Abstract
Background
The role of pembrolizumab in the treatment of poor performance status (PS) patients remains unclear.
Patients and methods
We conducted a phase II trial to investigate the efficacy and safety of pembrolizumab as first-line therapy for non-small-cell lung cancer (NSCLC) patients with PSs of 2–3 and programmed cell death ligand 1 (PD-L1) expression ≥ 50%. The primary endpoint of this study was the objective response rate (ORR).
Results
Fourteen patients treated at eight institutions were enrolled. Most patients had PS 2 (12/14; 86%) and others had PS 3 (2/14; 14%). The ORR was 57.1% (95% confidence interval 28.9–82.3%), which met the primary endpoint. The median progression-free survival (PFS) and 1-year PFS rates were 5.8 months and 20.0%, respectively. At the time of data cut-off, one patient had received treatment for more than 1 year; another patient had received treatment for more than 2 years. Nine patients had improved PS with treatment (Wilcoxon signed-rank test, p = 0.003). Two patients had immune-related adverse events ≥ grade 3: grades 5 and 3 elevation in alanine and aspartate aminotransferases. Two PS 3-stage patients were diagnosed with clinically progressive disease prior to initial computed tomography; both died within 2 months.
Conclusion
Pembrolizumab was effective for the treatment of NSCLC patients with a poor PS and PD-L1 level ≥ 50%. However, given the poor outcomes of the PS 3 patients, the drug is not indicated for such patients. Adverse events, including liver dysfunction, should be carefully monitored.
Registration ID
UMIN000030955.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pembrolizumab is the standard first-line treatment for advanced non-small cell lung cancers (NSCLCs) exhibiting high-level expression of programmed cell death ligand 1 (PD-L1). The pivotal phase III KEYNOTE-024 clinical trial revealed that pembrolizumab afforded significantly better outcomes than did standard platinum-based, doublet first-line treatment for advanced NSCLCs evidencing high-level PD-L1 expression [an objective response rate (ORR) of 44.8%, median progression-free survival (PFS) of 10.3 months, and overall survival (OS) of 26.3 months]. In another phase III clinical trial (KEYNOTE-189), a combination of pembrolizumab and platinum-based doublet therapy significantly improved the ORR (47.6% of patients), the PFS (to 8.8 months), and the OS (to 22.0 months), regardless of the PD-L1 expression level [1]. Therefore, the standard therapy for treatment-naïve NSCLC patients with high PD-L1 expression levels involves pembrolizumab with or without platinum-based doublet therapy.
However, the aforementioned trials excluded patients with poor performance status (PS). Therefore, the treatment of patients with poor PS has lagged behind the current advances in NSCLC treatment. Because of the aging population in developed countries, the number of advanced NSCLC patients with poor PS is expected to increase. Therefore, effective treatment strategies for NSCLC patients with poor PS are urgently required.
To determine the efficacy and safety of pembrolizumab as the first-line treatment for advanced NSCLC patients with poor PS and high PD-L1 expression, we performed a phase II study to evaluate pembrolizumab treatment for treatment-naïve advanced NSCLC patients with PS 2 or 3 and high PD-L1 expression.
Methods
Study design and participants
This was a multi-institutional, single-arm, non-randomized, open-label phase II trial. This study included patients who met the following criteria: age ≥ 20 years, provision of written informed consent, presence of histologically or cytologically confirmed NSCLC, presence of postoperative recurrence or incurable advanced stage cancer, no prior systemic anti-cancer therapy, PD-L1 tumor proportion score ≥ 50%, no sensitizing epidermal growth factor receptor mutations or anaplastic lymphoma kinase rearrangement, Eastern Cooperative Oncology Group PS of 2–3, and adequate organ function. Patients were excluded if they met any of the following criteria: current autoimmune disease complications, history of chronic/recurrent autoimmune diseases, interstitial lung disease, symptomatic brain lesions, and/or leptomeningeal carcinomatosis. PD-L1 expression levels were determined by immunohistochemistry using anti-22C3 antibodies.
Intervention and assessment
Patients received 200 mg of pembrolizumab intravenously, once every 3 weeks for up to 2 years, until disease progression or interruption due to toxicity. All adverse events were recorded in accordance with the 4th version of the Common Terminology Criteria for Adverse Events. The efficacy of pembrolizumab was determined via radiological assessment by computed tomography and version 1.1 of the Response Evaluation Criteria in Solid Tumors (RECIST); assessments were performed once every 6 weeks for the first 48 weeks and once every 12 weeks thereafter.
