Abstract
Background
Thrombospondin-1 (THBS1) is a secretory adhesive glycoprotein involved in the progression of multiple malignancies, including breast cancer. However, the clinical significance and prognostic role of plasma THBS1 in breast cancer have yet to be clarified.
Methods
Plasma THBS1 levels in 627 breast cancer patients were analyzed by enzyme-linked immunosorbent assay. Bone marrow blood was drawn from the anterior/posterior superior iliac spine to detect the presence of disseminated tumor cells (DTCs). The effects of plasma THBS1 on the clinicopathological characteristics and survival prediction of breast cancer patients were explored.
Results
Plasma THBS1 did not correlate with overall survival, breast cancer-specific survival (BCSS), and distant disease-free survival (DDFS) in the entire breast cancer cohort. Notably, HER2-enriched patients with high-plasma THBS1 levels had significantly shorter BCSS (P = 0.027) and DDFS (P = 0.011) than those with low levels. Multivariate analyses revealed that plasma THBS1 was an independent prognostic marker of BCSS (P = 0.026) and DDFS (P = 0.007) in HER2-enriched patients. THBS1 levels were 24% higher in positive DTC patients than in negative DTC patients (P = 0.031), and high levels were significantly associated with poor BCSS in positive DTC patients (HR 2.08, 95% CI 1.17–3.71; P = 0.019). Moreover, high-plasma THBS1 levels were specifically associated with an increased occurrence of brain metastasis in HER2-enriched patients (P = 0.041).
Conclusion
These findings suggest that plasma THBS1 may be serving as an unfavorable prognosis predictor for HER2-enriched breast cancer and justifies the need for further research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Progress in early detection, diagnosis, and treatment monitoring has improved the efficacy of treatment for invasive breast cancer. However, recurrence and distant metastasis are still reported in about 20% of breast cancer patients [1]. Even with advances in multidisciplinary treatment, brain metastasis is still the leading cause of death in patients with advanced breast cancer, with a 3-year overall survival (OS) rate of only 5–8% [2, 3]. Some genomic analyses have demonstrated that unique chromosomal aberrations, copy number changes, and hypomethylation may be related to the occurrence of breast cancer brain metastasis [4, 5]; nonetheless, the mechanism underlying the distant metastasis of the central nervous system in breast cancer patients has yet to be determined.
Thrombospondin-1 (THBS1) is a secreted-matrix glycoprotein encoded by the human THBS1 gene. As a protein released by activated platelets, THBS1 can effectively promote tissue fibrosis and attenuate angiogenesis [6]. THBS1 is the most abundant protein in the α-granules of platelets; however, considerably low-THBS1 levels are found in normal plasma. THBS1 expression in other cell types and tissues is induced by trauma, atherosclerotic lesions, ischemia, rheumatoid synovium, glomerulonephritis, and malignant tumor cells. THBS1 expression increases with age and age-related diseases, such as type 2 diabetes and cardiovascular diseases. THBS1 is positively regulated by TGF-β1, retinoic acid, vitamin A, and progesterone and inhibited by Hantavirus infection, vascular endothelial growth factor (VEGF), and Myc [7, 8].
The effect of THBS1 on tumor progression is multifaceted and at times the opposite. THBS1 is reported to inhibit tumor occurrence and metastasis in several tumor models [9]. THBS1 deficiency is associated with increased tumorigenesis, and its overexpression or exogenous administration inhibits tumor formation and progression [10, 11]. In medulloblastoma, THBS1 regression caused by carcinogenic Myc may be a key determinant of the metastatic phenotype [8]. Notably, THBS1 also promotes metastasis and diffusion by mediating tumor cell embolization [12]. An increasing number of studies tend to use THBS1 as a marker of poor prognosis and recurrence in multiple malignancies, including glioma [13], melanoma [14], ovarian cancer [15], and pancreatic cancer [16].
The role of THBS1 in the development and progression of breast cancer is also complex and inconsistent. THBS1 expression in tumor stroma has been reported to be negatively correlated with axillary lymph node metastasis [17]. THBS1 production can significantly inhibit tumor progression in the MDA-MB-435 breast cancer cell line, which may be attributable to a decrease in angiogenesis [18]. Meanwhile, breast cancer patients with high-THBS1 expression have poor prognoses, and THBS1 mediated by tRF-17 induces the TGF-β1/smad3 pathway to promote breast cancer distant metastasis [19]. Wang et al. reported that integrin β1/mTOR activation mediated by THBS1 upregulation following neoadjuvant chemotherapy was related to chemotherapy resistance in breast cancer patients [20]. THBS1 inhibition increases CD8+ tumor-infiltrating lymphocytes (TILs) and immunotherapy efficacy, as well as represses TGF-β activation and breast cancer cell metastasis in triple-negative breast cancer [21].
The circulating THBS1 levels could be used as a biomarker for tumor prognosis. THBS1 serum levels reflect the invasiveness of non-small cell lung carcinoma and serve as a potential prognostic marker for patients with primary lung cancer [22]. Zhu et al. found that serum THBS1 was relatively low in acute myeloid leukemia, and patients with low serum THBS1 had reduced survival time [23]. Serum methylated THBS1 DNA has also been recently suggested to predict peritoneal dissemination, a potential risk factor in patients with gastric cancer [24]. Suh et al. conducted a comparative profiling study of preoperative plasma proteome from breast cancer patients and age-matched normal healthy women by mTRAQ-based stable isotope-labeling mass spectrometry and confirmed the diagnostic value of THBS1 in detecting breast cancer [25]. In addition, preoperative high circulating THBS1 levels may play a pro-angiogenic rather than anti-angiogenic role in breast cancer and seems to be a marker of breast cancer aggressiveness [26]. However, the aforementioned studies did not consider the influence of plasma THBS1 on the prognosis of breast cancer patients. In the current study, we examined the preoperative plasma THBS1 levels in patients with primary nonmetastatic breast cancer and analyzed the relationship of plasma THBS1 with clinical characteristics, prognosis, and metastasis.
