no significant differences were observed in OS between the PSM and NSM groups (all p > 0.05, according to the IPTW-adjusted log-rank test; Fig. 3B).
Histological subgroup analysis
Supplementary Fig. 1 illustrates the DFS of patients with NSM and PSM, categorized by histological subtype. In patients with clear cell Renal Cell Carcinoma those in the PSM group exhibited a significantly shorter median DFS compared to the NSM group (p = 0.03). Among patients with papillary Renal Cell Carcinoma, the Kaplan–Meier curve indicated that DFS in the PSM group was lower than in the NSM group, though the difference was not statistically significant (p = 0.09) (Supplementary Fig. 1B). For patients with chromophobe Renal Cell Carcinoma (chRCC), there was no significant difference in DFS between the PSM and NSM groups (p = 0.98) (Supplementary Fig. 1C).
Discussion
The effectiveness of any curative cancer surgery hinges on the complete excision of the tumour. The presence of cancer cells at the surgical margin often indicates a less favourable prognosis for the patient. Despite advancements in surgical techniques, including the adoption of robotic approach, PSM in PN specimens remain a relatively common occurrence. This raises concerns about the potential for increased recurrence risk, and the implications for long-term oncologic outcomes are still not fully understood. In our multi-center study, we analysed positive surgical margins following partial nephrectomy over a median follow-up period of 51 months. We noted a PSM rate of 5.4% and observed that, although positive margins significantly increased the recurrence rate, they did not affect overall survival.
The results of this study are consistent with earlier research examining the effect of PSM on patients with non-metastatic RCC undergoing PN. A retrospective analysis by Carvalho et al. of 388 patients who underwent PN at a single institution revealed a significant increase in the recurrence rate (18.7% compared to 4.2%, p = 0.007) and the rate of subsequent total nephrectomy (25% versus 4.4%, p < 0.001) in patients with PSM compared to those with negative surgical margins (NSM) [14]. In a similar vein, research by Khalifeh et al. identified a correlation between PSM and elevated rates of local recurrence and metastasis (p < 0.001) [15]. Nonetheless, the overall survival rates were comparable in both groups.
Contrastingly, a matched-pair study by Bensalah et al., which included 101 cases of PSM and 102 of NSM, matched by surgical indication, tumour size, and grade, showed no impact of PSM on 5-year recurrence-free survival (RFS), 5-year cancer-specific survival (CSS), or 5-year overall survival (OS) [5]. Yossepowitch et al. evaluated 77 PSM cases in a pool of 1,390 PN procedures over a median follow-up period of 40.8 months and found no link between PSM and diminished RFS or metastasis-free survival (MFS) [16]. Furthermore, Rothberg et al. compared outcomes of 797 NSM patients with 42 PSM patients after robotic partial nephrectomy and concluded that the oncological results for patients with PSM were not inferior to those with NSM after a median follow-up of 18.8 months [17].
Our study, benefiting from an extended follow-up period, is among the few to demonstrate an impact on overall survival. However, a weighted propensity score analysis revealed no significant differences in overall survival between groups with positive and negative surgical margins. This apparent contradiction may be attributed to confounding variables. In fact, patients with a PSM who experience disease recurrence could be undergo additional treatments, such as ablative treatment or further surgeries [18]. These interventions may effectively manage the recurrent disease, delaying its progression. While these treatments may improve DFS by controlling the disease, their impact on overall survival (OS) may be less pronounced if they successfully manage the disease without significantly affecting the patient’s overall health. Additionally, although the recurrence may necessitate ongoing surveillance and treatment, its impact on OS may be mitigated by the relatively slow progression of the disease and the availability of effective salvage treatments to manage it. These findings are in line with a recent meta-analysis by Bai et al. of 39 studies, which showed no significant correlation between PSM and overall survival [19].
RCC is a heterogeneous and intricate disease, with each histologic subtype exhibiting unique genetic, pathological, and clinical characteristics. Consequently, the influence of surgical margin status on RCC outcomes may vary depending on the specific subtype. In this context, our study stands out as it is the first to investigate the prognostic significance of PSM across different histologic subtypes (chromophobe or papillary), drawing upon extensive data from multiple centers. Our findings indicate that the association between surgical margin status and RCC prognosis could be influenced by the subtype. Our results suggest that the association between surgical margin status and RCC prognosis may be influenced by subtype. Specifically, we observed distinct differences in the effect of PSM on DFS among different RCC histological subtypes. We reported trends suggesting lower DFS in the PSM group compared with the NSM group for patients with papillary RCC, and no significant difference in DFS between the PSM and NSM groups for patients with chromophobe RCC. However, we acknowledge that the number of patients in these subgroups was limited, which may introduce uncertainty in the results regarding DFS between the PSM and NSM groups.
This study has limitations. The retrospective nature of data collection resulted in some missing information. Surgical approach, resection techniques, and type of clamping varied according to surgeon preference and were not standardized across centers. In addition, cause of death was not assessed, and patients in the PSM group were older, which could bias the study due to a higher risk of death from any cause. We acknowledge the potential for imprecision in the results for the subgroups (papillary and chromophobe) due to the small sample size. There is a potential overinterpretation of these findings and emphasize the need for larger studies specifically focusing on patients with papillary or chromophobe RCC to validate our observations. The low incidence of PSM precluded the use of a multivariate model to identify predictive factors for PSM. However, this contemporary study is significant because of its large size and long follow-up of more than 20 years.
