Abstract
Background
The present study aimed to compare the efficacy and safety of nivolumab (NIVO) and irinotecan (IRI) and to identify clinical factors that facilitate treatment selection.
Methods
Patients with advanced gastric cancer (AGC) who underwent NIVO or IRI treatment between November 2016 and June 2018 at three institutions were retrospectively reviewed. The inclusion criteria were histologically confirmed gastric/gastroesophageal adenocarcinoma pretreated with fluoropyrimidines and taxanes, no previous NIVO or IRI treatment, and adequate organ function. Main outcome measures were objective response rate (ORR), progression-free survival (PFS), overall survival (OS), and adverse events. Interaction between treatment groups and clinical factors regarding OS were tested using a multivariate Cox proportional hazards model adjusted for relevant variables.
Results
Both NIVO (n = 71) and IRI (n = 61) groups had similar baseline characteristics, except for sex distribution. NIVO and IRI groups had ORR of 20% and 6%, median PFS of 1.6 and 1.8 months, and median OS of 6.4 and 6.4 months, respectively. Interaction analysis did not reveal any significant interaction between NIVO and IRI related to OS for various factors. NIVO group tended to have fewer ≥ grade 3 adverse events than IRI group, especially neutropenia (3% vs. 28%) and febrile neutropenia (1% vs. 8%). In the NIVO group, one patient developed pneumonitis, and four patients developed skin reactions.
Conclusions
Although no remarkable differences in efficacy were found between IRI and NIVO for AGC, NIVO had a better safety profile compared to IRI. We found no clinical markers that can assist treatment decisions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is the fifth most frequently diagnosed cancer and the third most frequent cause of cancer-related deaths worldwide [1]. In Japan, gastric cancer was the second most common cancer in 2016 and the third leading cause of cancer-related deaths in 2018 [2]. Although early-stage gastric cancer is amenable to cure by endoscopic or surgical excision, a substantial proportion of patients present with incurable or recurrent disease. Systemic chemotherapy is the current therapeutic option for advanced gastric cancer (AGC), but the prognosis is still poor (5-year survival rate: < 10%) [3].
Doublet combination regimens with fluoropyrimidine plus platinum and ramucirumab in combination with paclitaxel or a single-agent regimen are recommended as first- and second-line treatment for fit patients with AGC [4]. In the placebo-controlled randomized phase III trials ATTRACTION-2 and TAGS, nivolumab targeting the programmed cell death 1 (PD-1) and trifluridine/tipiracil extended the survival of patients with AGC refractory to, or intolerant of, at least two previous regimens [5, 6]. These results provide robust level I evidence for the use of nivolumab or trifluridine/tipiracil in this clinical setting [4]. There is a lack of high-quality evidence supporting the use of irinotecan monotherapy, but it has shown tolerable safety profile and modest efficacy against AGC [7,8,9]. Recently, nivolumab combined with chemotherapy demonstrated superior survival benefit versus chemotherapy alone in treatment-naïve patients with human epidermal growth factor receptor 2 (HER2)-negative AGC; however, an exploratory analysis suggested that survival benefit with nivolumab was modest in patients whose tumors express programmed death-ligand 1 (PD-L1) with a combined positive score (CPS) < 5 or < 1 [10]. In two pivotal randomized phase III trials, CheckMate-649 and KEYNOTE-062, reconstructed Kaplan–Meier plots in unreported PD-L1 CPS subgroups suggest the lack of survival benefit in addition to immune checkpoint inhibition to chemotherapy for patients with low PD-L1–expressing tumors [11]. These data indicate that cytotoxic chemotherapies with trifluridine/tipiracil or irinotecan, or anti-PD-1 inhibitor nivolumab are still viable options for patients with low PD-L1 tumor in the later-line treatment setting. In these studies, the median overall survival (OS) values were just approximately 6 months and still need to be improved.