Materials and methods
Patients and clinicopathological information
This study was reviewed and approved by the Ethics Committee of Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine and was conducted in accordance with the Declaration of Helsinki. No animal experiment was involved in the current study. Informed consent signed by every subject for voluntary participation was obtained during the first interview. Two cohorts were analyzed in this study: (i) a retrospective cohort comprising 627 patients with primary nonmetastatic breast cancer and (ii) a second cohort consisting of 74 fresh breast cancer tissues matched with adjacent normal tissues (ANTs). In addition, the corresponding 74 preoperative breast cancer plasma samples were collected. The original follow-up information was obtained from the Registry Center and the outpatient doctor.
The subjects of this study should meet the following criteria: patients with breast cancer confirmed by histopathological examination; no obvious distant metastasis after imaging evaluation before operation; bone marrow puncture at the initial diagnosis of breast cancer; no serious uncontrollable cardiovascular and cerebrovascular complications or other systemic diseases; no previous or current history of other malignancies; appropriate adjuvant therapy was completed after operation in accordance with the National Comprehensive Cancer Network guidelines at the time, mainly including adjuvant chemotherapy (anthracyclines, cyclophosphamide, fluorouracil, and taxanes), endocrine therapy for estrogen receptor (ER)- and progesterone receptor (PR)-positive tumors (tamoxifen and aromatase inhibitor), trastuzumab targeted therapy in patients with immunohistochemical overexpression of human epidermal growth factor receptor 2 (HER2) or positive HER2 determined by fluorescence in situ hybridization (FISH), and radiotherapy, if necessary. Patients receiving neoadjuvant chemotherapy, endocrine therapy, or targeted therapy were excluded. Pathology reports and medical records were reviewed to evaluate molecular subtypes, pathologic types, histologic grades, and tumor–node–metastasis staging.
ER and PR with more than 1% of cells staining positive were defined as positive. Levels < 20% indicated a low PR status. Immunohistochemistry was performed to determine the HER2 expression levels, which were defined as positive when the score was 3 + or negative when the score was either 1+ or 0. Tumors with 2+ moderate staining were classified as HER2-positive only if positive results were obtained in alternative FISH. Ki-67 levels with a cut-off of ≥ 20% positive staining were considered high.
Intrinsic molecular subtypes
Breast cancer patients were subdivided into four groups in accordance with the St. Gallen Consensus 2013 [27]: luminal A: ER- and PR-positive, HER2-negative, and Ki-67 < 20%; luminal B (HER2-negative): ER-positive, PR-negative or low, HER2-negative, and Ki-67 ≥ 20%; luminal B (HER2-positive): ER-positive, HER2-positive, any Ki-67, and any PR; HER2-enriched: ER-negative, PR-negative, and HER2-positive; triple-negative breast cancer: ER-negative, PR-negative, and HER2-negative.
Collection of plasma
Approximately 10 mL of venous blood was preoperatively collected from each breast cancer patient in the fasting state using a VACUETTE butterfly retraction blood sampling needle (Greiner Bio-One Suns Co., Ltd.). The blood samples were collected into BD Vacutainer EDTA tubes (Becton Dickinson Diagnostics), immediately placed on ice, and fractionated within 30 min via two centrifugation steps at 2500 × g for 20 min at 4 °C to avoid platelet degranulation [28]. The isolated plasma was stored at − 80 °C and then analyzed for THBS1 during batch processing.
Preparation of tissue cytosols
A tumor sample of 500 mg was transferred into a mortar, sliced into thin sections, and crushed with a porcelain pestle until only a small amount of particles remained. A protease inhibitor solution was added to the samples, and the blend was finely mixed. NP40 was then added, and the samples were placed onto a mechanical wheel for at least 1 h. The samples were centrifuged at 15,000 g for 10 min at 4 ℃. The supernatant was carefully transferred into Eppendorf tubes and then stored at − 70 ℃.
Detection of disseminated tumor cells
Before the operation of the primary breast tumor, 10 mL of bone marrow blood was extracted from the anterior/posterior superior iliac spine of each patient under local, epidural, or general anesthesia. The samples were then processed within 24 h. The bone marrow blood was separated by Ficoll–Hypaque density gradient centrifugation at 400 × g for 30 min. Mononuclear cells were isolated. Red blood cells were destroyed with a 10 mmol/L cell lysate, washed repeatedly with a phosphate buffer, and fixed with acetone. Disseminated tumor cells (DTCs) in slides were detected by immunocytochemistry with the pan-cytokeratin antibody (A45-B/B3). The results were evaluated using the ARIOL system (Applied Imaging) in accordance with the diagnostic standard set by the International Society for Hematotherapy and Graft Engineering [29].
Detection of THBS1 by enzyme-linked immunosorbent assay
Plasma and tissue THBS1 levels were assessed using an enzyme-linked immunosorbent assay in accordance with the manufacturer’s protocol (ThermoFisher, Vienna, Austria). Plasma or tissue supernatant was diluted, transferred to appropriate wells, and incubated at room temperature for 2.5 h. HRP-conjugate was added to each well, which was then incubated at room temperature for 2 h. After washing Microwell strips five times, we added the 3, 3′, 5, 5′-tetramethylbenzidine substrate solution into each well. After adding the stop solution, we measured the absorbance of each microwell on a microplate reader, with 450 nm as the primary wavelength. A standard curve was generated by plotting the mean absorbance for each standard concentration on the ordinate against the human THBS1 concentration on the abscissa. The concentration of THBS1 in plasma or tissue supernatant was determined based on the standard curve.
Real-time quantitative polymerase chain reaction
Total RNA was extracted from fresh tissues using TRIzol Reagent (Life Technologies). Reverse transcription was performed to synthesize complementary DNA using the PrimeScript RT-PCR Kit (TaKaRa). Real-time quantitative polymerase chain reaction (RT-qPCR) was accomplished using the QuantiFast SYBR Green RT-PCR Kit (QIAGEN). The primer sequences of THBS1 were as follows: 5′-AGACTCCGCATCGCAAAGG-3′ (forward); 5′-TCACCACGTTGTTGTCAAGGG-3′ (reverse). GAPDH served as the internal control, and the primer sequences were 5′-AATGGACAACTGGTCGTGGAC-3′ (forward) and 5′-CCCTCCAGGGGATCTGTTTG-3′ (reverse). PCR was conducted on the QuantStudio 5 Real-Time PCR System (Applied Biosystems), and the relative expression of THBS1 was analyzed using the 2–ΔΔCT method.