Outcomes
This study evaluated the efficacy of pembrolizumab monotherapy in advanced NSCLC patients with PS 2 or 3 and high PD-L1 expression. The primary endpoint was the ORR, defined as the rate of RECIST complete and partial responses. The secondary endpoints were disease control rate, PFS, OS, safety (evaluated using the 4th version of the Common Terminology Criteria for Adverse Events), and a change in PS. PFS was defined as the time from registration to disease progression or death from any cause. OS was defined as the time from registration to death from any cause. PFS may be an appropriate primary endpoint when evaluating the efficacies of immune checkpoint inhibitors. However, patients with a poor PS may drop out prior to PFS determination; the ORR (which is rapidly determined) thus served as the primary endpoint and PFS as the secondary endpoint.
Statistical analysis
The KEYNOTE-024 trial [2] reported an ORR of 45% in patients with good PS. The ORR to cytotoxic chemotherapeutic agent monotherapy in patients with PS 2 is approximately 10% [3]. Therefore, using an expected ORR of 35%, threshold of 10%, one-sided significance level of 0.05, and detecting ability of 80%, it was estimated that 13 patients would be required to reach statistical significance. Assuming dropout of one patient, 14 patients were enrolled in this study. This study was conducted in compliance with the Declaration of Helsinki; it was approved by the Institutional Review Boards of each participating institution. Written informed consent was obtained from all patients. The study protocol was registered at the website of the University Hospital Medical Information Network, Japan (ID: UMIN000030955).
Results
Patient characteristics
Fourteen patients from eight institutions were enrolled between July 2018 and September 2020. Table 1 summarizes the baseline characteristics of study participants. The median age of study participants was 77 years (range 62–87 years); 12 of the 14 participants were men. Twelve patients had PS 2, while the remaining two patients had PS 3. Thirteen patients had stage IV disease, and one patient had disease recurrence. The PD-L1 expression level was ≥ 50% in all patients (median 80%; range 50–100%).
Response and survival
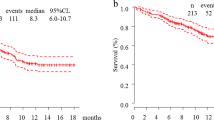
Table 2 summarizes the treatment responses according to RECIST (version 1.1). Eight patients had partial response (57.1%), 2 patients had stable disease (14.2%), and 4 patients had progressive disease (28.6%). The ORR was 57.1% (95% confidence interval [CI] 28.9–82.3%), which met the primary endpoint. The disease control rate was 71.4% (95% CI 41.9–91.6%). Waterfall plot analysis demonstrated that 12 of the 14 participants had a reduction in tumor size following pembrolizumab treatment (Fig. 1). The median PFS duration was 5.8 months (95% CI 0.9–11.0; Fig. 2A). Ten patients (71.4%) experienced disease progression or death by the time of survival analysis. The 1-year PFS rate was 20.0% (95% CI 3.4–46.5). The median OS duration and 1-year OS rate were 9.9 months (95% CI 1.3–not reached; Fig. 2B) and 22.9% (95% CI 3.9–51.2), respectively. Finally, we investigated PS 3 patient survival. Although PS 2 and PS 3 patients were eligible for inclusion, only two were of PS 3 status. Both were diagnosed with clinically progressive disease prior to initial computed tomography; both died within 2 months.
Change in PS following pembrolizumab treatment
Figure 3 depicts the change in PS following treatment with pembrolizumab. Eight of the 14 patients (51.7%) showed improved PS following pembrolizumab treatment. The two PS 3 patients evidenced no PS improvement.
Adverse effects
The adverse events are listed in Table 3. There were 11 immune-related adverse events (irAEs) of any grade and 11 non-irAEs. Two irAEs were grade ≥ 3, and both involved liver dysfunction (14%). No other severe irAEs were reported. One patient who experienced an irAE died of liver dysfunction. This patient had a PS of 2 at study enrollment but did not have previous liver diseases or other causes of liver dysfunction (e.g., hepatitis virus infection or chronic liver disease). Therefore, liver dysfunction was considered an irAE. Grade ≥ 3 non-irAEs included two lung infections (14%), which may have been caused by an immunocompromised condition in patients with poor PS; they also included one gastric hemorrhage because of lung cancer metastasis to the stomach (7%), one spinal fracture (7%), and one perianal abscess (7%).
The two PS 3 patients exhibited no irAE and one non-irAE (a grade 3 perianal abscess).
Treatment delivery
At the final follow-up, the median duration of pembrolizumab treatment was 3.8 months (range 0.7–8.4 months). Pembrolizumab was stopped in four patients (28.6%) because of adverse events (liver dysfunction in two, drug-induced pneumonitis in one, and perianal abscess in one).