In general, immune checkpoint inhibitors are associated with fewer severe adverse events and more sustained response compared to cytotoxic chemotherapies. Long-term follow-up data of the ATTRACTION-2 trial showed promising 1-year and 2-year OS rates in the nivolumab arm (27.3% and 10.6%, respectively) [12]. However, owing to the paucity of evidence supporting the use of specific agents, selection of therapy in the third- or later-line setting is a clinical challenge. Therefore, in this study, we retrospectively compared the efficacy and safety of nivolumab and irinotecan in patients with AGC in the third- or later-line setting.
Patients and methods
Patient population
Data pertaining to AGC patients who received nivolumab or irinotecan monotherapy as third- or later-line treatment between November 2016 and June 2018 at three institutions were retrospectively reviewed. The inclusion criteria were: age ≥ 18 years; Eastern Cooperative Oncology Group Performance Status (ECOG PS): 0 to 2; histologically confirmed gastric/gastroesophageal adenocarcinoma; disease refractory to or intolerant of fluoropyrimidines and taxanes; no previous treatment with either nivolumab or irinotecan; and adequate organ function. The following clinical data were collected: age, sex, ECOG PS, histological type of tumors, HER2 status, oral intake (adequate or inadequate), history of gastrectomy, number of metastatic sites, sites of metastasis, the severity of ascites, history of chemotherapy, time from the start of first-line therapy, history of antibiotics within 30 days before treatment, neutrophil/lymphocyte ratios (NLR), lactate dehydrogenase (LDH) level, Glasgow Prognostic Score (GPS), and subsequent therapies. The need for total parenteral nutrition was defined as inadequate oral intake. The severity of ascites on CT scan was graded as none, mild, moderate, or massive: “mild” ascites was localized in only the upper abdominal space or pelvis; “moderate” was neither mild nor massive; “massive” extended throughout the abdominal cavity. The NLR was calculated as the absolute neutrophil count divided by the absolute lymphocyte count. The cutoff values for NLR and LDH level were determined according to a median value and upper limit of the normal, respectively. The GPS was graded as 2 in presence of both elevated C-reactive protein (CRP) level (> 1.0 mg/dL) and hypoalbminemia (< 3.5 g/dL), 1 in presence of either elevated CRP level or hypoalbminemia, and 0 in the presence of neither of these [13]. All patients provided written informed consent prior to receiving treatment. This study was approved by the Institutional Review Board of the Aichi Cancer Center Hospital, Saitama Cancer Center, and Kobe City Medical Center General Hospital.
Treatment and assessments
Patients received nivolumab 3 mg/kg or 240 mg (since November 2018) administered as 30-min intravenous infusion every 14 days or irinotecan 150 mg/m2 as 90-min intravenous infusion every 2 weeks. Treatment was continued until the occurrence of confirmed disease progression, unacceptable toxicity, patient’s refusal, or at the investigator’s discretion. Tumor response was assessed in patients with measurable disease by the attending doctors according to the Response Evaluation Criteria in Solid Tumors (RECIST) ver. 1.1 [14]. Treatment toxicity was evaluated according to Common Terminology Criteria for Adverse Events ver. 4.0.
Statistical analysis
Between-group differences with respect to clinicopathological factors were compared using the Fisher’s exact test for categorical variables and the Mann–Whitney U test for continuous variables. Objective response rate (ORR) was defined as proportion of patients with confirmed complete response (CR) or partial response (PR). Disease control rate (DCR) was defined as proportion of patients with CR, PR, or stable disease (SD). PFS was calculated from the date of the initiation of treatment until the date of disease progression or death from any cause. OS was calculated from the date of the initiation of treatment until the date of death from any cause. Patients who were still alive were censored at the last follow-up. The PFS and OS curves were estimated using the Kaplan–Meier method and between-group differences assessed using the log-rank test. Survival differences between treatment groups were also evaluated by multivariate analyses using the Cox proportional hazards model, adjusted for relevant variables that were associated with p values < 0.05 in univariate analyses. Interaction between treatment groups and demographic factors regarding OS was tested using a multivariate Cox proportional hazards model adjusted for relevant variables. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) [15]. Two-sided p values < 0.05 were considered indicative of statistical significance.