Statistical analysis
The experimental results were expressed as mean ± standard deviation unless otherwise stated. A comparison between two independent samples was conducted using the Mann–Whiney U test, and a comparison of three or more samples was performed with ANOVA. The patients were divided, based on the median plasma THBS1 level in the whole cohort, into the high-THBS1 and low-THBS1 groups. OS is used to denote the time from the initial operation to death regardless of the cause. Breast cancer-specific survival (BCSS) is defined as the time from breast cancer surgery to the date of death related directly to the tumor. Distant disease-free survival (DDFS) refers to the time from the date of breast cancer surgery to the first detection of distant metastasis from breast cancer. Survival curves were plotted using the Kaplan–Meier method and then compared using the log-rank test. Univariate and multivariate Cox regression analyses were adopted to assess the correlation between various parameters and survival outcomes. Variables with P < 0.1 in univariate regression analysis were screened and included in the subsequent multivariate analysis. Correlation analysis was performed using the Spearman correlation test. The invasive breast cancer dataset in The Cancer Genome Atlas (TCGA) database was selected and analyzed using the cBioPortal platform (http://cbioportal.org/). The mRNA expression profiles associated with 2217 cases of invasive breast carcinoma from three cohorts were acquired to analyze the correlation between THBS1 and potential molecules [30]. The data were statistically analyzed using IBM SPSS Statistics 25, and survival curves were plotted and analyzed with GraphPad Prism 8.0.2. P < 0.05 was considered statistically significant.
Results
Patient characteristics
A total of 627 breast cancer patients participated in this study. The biologic features and clinical data at the baseline are listed in Table 1. All subjects were female, with a median age of 58.5 y (range 24–87 y), and 70.2% (440/627) of the patients were postmenopausal. Eight of these patients lost contact during follow-up. Therefore, 619 patients had complete survival data, and the median follow-up time was 8.33 y (range 0.13–11.67 y), and 63.2% (396/627) of the patients had a T1 tumor. Positive axillary lymph nodes were detected in 37.2% (233/627) of the patients; 58.9% (369/627) of the tumors had moderately histologic grading. ER and PR expression were positive in 61.4% (385/627) and 47.4% (297/627) of the tumors. Positive HER2 expression was observed in 42.9% (269/627) of the tumors. For the entire cohort, 40.2% (252/627) of the cases were ER-positive and/or PR-positive and HER2-negative; 22.3% (140/627) were ER-positive and/or PR-positive and HER2-positive; 20.6% (129/627) were ER-negative, PR-negative, and HER2-positive, and 16.9% (106/627) were triple-negative breast cancer.
Plasma THBS1 levels and the correlation of THBS1 with clinicopathological characteristics
The THBS1 plasma levels in all patients were quantified, and all plasma THBS1 levels in 627 patients were measurable (Table 2). The distribution of plasma THBS1 levels in breast cancer patients is shown in Fig. 1. For the entire cohort, the mean plasma THBS1 level was 774.22 ± 141.59 ng/mL. THBS1 expression in the plasma was then analyzed based on clinicopathological indexes, molecular typing, and DTC status. The plasma THBS1 level in patients with axillary lymph node involvement was significantly higher than that in patients with negative lymph node patients (987.2 ± 186. 3 vs. 554.5 ± 104.6; P < 0.001). No correlation was found between the plasma THBS1 levels and ER, PR, HER2 status, and molecular subtype. In addition, the plasma THBS1 levels in the positive DTC group were significantly higher than those in the negative DTC group (826.3 ± 218.5 vs. 681.5 ± 144.3; P = 0.031). We analyzed the plasma THBS1 level between the distant and non-distant metastasis groups, and the difference was not statistically significant; however, the plasma THBS1 level in patients with brain metastasis was significantly higher than that in patients without brain metastasis (863.3 ± 193.9 vs. 603.2 ± 114.8; P = 0.007).
Relationship between THBS1 level in plasma and tumor tissue
To validate the expression of THBS1 in breast cancer tissues, we conducted RT-qPCR on 74 pairs of breast cancer and ANTs. The results showed that relative to ANTs, THBS1 was significantly upregulated in breast cancer tissues (P < 0.001; Fig. 2A). We then investigated the expression of THBS1 in 74 tumor tissues by ELISA and compared it with the THBS1 level in plasma. We found that the THBS1 level in the tumor was significantly higher than that in plasma, and the median level (3344 ng/mL) was more than four times that of plasma (P < 0.001; Fig. 2B). Moreover, we evaluated the correlation between THBS1 levels in tumor tissues and corresponding plasma THBS1 levels in 74 breast cancer patients. The levels of THBS1 in plasma were positively correlated with the THBS1 level in tumor (r = 0.711, P < 0.001; Fig. 2C).
Correlation between THBS1 levels in plasma and tumor tissue. A Real-time PCR results of THBS1 mRNA levels in paired breast cancer tissues and adjacent normal tissues (ANTs) (n = 74). B Comparison of THBS1 levels in plasma and tumor tissues of breast cancer patients. Median tumor THBS1 levels are more than four times higher than those observed in plasma (P < 0.001). C Correlation between THBS1 levels in tumor tissues and corresponding plasma THBS1 levels in breast cancer patients (n = 74). A strong correlation is observed between THBS1 levels in plasma and tumor tissues. (r = 0.711, P < 0.001)
Prognostic value of plasma THBS1 in breast cancer patients
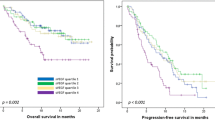
The study population was classified into high- and low-THBS1 groups on the basis of the measured median value to evaluate the prognostic significance of plasma THBS1. The mean THBS1 levels of the low- and high-plasma THBS1 groups were 564 ng/mL (range 147–785 ng/mL) and 983 ng/mL (range 786–1975 ng/mL), respectively. The median follow-up time was 8.33 y after surgery for primary breast cancer. During the follow-up, 95 (15.3%) patients died, consisting of 44 (14.2%) cases in the low-THBS1 group and 51 (16.5%) cases in the high-THBS1 group. Of the 95 deaths, 89 (93.4%) deaths were caused by breast cancer, including 40 deaths (12.9%) in the low-THBS1 group and 49 deaths (15.8%) in the high-THBS1 group. Distant metastasis occurred in 116 (18.7%) breast cancer cases, 56 (18.1%) of which were in the low-THBS1 group, and 60 (19.4%) were in the high-THBS1 group. To explore the relationship between plasma THBS1 levels and clinical outcomes, we generated survival curves using the Kaplan–Meier estimator. When all cases were included, plasma THBS1 levels had no statistical effect on OS, BCSS, and DDFS (Fig. 3A–C). However, in subgroup analyses based on the molecular subtypes, the high-plasma THBS1 levels had a significant effect on BCSS (P = 0.027) and DDFS (P = 0.011) in HER2-enriched patients but not in those with other molecular subtypes (Fig. 4). The aforementioned results demonstrate that high-THBS1 plasma levels are associated with impaired BCSS and DDFS in HER2-enriched patients.