Conclusion
The debate continues over the implications of positive surgical margins following partial nephrectomy. Our study reveals an increased recurrence risk linked to PSMs post-procedure. Nonetheless, after accounting for potential confounding factors, we found no substantial difference in overall survival outcomes.
References
Fergany AF, Hafez KS, Novick AC (2000) Long-term results of nephron sparing surgery for localized renal cell carcinoma: 10-year followup. J Urol 163(2):442–445
Mir MC, Derweesh I, Porpiglia F et al (2017) Partial nephrectomy versus radical nephrectomy for clinical T1b and T2 renal tumors: a systematic review and meta-analysis of comparative studies. Eur Urol 71(4):606–617. https://linkinghub.elsevier.com/retrieve/pii/S0302283816305334. Cité 5 Oct 2022
Minervini A, Campi R, Sessa F et al (2017) Positive surgical margins and local recurrence after simple enucleation and standard partial nephrectomy for malignant renal tumors: systematic review of the literature and meta-analysis of prevalence. Minerva Urol Nephrol 69(6). https://www.minervamedica.it/index2.php?show=R19Y2017N06A0523. Cité 17 Mai 2022
Ryan ST, Patel DN, Ghali F et al (2021) Impact of positive surgical margins on survival after partial nephrectomy in localized kidney cancer: analysis of the National Cancer Database. Minerva Urol Nephrol 73(2). https://www.minervamedica.it/index2.php?show=R19Y2021N02A0233. Cité 7 Mai 2022
Bensalah K, Pantuck AJ, Rioux-Leclercq N et al (2010) Positive surgical margin appears to have negligible impact on survival of renal cell carcinomas treated by nephron-sparing surgery. Eur Urol 57(3):466–473. https://linkinghub.elsevier.com/retrieve/pii/S0302283809003145. Cité 8 Mai 2022
Kwon EO, Carver BS, Snyder ME et al (2007) Impact of positive surgical margins in patients undergoing partial nephrectomy for renal cortical tumours. BJU Int 99(2):286–289. https://onlinelibrary.wiley.com/doi/https://doi.org/10.1111/j.1464-410X.2006.06623.x. Cité 17 Mai 2022
Kryvenko ON (2017) Positive surgical margins increase risk of recurrence after partial nephrectomy for high risk renal tumors. Shah PH, Moreira DM, Okhunov Z, Patel VR, Chopra S, Razmaria AA, Alom M, George AK, Yaskiv O, Schwartz MJ, Desai M, Vira MA, Richstone L, Landman J, Shalhav AL, Gill I, Kavoussi LR. J Urol. 2016 Aug;196(2):327–334. Urol Oncol Semin Orig Investig 35(6):449–450. https://linkinghub.elsevier.com/retrieve/pii/S107814391730114X. Cité 7 Mai 2022
Carbonara U, Amparore D, Gentile et al (2022) Current strategies to diagnose and manage positive surgical margins and local recurrence after partial nephrectomy. Asian J Urol 9(3):227. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9399527/. Cité 28 Déc 2023
Khene ZE, Bex A, Bensalah K (2022) Adjuvant therapy after surgical resection of nonmetastatic renal-cell carcinoma: One size does not fit all. Eur Urol 81(4):432–433. https://doi.org/10.1016/j.eururo.2021.10.033
Kutikov A, Uzzo RG (2009) The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol 182(3):844–853. http://linkinghub.elsevier.com/retrieve/pii/S0022534709011756. Cité 7 Nov 2016
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Austin PC (2009) Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 28(25):3083–3107
Austin PC (2014) The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med 33(7):1242–1258. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4285179/. Cité 12 Août 2021
Carvalho JAM, Nunes P, Tavares-da-Silva E et al (2020) Impact of positive surgical margins after partial nephrectomy. Eur Urol Open Sci 21:41–6
Khalifeh A, Kaouk JH, Bhayani S et al (2013) Positive surgical margins in robot-assisted partial nephrectomy: a multi-institutional analysis of oncologic outcomes (leave no tumor behind). J Urol 190(5):1674–1679. http://www.jurology.com/doi/https://doi.org/10.1016/j.juro.2013.05.110. Cité 26 Juin 2022
Yossepowitch O, Thompson RH, Leibovich BC et al (2008) Positive surgical margins at partial nephrectomy: predictors and oncological outcomes. J Urol 179(6):2158–2163. http://www.jurology.com/doi/https://doi.org/10.1016/j.juro.2008.01.100. Cité 27 Mai 2022
Rothberg MB, Peak TC, Reynolds CR et al (2020) Long-term oncologic outcomes of positive surgical margins following robot-assisted partial nephrectomy. Transl Androl Urol 9(2):879–886. http://tau.amegroups.com/article/view/33223/30264. Cité 7 Mai 2022
Brassier M, Khene ZE, Bernhard JC et al (2022) Percutaneous ablation versus surgical resection for local recurrence following partial nephrectomy for renal cell cancer: a propensity score analysis (REPART Study? UroCCR 71). Eur Urol Focus 8(1):210–216
Bai R, Gao L, Wang J et al (2022) Positive surgical margins may not affect the survival of patients with renal cell carcinoma after partial nephrectomy: a meta-analysis based on 39 studies. Front Oncol 12:945166. https://www.frontiersin.org/articles/https://doi.org/10.3389/fonc.2022.945166/full. Cité 2 Nov 2023
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Hulin, M., Audigé, V., Baghli, A. et al. Long-term consequences of positive surgical margin after partial nephrectomy for renal cell carcinoma: multi‐institutional analysis. Int J Clin Oncol (2024). https://doi.org/10.1007/s10147-024-02578-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10147-024-02578-0