Results
Patient characteristics
A total of 71 patients treated with nivolumab and 61 with irinotecan were eligible for this study. The last follow-up time was April 2019. The baseline patient characteristics are summarized in Table 1. There was no significant between-group difference with respect to baseline characteristics except for sex distribution. Numerically, more patients in the nivolumab group had peritoneal metastases, inadequate oral intake, and greater severity of ascites, compared with the irinotecan group.
Treatment exposure and subsequent treatment
The median follow-up duration was 12.1 (range 5.1–20.2) months in the nivolumab group and 16.9 (range 2.8–29.0) months in the irinotecan group. Treatment was discontinued in 67 (94%) patients with nivolumab and 61 (100%) with irinotecan. The main reasons for discontinuation were disease progression (92% and 97%) and adverse events (3% and 3%) in the nivolumab and irinotecan groups, respectively. The subsequent cancer therapy is listed in Table 2. Subsequent chemotherapy agents were administered to 32 (45%) patients in the nivolumab group (of which 17 patients received irinotecan) and 36 (59%) patients in the irinotecan group (of which 23 patients received nivolumab) (p = 0.22).
Tumor response and survival outcomes
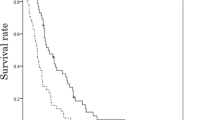
Tumor response in patients with target lesions is shown in Table 3. A trend toward better ORR was observed in the nivolumab group compared with irinotecan group (20% vs. 6%, p = 0.17). There was no significant between-group difference with respect to DCR (30% vs. 39%, p = 0.46). Median PFS was 1.6 (95% confidence interval [CI], 1.4–2.3) months with nivolumab and 1.8 (95% CI, 1.6–2.3) months with irinotecan (hazard ratio [HR] 0.93, 95% CI 0.65–1.32, p = 0.67) (Fig. 1a). After adjusting for relevant factors (Supplementary Table 1), the between-group difference was not statistically significant (adjusted HR 1.06, 95% CI 0.72–1.56, p = 0.76). A total of 54 (76%) patients receiving nivolumab and 55 (90%) patients receiving irinotecan had died at the time of data cutoff. The median OS was 6.4 (95% CI 5.0–8.2) months in the nivolumab group and 6.4 (95% CI 5.5–8.1) months in the irinotecan group (HR 0.88, 95% CI 0.60–1.28, p = 0.50) (Fig. 1b). After adjusting for relevant factors (Supplementary Table 2), a similar result was observed (adjusted HR 0.78, 95% CI 0.51–1.20, p = 0.26). The one-year survival rate in the nivolumab group was numerically better than that in the irinotecan group (26% vs. 19%, p = 0.61). Subgroup analysis of OS revealed no obvious interaction between treatment groups and various clinicopathological factors (Fig. 2). On the other hand, patients with one metastatic site, liver metastases, no history of antibiotics, and ALP levels above the upper limit of normal level had a better OS in the nivolumab group than those in the irinotecan group (interaction test p < 0.20). Patients with three or more of these factors exhibited significantly longer OS in the nivolumab group than in the irinotecan group (median, 6.6 vs. 4.7 months; HR, 0.36; 95% CI, 0.16–0.81; p = 0.01), but there was no significant difference between the patients in the two groups with less than three factors (median, 6.1 months vs. 8.0 months; HR, 1.03; 95% CI, 0.66–1.60; p = 0.89) (Supplementary Fig. 1). While analyzing patients who achieved PR or SD, OS (median 13.8 vs. 10.3 months; HR 0.56, 95% CI 0.20–1.58; p = 0.27) and PFS (median 6.7 vs. 4.0 months; HR 0.50, 95% CI 0.20–1.20; p = 0.12) showed a tendency to be prolonged in the nivolumab group compared with the irinotecan group.