Prognostic significance of the plasma THBS1 level in breast cancer patients with different molecular subtypes. A–D Kaplan–Meier curves for breast cancer-specific survival based on the plasma THBS1 level in patients with different molecular subtypes of breast cancer. E–H Kaplan–Meier curves for distant disease-free survival based on the plasma THBS1 level in patients with different molecular subtypes of breast cancer
Univariate analyses indicated that HER2-enriched breast cancer patients with high-plasma THBS1 levels were more likely to have worse BCSS and DDFS than those with low plasma THBS1 levels; moreover, multivariate regression analyses revealed that high-plasma THBS1 levels were significantly correlated with poor BCSS (HR 2.28, 95% CI 1.35–3.65; P = 0.026) and DDFS (HR 2.46, 95% CI 1.18–4.40; P = 0.007) in HER2-enriched patients (Tables 3 and 4). These results indicate that high-plasma THBS1 levels can be an independent unfavorable prognostic factor for HER2-enriched patients.
Effect of DTCs on the prognostic value of THBS1
The presence of DTCs in the bone marrow blood of each patient was determined, and 241 (38.5%) patients were identified as DTC-positive (p-DTC). The plasma THBS1 levels were higher in p-DTC cases than in negative DTC (n-DTC) cases (P = 0.031; Fig. 5A). The status of DTCs exerted a significant effect on the prognostic value of plasma THBS1 in breast cancer patients. In contrast to low plasma THBS1 levels, high-THBS1 plasma levels were significantly associated with poor BCSS in p-DTC patients (P = 0.019; Fig. 5B). No difference in the survival rate of breast cancer patients was observed regardless of plasma THBS1 levels in n-DTC patients (Fig. 5C). Subsequently, we analyzed the effect of DTC on the prognostic significance of plasma THBS1 levels in HER2-enriched patients. For HER2-enriched patients with p-DTC, high-THBS1 levels predicted poor prognosis, which was the same as the prognostic significance of p-DTC in the overall cohort and had more obvious statistical power (P = 0.012; Fig. 5D). However, the plasma THBS1 level exerted no significant effect on the prognosis for HER2-enriched breast cancer patients with n-DTC (Fig. 5E). Moreover, multivariate analysis confirmed the prognostic significance of high-THBS1 plasma in the p-DTC population (HR 2.51, 95% CI 1.18–5.32; P = 0.016; Fig. 5F).
Effect of the status of disseminated tumor cells (DTCs) on the prognostic value of THBS1. A Plasma THBS1 levels increase in the positive DTC (p-DTC) breast cancer patients (P = 0.031). Values shown as mean ± SEM. B In patients with p-DTCs, the high plasma THBS1 group shows poorer breast cancer-specific survival (BCSS) than the low plasma THBS1 group (P = 0.019). C In negative DTC (n-DTC) patients, the plasma THBS1 level exerts no effect on BCSS (P = 0.658). D In HER2-enriched breast cancer patients with p-DTCs, high plasma THBS1 level predicts poor prognosis (P = 0.012). E In HER2-enriched breast cancer patients with n-DTCs, the plasma THBS1 level has no significant effect on the prognosis (P = 0.291). F Cox multivariate analysis confirms the independent prognostic value of plasma THBS1 in BCSS for p-DTC breast cancer patients (P = 0.016)
Plasma THBS1 levels and distant metastasis in HER2-enriched breast cancer patients
On the basis of the aforementioned results, high-plasma THBS1 levels were associated with increased distant metastasis in HER2-enriched breast cancer patients, contrary to low plasma THBS1 levels. We continue to explore the role of plasma THBS1 in lung, brain, and bone metastasis in HER2-enriched breast cancer patients. According to the results of imaging examination, a total of 45 HER2-enriched patients exhibited distant metastasis during the follow-up period, including 19 cases of lung metastasis, 14 cases of brain metastasis, 9 cases of bone metastasis, 2 cases of combined lung and brain metastasis, and 1 case of combined lung and bone metastasis. Plasma THBS1 level has no effect on lung (P = 0.319; Fig. 6A) and bone (P = 0.656; Fig. 6B) metastasis in HER2-enriched breast cancer patients. Among the HER2-enriched patients with brain metastasis, 12 cases of brain metastasis occurred in the high-plasma THBS1 group and only 4 cases in the low plasma THBS1 group (HR 3.04, 95% CI 1.14–8.10; P = 0.041; Fig. 6C). These findings indicate that high-plasma THBS1 levels were associated with increased brain metastasis in HER2-enriched breast cancer patients.
Relationship between the plasma THBS1 level and distant organ metastasis in HER2-enriched breast cancer. Kaplan–Meier curves for lung metastasis-free survival (A), bone metastasis-free survival (B), and brain metastasis-free survival (C) based on the plasma THBS1 level in HER2-enriched breast cancer patients. Brain metastasis occurs significantly more often in HER2-enriched patients with high-THBS1 levels than in those with low-THBS1 levels (P = 0.041). No differences in lung and bone metastasis are observed
Discussion
The study including 627 breast cancer patients showed that high-plasma THBS1 levels predicted poor prognosis and a higher risk of developing brain metastasis in HER2-enriched breast cancer. We revealed that high-THBS1 levels in plasma strongly correlated to lymph node involvement and brain metastasis, suggesting that breast cancer with high-THBS1 levels is more aggressive. Patients with p-DTC breast cancer had higher plasma THBS1 levels than patients with n-DTC breast cancer. The plasma THBS1 level was not related to age, menstrual status, histologic grade and typing, tumor size, hormone receptor and HER2 status, and molecular subtyping. No association was found between plasma THBS1 levels and survival analyses, including OS, BCSS, and DDFS. In the subgroup analyses, high-plasma THBS1 levels correlated with poor BCSS and DDFS in HER2-enriched breast cancer patients but not in patients with other subtypes of breast cancer. Notably, this study identified plasma THBS1 as a latent indicator of brain metastasis for HER2-enriched breast cancer patients.