Discussion
We conducted a prospective, phase II study to evaluate the efficacy of first-line pembrolizumab treatment for NSCLC patients with poor PS and high PD-L1 expression. We found an ORR of 57.1% with pembrolizumab treatment, which met the primary endpoint. A durable response to pembrolizumab treatment can be expected in patients with poor PS, as demonstrated by the finding that 22.9% of patients were alive and 20.0% of patients had PFS at 12 months. This is the first prospective study to statistically investigate the efficacy of first-line pembrolizumab in patients with a PD-L1 level ≥ 50% and those of poor PS.
Previous studies evaluated the utility of chemotherapy in patients of poor PS. In the subgroup analysis of the CALGB 9730 study, the ORR and median OS of PS 2 patients given paclitaxel and carboplatin were 24% and 4.7 months, respectively [4]. In the ECOG 1599 randomized phase II study, the ORRs, median PFSs, and median OSs of PS 2 patients given gemcitabine plus cisplatin, and paclitaxel plus carboplatin, were 23 and 24%, 3.0 and 3.5 months, and 6.9 and 6.2 months, respectively [5]. In another study, PS 2 patients evidenced an ORR of 24%, a PFS of 5.8 months, and an OS of 9.3 months on carboplatin and pemetrexed combination therapy [6]. These durations are less than or similar to those of the present study (ORR 57.1%, median PFS 5.8 months, and median OS 9.9 months), although we included both PS 2 and PS 3 patients, whereas the cited work did not include PS 3 patients. In addition, the response continued in 20% of our patients to 24 months; previous studies reported disease progression (even with chemotherapy) in most patients by about 20 months [5, 6]. The response to pembrolizumab is durable, even in patients of poor PS.
Earlier retrospective studies reported poor outcomes after immune checkpoint inhibitors were prescribed for patients of poor PS [7]. We previously reported the results of a multicenter, retrospective cohort study that investigated the efficacy of immune checkpoint inhibitors as treatments for NSCLC patients of poor PS [8]. In that work, poor PS was significantly associated with reduced survival. However, first-line pembrolizumab treatment in NSCLC patients of PS 2 and a PD-L1 expression level ≥ 50% (comparable to the population of the current study) revealed a median PFS of 7.5 months and a 1-year PFS rate of approximately 20%. Our current phase 2 study prospectively confirmed the reproducibility of our previous findings, demonstrating the efficacy of pembrolizumab in NSCLC patients of PS 2 with a PD-L1 expression level ≥ 50%.
A previous prospective study (PePS2) investigated the efficacy of pembrolizumab as treatment for advanced NSCLC patients irrespective of the PD-L1 expression level and the treatment line [9]. That study included only patients of PS 2; the ORR was 47%, the PFS 12.6 months, and the OS of 14.6 months for patients with PD-L1 expression levels ≥ 50%. The results support the use of pembrolizumab for NSCLC patients of PS 2 and PD-L1 expression levels ≥ 50%.
In the current study, irAEs were less frequent than previously reported for patients of good PS. However, one patient developed fatal liver dysfunction (grade 5). The PePS2 study included more than 100 patients of PS 2 treated with pembrolizumab, but did not report any grade 5 adverse event [9]; thus, patients of PS 2 may not be at an increased risk of fatal adverse effects on pembrolizumab treatment. However, caution must be exercised when using the drug.
Although there were only two PS 3 patients in our study, both evidenced poor outcomes (OS less than 2 months). In our earlier, multicenter retrospective study of patients of poor PS [8], those of PS 3 or higher had poor prognoses despite high PD-L1 expression levels. Therefore, immune checkpoint inhibitors should not be given to NSCLC patients of PS 3 or higher, even those expressing high levels of PD-L1.
One limitation of the current study is the primary endpoint. This was the ORR; the PFS would better indicate the long-term efficacy of an ICI. The reason that we chose the ORR is that we targeted patients of poor PS who might drop out prior to determination of the PFS (e.g., on transfer to a palliative care hospital). Therefore, we chose the ORR (which can be determined quickly) as the primary endpoint. This was met; we conclude that pembrolizumab induces a promising tumor response. On the other hand, as the PFS was only a secondary endpoint and as our sample size was small, it is difficult to identify any long-term benefit of the drug. Thus, we do not confirm a long-term response; we rather speculate that this may exist. Further study is required to confirm our findings in larger samples of NSCLC patients of poor PS.
Conclusion
This phase II study demonstrated that pembrolizumab treatment of previously untreated advanced NSCLC patients with PS 2 or 3 and high PD-L1 expression produced a good response. Given the poor outcomes of our PS 3 patients, the drug is not indicated for such patients.
Data availability
The datasets generated and/or analyzed in this report are available from the corresponding author on reasonable request.