Adverse events
The adverse events in our study population are listed in Table 4. Any grade 3 or 4 adverse events occurred in 22 (31%) patients in the nivolumab group and 31 (51%) in the irinotecan group (p = 0.02). The frequency of grade 3 or 4 neutropenia in the nivolumab group was significantly lower than that in the irinotecan group (3% vs. 28%, p < 0.01). Febrile neutropenia in the irinotecan group was numerically more frequent compared with the irinotecan group (8% vs. 1%). The frequency of any grade neutropenia, nausea, diarrhea, constipation, fatigue, and anorexia in the nivolumab group was significantly lower than that in the irinotecan group. Immune-related adverse events in the nivolumab group were pneumonitis in one patient (grade 1) and rash in four patients (grade 1 in 3 and grade 2 in 1 patient). In one patient in the nivolumab group, the treatment was stopped because of liver disorder diagnosed by computed tomography. There was no treatment-related death in either group.
Discussion
In the present study, we compared the efficacy and safety of nivolumab and irinotecan in patients with AGC treated in third- or later-line setting. The efficacy results were similar between nivolumab and irinotecan, but the one-year survival rate (26%) in the nivolumab group was numerically higher than that in the irinotecan group (19%) and was comparable to that (27%) in the ATTRACTION-2 trial [12]. In terms of safety, toxicity profiles of both groups were consistent with results from previous studies [5, 7]. As expected, the irinotecan group experienced a higher frequency of all grade 3 or 4 adverse events compared with the nivolumab group. The frequencies of any grade neutropenia, nausea, diarrhea, fatigue, and anorexia were higher in the irinotecan group. It was observed that the frequency of immune-related adverse events reported in the current study was lower than that reported in previous studies [16, 17]. This difference can be attributed to the retrospective nature of the study and the possibility that the data regarding grade 1–2 adverse events were not adequately collected from the medical records and the mild adverse events were not properly diagnosed as this study was conducted when nivolumab was just approved. Although estimating the frequency of such events was difficult to judge because of the small number of subjects, we do not believe that there is a marked difference between the frequency reported in this study and the frequency in the ATTRACTION-2 trial [5].
In the JAVELIN 300 trial, avelumab, which is an antibody targeting PD-L1, failed to demonstrate superiority over chemotherapy in terms of OS in third-line treatment of AGC [18]. However, this is not the case with nivolumab because avelumab leaves the PD-1/PD-L2 pathway intact in spite of frequent expression of PD-L2 in gastric cancer tissue in the absence of PD-L1 and functional PD-1/PD-L2 interactions in the anti-tumor response of cytotoxic T lymphocytes [19, 20]. Nivolumab in combination with chemotherapy as first-line treatment was shown to improve the OS and PFS compared with chemotherapy alone in patients with AGC and became one of standard treatment option for these patients [10]. However, patients with HER2-positive or low expression of PD-L1 CPS tumors may still receive nivolumab as third- or later-line treatment and, therefore, the choice between irinotecan and nivolumab remains a clinical challenge. In another study comparing the two agents as third-line treatment, nivolumab was more effective when patients had factors such as good PS, no liver metastases, and low baseline tumor volume. On the contrary, irinotecan was found to be more effective in patients with two or more of these factors [21]. In the present study, nivolumab was significantly better than irinotecan in patients with three or more clinical factors, including having one metastatic site, liver metastases, no history of antibiotics, and elevated ALP; however, this is only an exploratory study, and the contradictory results for liver metastasis with previous reports need to be validated in a larger cohort of patients. Since the prognosis was favorable in cases where disease control was achieved, further investigation on biomarkers to predict their efficacy, including PD-L1 CPS, is required in the future. The 6-month PFS rate (15.5% vs. 6.6%) and 1-year OS rate (26.3% vs. 19.3%) were better in the nivolumab group, and with a longer observation period, the nivolumab group may have had a better outcome as previously reported [21].
In general, cytotoxic drugs are associated with short-term adverse events, such as myelosuppression and gastrointestinal toxicity, whereas immune checkpoint inhibitors produce immune-related events that are less frequent but require long-term management. Although these differences in safety profiles need to be taken into account in treatment selection, nivolumab may be more suitable as third- or later-line treatment for nivolumab-naïve AGC patients because of the lower toxicity profile, the long-term survival expected in a certain proportion of patients, and the suggested enhancement of subsequent treatment efficacy [22, 23].