The biologic role of THBS1 in the progression and metastasis of breast cancer is complicated to a certain extent. Previous studies have found that THBS1 as a positive or negative factor determines the survival of patients with breast cancer. The change in cell adhesion is a major step in tumor invasion and metastasis [31]. THBS1 can regulate cell adhesion in different cell types and control the adhesion, migration, and proliferation of tumor cells [32]. Knockout of THBS1 expression has been found to inhibit FAK phosphorylation, reduce the local adhesion of breast cancer cells, and weaken the migratory and invasive capability of tumor cells [33]. By contrast, other studies have described the anti-adhesive effect of THBS1. THBS1 may block the formation of focal adhesion or lose preformed focal adhesion [34] via PI3K-dependent ERK activation to destroy the stability of cell adhesion [35]. Exosome THBS1 enhances local vascular permeability, destroys integrity, promotes breast cancer cell spillage, and helps grow distant organs and form tiny metastases by disrupting normal connections between vascular endothelial cells [36]. In the TCGA invasive breast cancer database, the THBS1 level in stage III breast cancer was significantly higher than that in normal breast specimens, and the overall survival of cases with high-THBS1 expression was significantly poor [19]. However, other studies found that THBS1 production by tumor cells has been shown to significantly inhibit tumor progression, which may be partly attributable to the reduction of tumor angiogenesis [18]. The low-THBS1 level in breast carcinoma stroma is associated with axillary lymph node involvement, indicating the inhibitory effect of THBS1 on tumor progression [17].
The DTC status exerted a significant effect on the prognostic value of plasma THBS1 in breast cancer patients. Among the p-DTC patients, those with high-plasma THBS1 had a poorer prognosis than those with low plasma THBS1, while in n-DTC patients, plasma THBS1 level had no such influence. After adjustments with other clinicopathological parameters, the prognostic significance of plasma THBS1 level in the p-DTC population remained unchanged. These findings suggest that the biologic function of plasma THBS1 may be closely related to DTC activation or malignant transformation. The persistence of DTCs in the bone marrow before the operation and during follow-up is an important prognostic index in tumors [37]. Even after the complete resection of breast tumors, up to 40% of cases still had a high risk of recurrence, probably owing to the reactivation of dormant DTCs [38]. Evidence suggests the presence of HER2 and PR double-positive breast cancer cells secreting important cell migration promoting factor RANKL in pre-invasion or primary lesions, which subsequently activate the migration signals and initiate the dissemination of HER2-positive cancer cells that do not express PR; meanwhile, THBS1 can positively regulate RANKL expression [39, 40]. The dormancy of DTCs depends on the interrelation between cancer cells and bone marrow stroma. Stable microvascular niches constitute the dormant chamber of DTCs. Mature vascular endothelial cells secrete THBS1, which can induce sustained dormancy or quiescence in breast cancer cells, rendering them highly resistant to standard treatments, including chemotherapy and anti-HER2-targeted therapy [41]. In addition, high-THBS1 levels predicted a poor prognosis for HER2-enriched breast cancer patients with p-DTCs. Meanwhile, the plasma THBS1 level had no significant effect on the prognosis of HER2-enriched breast cancer patients with n-DTCs. That is, the prognostic significance of the DTC status in the plasma THBS1 level of HER2-enriched patients is relatively important. Targeted therapy, such as trastuzumab and/or pertuzumab, increases the THBS1 levels [42], and THBS1 promotes dormancy and reduces the proliferation of DTCs to evade chemotherapy or targeted therapy [43] and becomes a source of tumor recurrence after breast surgery. However, in subgroup analysis, the p-DTC and n-DTC patients in HER2-enriched breast cancer were relatively few. A further study needs to be conducted on the relationship between plasma THBS1 and DTCs in breast cancer patients.
Evidence has been reported on the relationship of ER and PR expression with plasma THBS1 in breast carcinoma and the detection of a higher THBS1 level in ER-negative breast cancer [25]. Our results indicate that no correlation existed between plasma THBS1 levels and ER, PR, and HER2 expression in breast cancer patients. However, a significant effect of plasma THBS1 on the progression of HER2-enriched patients was observed. The HER2 signaling pathway regulates the expression of pro-angiogenic (VEGF and IL-8) and anti-angiogenic (THBS1) factors in breast cancer cells [42]. By transfecting retrovirus-mediated small interfering RNA against HER2, Yang et al. found that HER2 knockdown could upregulate THBS1 expression in breast cancer cells [44]. The molecular mechanism by which HER2 regulates THBS1 expression has yet to be fully elucidated. Activation of the p38 MAP kinase (p38 MAPK) pathway promotes the metastatic and invasive phenotype of various malignant tumors [45] but inhibits the early metastasis of breast cancer in the MMTV-Her2 mouse model [46]. Previous research has elucidated that p38 MAPK signaling induces upregulation of Egr-1, which can potentially enhance THBS1 promoter activity and produce THBS1 mRNA and protein [47]. HER2 promotes proteasome reduction of Tpl2 (a p38 MAP3K) via E3 ubiquitin–protein ligase Skp2, thereby inhibiting p38 activity and reducing THBS1 expression [48]. Notably, trastuzumab significantly increases THBS1 expression via delayed but sustained activation of the p38 MAPK pathway [42]. Affymetrix microarray analyses have shown that trastuzumab treatment of BT474 cells with high HER2 expression can increase THBS1 expression [49]. In animal models of human breast cancer, trastuzumab induces the normalization and degeneration of the tumor vascular system by increasing the expression of the anti-angiogenic element THBS1 [50]. However, the increase in THBS1 caused by trastuzumab treatment is a double-edged sword. THBS1 is a major activator of transforming growth factor beta (TGF-β), a potent immunosuppressive and pro-metastatic cytokine [21]. THBS1 increases the formation of plasmin, and as a secreted adhesion protein, it can create an environment to promote cell invasion [51]. Besides, THBS1 signaling through CD47 is the endogenous inhibitor that negatively regulated T cell activation in the immune response to cancer metastasis [52]. However, HER2-positive luminal B breast cancer patients also received HER2-targeted therapy and why does the plasma THBS1 level exerts no effect on the prognosis of patients with luminal B? The reason could be that oral endocrine therapy drugs, such as tamoxifen or aromatase inhibitor, can improve the poor prognosis of breast cancer caused by high-THBS1 levels. This argument needs to be verified using a large-scale study.