References
Gandhi L, Rodríguez-Abreu D, Gadgeel S et al (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378:2078–2092. https://doi.org/10.1056/NEJMoa1801005
Reck M, Rodríguez-Abreu D, Robinson AG et al (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375:1823–1833. https://doi.org/10.1056/NEJMoa1606774
Gridelli C, Ardizzoni A, Le Chevalier T et al (2004) Treatment of advanced non-small-cell lung cancer patients with ECOG performance status 2: results of an European Experts Panel. Ann Oncol Off J Eur Soc Med Oncol 15:419–426. https://doi.org/10.1093/annonc/mdh087
Lilenbaum RC, Herndon JE, List MA et al (2005) Single-agent versus combination chemotherapy in advanced non-small-cell lung cancer: the cancer and leukemia group B (study 9730). J Clin Oncol 23:190–196. https://doi.org/10.1200/JCO.2005.07.172
Langer CJ, Li S, Schiller J et al (2007) Randomized phase II trial of paclitaxel plus carboplatin or gemcitabine plus cisplatin in Eastern Cooperative Oncology Group performance status 2 non-small-cell lung cancer patients: ECOG 1599. J Clin Oncol 25:418–423. https://doi.org/10.1200/JCO.2005.04.9452
Zukin M, Barrios CH, Pereira JR et al (2013) Randomized phase III trial of single-agent pemetrexed versus carboplatin and pemetrexed in patients with advanced non-small-cell lung cancer and Eastern Cooperative Oncology Group performance status of 2. J Clin Oncol 31:2849–2853. https://doi.org/10.1200/JCO.2012.48.1911
Ahn B-C, Pyo K-H, Xin C-F et al (2019) Comprehensive analysis of the characteristics and treatment outcomes of patients with non-small cell lung cancer treated with anti-PD-1 therapy in real-world practice. J Cancer Res Clin Oncol 145:1613–1623. https://doi.org/10.1007/s00432-019-02899-y
Kano H, Ichihara E, Harada D et al (2020) Utility of immune checkpoint inhibitors in non-small-cell lung cancer patients with poor performance status. Cancer Sci 111:3739–3746. https://doi.org/10.1111/cas.14590
Middleton G, Brock K, Savage J et al (2020) Pembrolizumab in patients with non-small-cell lung cancer of performance status 2 (PePS2): a single arm, phase 2 trial. Lancet Respir Med 8:895–904. https://doi.org/10.1016/S2213-2600(20)30033-3
Acknowledgements
The authors thank Masafumi Fujii of the Kawasaki College of Allied Health Professions (a member of the Safety Review Committee). We also thank all investigators at the participating institutions. All authors contributed to study coordination among the various hospitals. We received no specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
E. Ichihara has received honoraria from Eli Lilly Japanand research funds from MSD, Ono Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., and Janssen Pharmaceutical K.K. T. Kubo has received honoraria from Chugai Pharmaceutical Co. Ltd. T. Kozuki has received honoraria from AstraZeneca, Eli Lilly Japan, and Ono Pharmaceutical Co. Ltd., and research funds from MSD, Chugai Pharmaceutical Co. Ltd., Taiho Pharmaceutical Co. Ltd., Daiichi-Sankyo Co. Ltd and Merck. S. Kuyama has received honoraria from Chugai Pharmaceutical Co. Ltd. K. Hotta received honoraria from Pfizer, AZ, Chugai, Lilly, Takeda, MSD, BMS, Ono, Taiho, and Boehringer-Ingelheim, and research funds from MSD, AZ, Chugai, Lilly, BMS, and Abbvie outside the submitted work. A. Bessho has received research funds from Ono Pharmaceutical Co. Ltd., AstraZeneca, MSD, and Chugai Pharmaceutical CO. Ltd. Y. Maeda has received honoraria from Novartis and research funds from Otsuka Pharmaceutical Co. Ltd., Eisai, MSD, Asahi Kasei Pharma, Chugai Pharmaceutical Co. Ltd., Nippon Shinyaku Co. Ltd., Takeda Pharmaceutical Co. Ltd., Kyowa Kirin, Astellas, Novartis, AstraZeneca, Janssen Pharmaceutical K.K., and Mundipharma K.K. K. Kiura has received honoraria from MSD and research funds from Nippon Kayaku Pharmaceutical Co. Ltd., Ono Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., Bristol Myers Squibb, Takeda Pharmaceutical Co. Ltd., Taiho Pharmaceutical Co. Ltd., Shionogi Pharmaceutical Co. Ltd., Boehringer Ingelheim, and MSD.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Hosokawa, S., Ichihara, E., Harada, D. et al. Pembrolizumab in advanced NSCLC patients with poor performance status and high PD-L1 expression: OLCSG 1801. Int J Clin Oncol 27, 1139–1144 (2022). https://doi.org/10.1007/s10147-022-02164-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-022-02164-2