Some limitations of our study should be acknowledged. First, this was a retrospective nonrandomized analysis. Second, none of the cases were treated with trifluridine/tipiracil because these drugs were not yet approved for use in Japan during the study reference period. In the TAGS trial, trifluridine/tipiracil extended survival of patients with AGC refractory to, or intolerant of, at least two previous chemotherapy regimens compared to placebo [6]. At present, trifluridine/tipiracil is also used as third- or later-line therapy for AGC, but no direct comparisons between them have been made. Therefore, there is room for further studies on the selection of trifluridine/tipiracil and nivolumab in the late-line setting. Third, we did not measure the markers such as tumor mutation burden, high frequency of microsatellite instability or PD-L1 CPS that are potential predictive factors for efficacy of immune checkpoint inhibitors [24,25,26]. The PD-L1 CPS has been consistently suggested to be a significant efficacy biomarker in first- and second-line treatment setting for AGC [10, 24]. Larger studies are required to investigate the usefulness of these clinical or molecular biomarkers for treatment selection.
In conclusion, in this study, nivolumab as third- or later-line treatment for AGC showed better safety profile and a tendency toward better efficacy compared to irinotecan; however, we did not identify any clinical factors that may help inform the choice of one drug over the other. Future studies should seek to identify biomarkers that may be useful for selection of nivolumab, irinotecan, and/or trifluridine/tipiracil in this setting.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Cancer registry and statistics. Cancer Information Service NCC, Japan.
Ito Y, Miyashiro I, Hosono S et al (2014) Long-term survival and conditional survival of cancer patients in Japan using population-based cancer registry data. Cancer Sci 105:1480–1486
Muro K, Van Cutsem E, Narita Y et al (2019) Pan-Asian adapted ESMO clinical practice guidelines for the management of patients with metastatic gastric cancer: a JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann Oncol 30:19–33
Kang YK, Boku N, Satoh T et al (2017) Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 390:2461–2471
Shitara K, Doi T, Dvorkin M et al (2018) Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 19:1437–1448
Makiyama A, Arimizu K, Hirano G et al (2018) Irinotecan monotherapy as third-line or later treatment in advanced gastric cancer. Gastric Cancer 21:464–472
Nishimura T, Iwasa S, Nagashima K et al (2017) Irinotecan monotherapy as third-line treatment for advanced gastric cancer refractory to fluoropyrimidines, platinum, and taxanes. Gastric Cancer 20:655–662
Kawakami T, Machida N, Yasui H et al (2016) Efficacy and safety of irinotecan monotherapy as third-line treatment for advanced gastric cancer. Cancer Chemother Pharmacol 78:809–814
Janjigian YY, Shitara K, Moehler M et al (2021) First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 398:27–40
Zhao JJ, Yap DWT, Chan YH et al (2022) Low programmed death-ligand1-expressing subgroup outcomes of first-line immune checkpoint inhibitors in gastric or esophageal adenocarcinoma. J Clin Oncol 40:392–402
Chen LT, Satoh T, Ryu MH et al (2020) A phase 3 study of nivolumab in previously treated advanced gastric or gastroesophageal junction cancer (ATTRACTION-2): 2-year update data. Gastric Cancer 23:510–519
Hwang JE, Kim HN, Kim DE et al (2011) Prognostic significance of a systemic inflammatory response in patients receiving first-line palliative chemotherapy for recurred or metastatic gastric cancer. BMC Cancer 11:489
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–458
Masuda K, Shoji H, Nagashima K et al (2019) Correlation between immune-related adverse events and prognosis in patients with gastric cancer treated with nivolumab. BMC Cancer 19:974