It is widely accepted that HER2-enriched and triple-negative breast cancer is associated with an increased risk of developing brain metastasis [53]. In the current study, the plasma THBS1 level in subjects with brain metastasis was higher than in those without brain metastasis. More importantly, patients with high-plasma THBS1 levels had a significantly increased risk of developing brain metastasis compared with patients having low-THBS1 levels in HER2-enriched breast cancer patients. The application of targeted therapy such as trastuzumab and pertuzumab has substantially improved the survival of HER2-positive breast cancer patients; however, a number of patients have eventually developed brain metastasis [54]. Wen et al. found that retroviral-mediated HER2 RNA (siHER2) increased THBS1 expression in BT474 breast cancer cells with high levels of HER2 expression and trastuzumab inhibited the growth of BT474 xenografts and increased the THBS1 expression [42]. THBS1 could enhance the ability of invasive breast carcinoma cells to migrate via collagen gel [55]. Longitudinal analysis revealed that in the plasma of the mouse model of glioblastoma multiforme (GBM), 25 abnormally expressed proteins were identified, including Thbs 1, which was closely related to the brain metastasis of GBM [56]. As an upstream molecule, THBS1 induces the transduction of the FAK/AKT signal pathway, which can promote the brain metastasis of melanoma cells [57]. In the central nervous system, astrocytes also secrete THBS1 to promote synaptogenesis. Phosphorylated-STAT3 positive tumor-associated astrocytes prevent CD8+ cytotoxic T cells from entering cancer cells by secreting immunosuppressive molecules—THBS1 and VEGF-A [58]. Chen et al. demonstrated that THBS1 mediated selective tight junction protein autophagy and promoted its degradation, and finally weakened the integrity of the blood–brain barrier [59]. In addition, THBS1/TNF-R1 signaling induces apoptosis of microvascular endothelial cells [60], and THBS1/CD47/Nox1 axis promotes endothelial cell senescence [61]. Therefore, THBS1 may activate various signal pathways, resulting in the destruction of the blood–brain barrier, which provides necessary conditions for cancer cell brain metastasis. THBS1 has been shown to synergize with HER2/HER3 heterodimers to mediate the marked activation of PI3K-Akt signaling, and the latter contributes to breast cancer brain metastasis [62, 63]. We hypothesized that inhibition of plasma THBS1 in HER2-positive breast cancer cases receiving trastuzumab targeted therapy might reduce the occurrence of brain metastasis.
Furthermore, the aforementioned correlation between THBS1 and potential molecules was analyzed based on the mRNA expression using the cBioPortal database. In the co-expression analysis, the mRNA expression levels of THBS1 in breast cancer patients were positively correlated with the expression of the aforementioned molecules (Fig. S1), including PIK3CA, TGFB1, MAPK14, STAT3, TNFRSF1A, and EGR1; however, further work is needed to confirm this correlation.
Our study has several limitations. First, this study did not consist of an age-matched control group, which can further explain THBS1 alterations in normal healthy aging. Second, sequential measurements of THBS1 and DTCs in the study cohort, including various periods of postoperative examination, were desirable because they could provide information on how changes in THBS1 and DTCs could influence the prognosis. However, these requirements were difficult to fulfill because some patients with their respective time frames were mostly monitored and followed up outside our clinic. Detection of DTCs in bone marrow is an invasive procedure that needs to be completed after anesthesia. Performing this operation for a large number of postoperative breast cancer patients in the outpatient department also presented a challenge. Third, brain metastasis likely occurs decades after the initial diagnosis, although in the current study, the occurrence was observed after the median follow-up of 8.33 y. We cannot exclude the possibility that any subsequent brain metastasis may affect our results. Finally, the plasma THBS1 levels vary considerably, which may limit the ability of plasma THBS1 as a laboratory marker. The heterogeneity of breast cancer may cause a wide range of changes in plasma THBS1 levels. Antibody selection and detection may also be related to considerable changes in plasma THBS1 levels. A large number of samples need to be verified to explain the variance in plasma THBS1 levels seen among breast cancer patients.
In this preliminary study, we provide proof of the concept that plasma THBS1 quantification opens a window for a noninvasive biomarker in a liquid biopsy of breast cancer. It can also be potentially used for early diagnosis, monitoring treatment response, and detecting brain metastasis. We underscore the importance of sufficiently powered studies to establish the clinical diagnostic criteria of plasma THBS1 in breast cancer patients and to test the utility of plasma THBS1 in identifying breast cancer brain metastasis.
In conclusion, our findings indicate that high-plasma THBS1 level is an independent unfavorable prognostic biomarker and is significantly associated with brain metastasis in HER2-enriched breast cancer patients. Plasma THBS1 may have a specific effect on biological characteristics and brain metastasis in HER2-enriched breast cancer, and further studies are warranted to identify specific breast cancer populations that may benefit from THBS1 inhibition.
References
Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249
Achrol AS, Rennert RC, Anders C et al (2019) Brain metastases. Nat Rev Dis Primers 5:5
Nayak L, Lee EQ, Wen PY (2012) Epidemiology of brain metastases. Curr Oncol Rep 14:48–54
Nik-Zainal S, Morganella S (2017) Mutational signatures in breast cancer: the problem at the DNA level. Clin Cancer Res 23:2617–2629
Harrell JC, Prat A, Parker JS et al (2012) Genomic analysis identifies unique signatures predictive of brain, lung, and liver relapse. Breast Cancer Res Treat 132:523–535
Muth M, Engelhardt BM, Kröger N et al (2011) Thrombospondin-1 (TSP-1) in primary myelofibrosis (PMF) - a megakaryocyte-derived biomarker which largely discriminates PMF from essential thrombocythemia. Ann Hematol 90:33–40
Khaiboullina SF, Morzunov SP, St Jeor SC et al (2016) Hantavirus infection suppresses thrombospondin-1 expression in cultured endothelial cells in a strain-specific manner. Front Microbiol 7:1077
Zhou L, Picard D, Ra YS et al (2010) Silencing of thrombospondin-1 is critical for myc-induced metastatic phenotypes in medulloblastoma. Cancer Res 70:8199–8210
Yee KO, Streit M, Hawighorst T et al (2004) Expression of the type-1 repeats of thrombospondin-1 inhibits tumor growth through activation of transforming growth factor-beta. Am J Pathol 165:541–552
Kazerounian S, Yee KO, Lawler J (2008) Thrombospondins in cancer. Cell Mol Life Sci 65:700–712
Martin-Manso G, Galli S, Ridnour LA et al (2008) Thrombospondin 1 promotes tumor macrophage recruitment and enhances tumor cell cytotoxicity of differentiated U937 cells. Cancer Res 68:7090–7099
Incardona F, Lewalle JM, Morandi V et al (1995) Thrombospondin modulates human breast adenocarcinoma cell adhesion to human vascular endothelial cells. Cancer Res 55:166–173
Perez-Janices N, Blanco-Luquin I, Tuñón MT et al (2015) EPB41L3, TSP-1 and RASSF2 as new clinically relevant prognostic biomarkers in diffuse gliomas. Oncotarget 6:368–380
Borsotti P, Ghilardi C, Ostano P et al (2015) Thrombospondin-1 is part of a Slug-independent motility and metastatic program in cutaneous melanoma, in association with VEGFR-1 and FGF-2. Pigment Cell Melanoma Res 28:73–81
Lyu T, Jia N, Wang J et al (2013) Expression and epigenetic regulation of angiogenesis-related factors during dormancy and recurrent growth of ovarian carcinoma. Epigenetics 8:1330–1346
Nie S, Lo A, Wu J et al (2014) Glycoprotein biomarker panel for pancreatic cancer discovered by quantitative proteomics analysis. J Proteome Res 13:1873–1884
Ioachim E, Damala K, Tsanou E et al (2012) Thrombospondin-1 expression in breast cancer: prognostic significance and association with p53 alterations, tumour angiogenesis and extracellular matrix components. Histol Histopathol 27:209–216
Weinstat-Saslow DL, Zabrenetzky VS, VanHoutte K et al (1994) Transfection of thrombospondin 1 complementary DNA into a human breast carcinoma cell line reduces primary tumor growth, metastatic potential, and angiogenesis. Cancer Res 54:6504–6511
Mo D, He F, Zheng J et al (2021) tRNA-derived fragment tRF-17-79MP9PP attenuates cell invasion and migration via THBS1/TGF-β1/Smad3 axis in breast cancer. Front Oncol 11:656078
Wang T, Srivastava S, Hartman M et al (2016) High expression of intratumoral stromal proteins is associated with chemotherapy resistance in breast cancer. Oncotarget 7:55155–55168
Marcheteau E, Farge T, Pérès M et al (2021) Thrombospondin-1 silencing improves lymphocyte infiltration in tumors and response to anti-PD-1 in triple-negative breast cancer. Cancers (Basel) 13:4059
Rouanne M, Adam J, Goubar A et al (2016) Osteopontin and thrombospondin-1 play opposite roles in promoting tumor aggressiveness of primary resected non-small cell lung cancer. BMC Cancer 16:483
Zhu L, Li Q, Wang X et al (2019) THBS1 is a novel serum prognostic factors of acute myeloid leukemia. Front Oncol 9:1567
Hu XY, Ling ZN, Hong LL et al (2021) Circulating methylated THBS1 DNAs as a novel marker for predicting peritoneal dissemination in gastric cancer. J Clin Lab Anal 35:e23936
Suh EJ, Kabir MH, Kang UB et al (2012) Comparative profiling of plasma proteome from breast cancer patients reveals thrombospondin-1 and BRWD3 as serological biomarkers. Exp Mol Med 44:36–44
Byrne GJ, Hayden KE, McDowell G et al (2007) Angiogenic characteristics of circulating and tumoural thrombospondin-1 in breast cancer. Int J Oncol 31:1127–1132
Goldhirsch A, Winer EP, Coates AS et al (2013) Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 24:2206–2223
Hayden K, Tetlow L, Byrne G et al (2000) Radioimmunoassay for the measurement of thrombospondin in plasma and breast cyst fluid: validation and clinical application. Ann Clin Biochem 37:319–325
Sutherland DR, Anderson L, Keeney M et al (1996) The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J Hematother 5:213–226
Cerami E, Gao J, Dogrusoz U et al (2012) The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2:401–404
Behrens J, Frixen U, Schipper J et al (1992) Cell adhesion in invasion and metastasis. Semin Cell Biol 3:169–178
Huang T, Sun L, Yuan X et al (2017) Thrombospondin-1 is a multifaceted player in tumor progression. Oncotarget 8:84546–84558
Shen J, Cao B, Wang Y et al (2018) Hippo component YAP promotes focal adhesion and tumour aggressiveness via transcriptionally activating THBS1/FAK signalling in breast cancer. J Exp Clin Cancer Res 37:175
Murphy-Ullrich JE, Höök M (1989) Thrombospondin modulates focal adhesions in endothelial cells. J Cell Biol 109:1309–1319
Orr AW, Pallero MA, Murphy-Ullrich JE (2002) Thrombospondin stimulates focal adhesion disassembly through Gi- and phosphoinositide 3-kinase-dependent ERK activation. J Biol Chem 277:20453–20460
Cen J, Feng L, Ke H et al (2019) Exosomal thrombospondin-1 disrupts the integrity of endothelial intercellular junctions to facilitate breast cancer cell metastasis. Cancers (Basel) 11
Hartkopf AD, Wallwiener M, Fehm TN et al (2015) Disseminated tumor cells from the bone marrow of patients with nonmetastatic primary breast cancer are predictive of locoregional relapse. Ann Oncol 26:1155–1160
Braun S, Vogl FD, Naume B et al (2005) A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med 353:793–802
Hosseini H, Obradović MMS, Hoffmann M et al (2016) Early dissemination seeds metastasis in breast cancer. Nature 540:552–558