Sato S, Oshima Y, Matsumoto Yu et al (2021) The new prognostic score for unresectable or recurrent gastric cancer treated with nivolumab: a multi-institutional cohort study. Ann Gastroenterol Surg 5:794–803
Bang YJ, Ruiz EY, Van Cutsem E et al (2018) Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol 29:2052–2060
Nakayama Y, Mimura K, Kua LF et al (2020) Immune suppression caused by PD-L2 expression on tumor cells in gastric cancer. Gastric Cancer 23:961–973
Yearley JH, Gibson C, Yu N et al (2017) PD-L2 expression in human tumors: relevance to anti-PD-1 therapy in cancer. Clin Cancer Res 23:3158–3167
Ishii T, Kawazoe A, Sasaki A et al (2020) Clinical and molecular factors for selection of nivolumab or irinotecan as third-line treatment for advanced gastric cancer. Ther Adv Med Oncol 12:1758835920942377
Kato K, Narita Y, Mitani S et al (2020) Efficacy of cytotoxic agents after progression on anti-PD-(L)1 antibody for pre-treated metastatic gastric cancer. Anticancer Res 40:2247–2255
Boku N, Satoh T, Ryu MH et al (2021) Nivolumab in previously treated advanced gastric cancer (ATTRACTION-2): 3-year update and outcome of treatment beyond progression with nivolumab. Gastric Cancer 24:946–958
Shitara K, Özgüroğlu M, Bang YJ et al (2018) Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 392:123–133
Yarchoan M, Hopkins A, Jaffee EM (2017) Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med 377:2500–2501
Marabelle A, Le DT, Ascierto PA et al (2020) Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol 38:1–10
Acknowledgements
The authors would like to thank Enago (www.enago.jp) for the English language review.
Funding
This study was conducted with no funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Shigenori Kadowaki has received grants and personal fees from Ono Pharmaceutical Co., Ltd., Daiichi Sankyo, Taiho Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Chugai Pharmaceutical Co., Ltd., MSD K.K.; grants from Janssen Pharmaceutical K.K., Nobelpharma Co., Ltd.; and personal fees from Bristol-Myers Squibb K.K., Bayer Yakuhin, Ltd., Eisai Co., Ltd., and Merck KGaA. Seiichiro Mitani has received grants from Taiho Pharmaceutical Co., Ltd. Yukiya Narita has received grants and personal fees from Ono Pharmaceutical Co., Ltd. and Bristol-Myers Squibb K.K.. Toshiki Masuishi has received grants and personal fees from Ono Pharmaceutical Co., Ltd. and received personal fees from Yakult Honsha. Hisateru Yasui has received grants from Ono Pharmaceutical Co., Ltd. and received personal fees from Bristol-Myers Squibb K.K. and Yakult Honsha. Hiroki Hara has provided an advisory role for Bristol-Myers Squibb K.K., Boehringer Ingelheim, Daiichi Sankyo, Dainippon Sumitomo, MSD K.K., and Ono Pharmaceutical Co., Ltd.; received grants from Amgen, Astellas, AstraZeneca K.K., Bayer Yakuhin, Ltd., BeiGene, Boehringer Ingelheim, Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo, Janssen Pharmaceutical K.K., MSD K.K., and Ono Pharmaceutical Co., Ltd.; and received honoraria from Asahi Kasei, Bayer Yakuhin, Ltd., Bristol-Myers Squibb K.K., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo, Eli Lily and Company, Merck Biopharma, MSD K.K., Ono Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., and Takeda Pharmaceutical Company. Kei Muro has received grants and personal fees from Ono Pharmaceutical Co., Ltd. and Bristol-Myers Squibb K.K.. The other authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Institutional review boards approval
This study was approved by the Institutional Review Boards of the Aichi Cancer Center Hospital, Saitama Cancer Center, and Kobe City Medical Center General Hospital.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Kumanishi, R., Kadowaki, S., Mitani, S. et al. Nivolumab versus irinotecan as third- or later-line treatment for advanced gastric cancer: a multi-center retrospective study. Int J Clin Oncol 28, 756–763 (2023). https://doi.org/10.1007/s10147-023-02330-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-023-02330-0