Liu X, Jin J, Liu Y et al (2021) Targeting TSP-1 decreased periodontitis by attenuating extracellular matrix degradation and alveolar bone destruction. Int Immunopharmacol 96:107618
Ghajar CM, Peinado H, Mori H et al (2013) The perivascular niche regulates breast tumour dormancy. Nat Cell Biol 15:807–817
Wen XF, Yang G, Mao W et al (2006) HER2 signaling modulates the equilibrium between pro- and antiangiogenic factors via distinct pathways: implications for HER2-targeted antibody therapy. Oncogene 25:6986–6996
Widner DB, Park SH, Eber MR et al (2018) Interactions between disseminated tumor cells and bone marrow stromal cells regulate tumor dormancy. Curr Osteoporos Rep 16:596–602
Yang G, Cai KQ, Thompson-Lanza JA et al (2004) Inhibition of breast and ovarian tumor growth through multiple signaling pathways by using retrovirus-mediated small interfering RNA against Her-2/neu gene expression. J Biol Chem 279:4339–4345
del Barco BI, Nebreda AR (2012) Roles of p38 MAPKs in invasion and metastasis. Biochem Soc Trans 40:79–84
Wen HC, Avivar-Valderas A, Sosa MS et al (2011) p38α signaling induces anoikis and lumen formation during mammary morphogenesis. Sci Signal 4:ra34
Zhao HY, Ooyama A, Yamamoto M et al (2008) Molecular basis for the induction of an angiogenesis inhibitor, thrombospondin-1, by 5-fluorouracil. Cancer Res 68:7035–7041
Wang G, Wang J, Chang A et al (2020) Her2 promotes early dissemination of breast cancer by suppressing the p38 pathway through Skp2-mediated proteasomal degradation of Tpl2. Oncogene 39:7034–7050
Le XF, Lammayot A, Gold D et al (2005) Genes affecting the cell cycle, growth, maintenance, and drug sensitivity are preferentially regulated by anti-HER2 antibody through phosphatidylinositol 3-kinase-AKT signaling. J Biol Chem 280:2092–2104
Izumi Y, Xu L, di Tomaso E et al (2002) Tumour biology: herceptin acts as an anti-angiogenic cocktail. Nature 416:279–280
Yee KO, Connolly CM, Duquette M et al (2009) The effect of thrombospondin-1 on breast cancer metastasis. Breast Cancer Res Treat 114:85–96
Miller TW, Kaur S, Ivins-O’Keefe K et al (2013) Thrombospondin-1 is a CD47-dependent endogenous inhibitor of hydrogen sulfide signaling in T cell activation. Matrix Biol 32:316–324
Kennecke H, Yerushalmi R, Woods R et al (2010) Metastatic behavior of breast cancer subtypes. J Clin Oncol 28:3271–3277
Zagar TM, Van Swearingen AE, Kaidar-Person O et al (2016) Multidisciplinary management of breast cancer brain metastases. Oncology (Williston Park) 30:923–933
Wang TN, Qian X, Granick MS et al (1996) Thrombospondin-1 (TSP-1) promotes the invasive properties of human breast cancer. J Surg Res 63:39–43
Anastasi F, Greco F, Dilillo M et al (2020) Proteomics analysis of serum small extracellular vesicles for the longitudinal study of a glioblastoma multiforme mouse model. Sci Rep 10:20498
Kircher DA, Trombetti KA, Silvis MR et al (2019) AKT1(E17K) activates focal adhesion kinase and promotes melanoma brain metastasis. Mol Cancer Res 17:1787–1800
Priego N, Zhu L, Monteiro C et al (2018) STAT3 labels a subpopulation of reactive astrocytes required for brain metastasis. Nat Med 24:1024–1035
Chen CY, Chao YM, Lin HF et al (2020) miR-195 reduces age-related blood-brain barrier leakage caused by thrombospondin-1-mediated selective autophagy. Aging Cell 19:e13236
Rege TA, Stewart J Jr, Dranka B et al (2009) Thrombospondin-1-induced apoptosis of brain microvascular endothelial cells can be mediated by TNF-R1. J Cell Physiol 218:94–103
Meijles DN, Sahoo S, Al Ghouleh I et al (2017) The matricellular protein TSP1 promotes human and mouse endothelial cell senescence through CD47 and Nox1. Sci Signal 10:eaaj1784
Alaoui-Jamali MA, Song DJ, Benlimame N et al (2003) Regulation of multiple tumor microenvironment markers by overexpression of single or paired combinations of ErbB receptors. Cancer Res 63:3764–3774
Hosonaga M, Saya H, Arima Y (2020) Molecular and cellular mechanisms underlying brain metastasis of breast cancer. Cancer Metastasis Rev 39:711–720
Acknowledgements
We sincerely thank the breast cancer patients who participated in this study.
Funding
This work was supported by the National Natural Science Foundation of China (82103630).
Author information
Authors and Affiliations
Contributions
YL: Conceptualization, Investigation, Methodology, Software, Validation, Resources, Funding acquisition, Writing (original draft, review, and editing). JQ: Conceptualization, Software, Resources, Validation. GC: Project administration, Visualization, Supervision, Investigation. WW: Conceptualization, Validation, Resources, Project administration, Formal analysis. XS: Conceptualization, Validation, Data curation, Visualization, Supervision, Project administration, Writing (review and editing).
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest or other disclosures.
Ethics approval and consent to participate
In the studies, all procedures involving human participants were performed in accordance with the ethical standards of the institutional and/or national research committee and in conformity with the 1964 Helsinki declaration, in addition to its later amendments or comparable ethical standards. No animal experiment was involved in the current study. The research protocol was reviewed and authorized by the Ethics Committee of the Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine. Informed consent was obtained from each participant.
Consent for publication
Patients signed informed consent to publishing their data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10147_2024_2472_MOESM1_ESM.tif
Fig. S1. The cBioPortal database was used to perform co-expression analysis of THBS1 and potential molecules on the basis of the mRNA expression (http://cbioportal.org/). The mRNA expression levels of THBS1 in breast cancer patients are found to be positively correlated with the expression of PIK3CA, TGFB1, MAPK14, STAT3, TNFRSF1A, and EGR1 (TIF 26208 KB)
About this article
Cite this article
Li, Y., Qin, J., Chen, G. et al. Plasma THBS1 as a predictive biomarker for poor prognosis and brain metastasis in patients with HER2-enriched breast cancer. Int J Clin Oncol 29, 427–441 (2024). https://doi.org/10.1007/s10147-024-02472-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-024-02472-9