Abstract
Objective
To evaluate the safety and efficacy of laparoscopic radical hysterectomy (LRH) for cervical cancer, in terms of morbidity and short-term oncologic outcome following LRH’s introduction into Japan.
Methods
We conducted a retrospective analysis of patients with early-stage cervical cancer (FIGO staging IA2, IB1, and IIA1) who underwent LRH from Dec 2014 to Dec 2016. We assessed the morbidity, overall survival (OS) and recurrence-free survival (RFS), and prognostic factors for RFS.
Results
A total of 251 patients were included from 22 facilities across Japan. There were 8 cases of stage IA2 cervical cancer, 226 of IB1, and 17 of IIA1. The median operating time was 343 min and the median blood loss was 190 ml. Two patients (0.8%) had a postoperative complication with a Clavien–Dindo classification of grade 3 or higher. After a median follow-up time of 15.6 months, the 2-year RFS was 87.4%, and the 2-year OS was 97.8%. When the 2-year RFS rate was compared with whether the patient pathologically had tumors of less than 2 cm, versus 2 cm or more, the RFS was 95.8% and 80.4%, respectively. Multivariate analysis found that tumor size and the route of lymph node removal were independent prognostic factors for recurrence.
Conclusion
When LRH was first introduced into Japan, we found that the route of lymph node removal was an independent prognostic factor for recurrence in addition to large tumors (≥ 2 cm). Our results suggest that prognosis may be secured by paying attention to the lymph node removal route.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radical hysterectomy (RH) is the accepted best treatment for early-stage cervical cancer. Various RH techniques have been utilized, based predominantly around an open radical hysterectomy (ORH), but more recently many retrospective studies have indicated the safety and feasibility of using laparoscopic radical hysterectomy (LRH). In the appropriately selected cervical cancer patient, LRH has proven benefits over ORH, including significant reductions of blood loss, pain, duration of hospital stay and wound complications, among other benefits [1]. However, there have been only a few studies comparing LRH and ORH regarding long-term recurrence and survival rates [2,3,4].
In 2018, the results of the Laparoscopic Approach to Carcinoma of the Cervix (LACC) were reported [5]. The study found that, compared to ORH, total laparoscopic RH (TLRH) and total robotic RH (TRRH) seemed to be associated with higher recurrence rates and worse overall survivals. Most previous studies of the safety and efficacy of LRH had been retrospective examinations of single-facility results, with the authors usually being seasoned laparoscopic surgeons, which might have skewed their results [6,7,8]. Significant concerns were immediately raised world-wide over the oncological safety of any minimally invasive surgery (MIS) for RH.
Unexpectedly at almost the same timing as the LACC report, in 2018, LRH first became a national-insurance-paid procedure in Japan. We set out to conduct a first of its kind in-depth nation-wide multicenter evaluation of the safety and efficacy of LRH, as routinely conducted under real-life situations by trained surgeons. Our study looked at data from 22 facilities nationwide involving 251 LRH conducted following LRH’s initial introduction into Japan. This period was concurrent with LRH being classified as an “advanced medical care procedure” for cervical cancer, but, importantly, prior to LRH becoming covered by national health insurance. We analyzed the data for perioperative complications and for oncological prognoses following the initial introduction period.
Methods
Study design and patients
This study was designed as a multi-institutional retrospective study of women with early-stage cervical cancer who underwent laparoscopic radical hysterectomy plus pelvic lymphadenectomy (LRH + PLN) from December 2014 to December 2016. We included only LRH for FIGO stage IA2, IB1, IIA1 cervical cancer, and only results from surgeons who completed three or more LRH surgeries during the study period. We excluded patients who received modified RH, or radiation or concurrent chemo-radiation therapy as their primary treatment, or who received neoadjuvant chemotherapy before the LRH.
Collected data and their definition
We collected patient, perioperative, and short-term prognosis data from medical records. The patient parameters collected were: age, number of previous pregnancies and parity, height, body weight, body mass index (BMI), operation time, perioperative blood loss, transfusion, type of surgery (i.e., nerve-sparing or ‘other’). Surgical data collected were: surgeon certification (endoscopic surgery and/or gynecologic oncology), use of a uterine manipulator, type of colpotomy, route of lymph node specimen removal, perioperative complications, duration of hospital stay, readmission, FIGO stage, histopathologic subtype, pTNM classification, pathological tumor size, stromal invasion, lymphovascular space invasion, lymph node involvement and adjuvant therapy. When utilized, adjuvant therapy consisted of concurrent chemoradiotherapy or chemotherapy.
Complications were subdivided into intraoperative and postoperative complications. Postoperative complications were further subdivided according to the Clavien–Dindo classification system into less than grade III (< III)—or grade III or higher (≥ III). Bladder dysfunction was defined as voiding difficulty requiring reinsertion of a Foley catheter or clean intermittent catheterization after 30 postoperative days.
Statistical analyses
Overall survival (OS) was defined as the time interval from initial surgery to death from any cause. Recurrence-free survival (RFS) was defined as the time from initial surgery to recurrence or cancer-related death, whichever occurred first. We considered RFS as being suitable for appraising the clinical value of the prognostic factors due to the rarity of deaths in this study.
Continuous and categorical variables were summarized by median (min–max) and frequency (percent), respectively. RFS was estimated using the Kaplan–Meier method. We performed comparisons using the log-rank test. The 95% confidence interval (CI) for a specific-time RFS rate was calculated using Greenwood’s formula. Univariate and multivariate analyses using the Cox proportional hazards regression model were performed to calculate the hazard ratio (HR) and 95% CI for RFS.
For multivariate analysis, Model 1 included five pre-specified prognostic factors (tumor type, pN, tumor diameter, vascular invasion, and method for lymph node removal). Model 2 included only the tumor diameter and the method of lymph node removal; in Model 1, these variables had p < 0.1. A value of p < 0.05 for the two-sided test was considered to be significant. All statistical analyses were performed using SAS software (version 9.4; SAS Institute, Inc., Cary, NC, USA).
Ethics
This study conformed to the Ethical Principles for Medical Research Involving Human Subjects and was approved by the Institutional Review Board of the Japanese Gynecologic Oncology Group (No. 16152-4).
Results
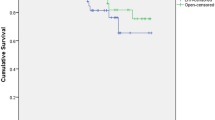
The characteristics of the 251 LRH cases we examined are summarized in Table 1. The distribution of FIGO stages during this time period was eight cases of FIGO stage IA2 (3.2%), 226 IB1 (90%) and 17 IIA1 (6.8%). During the same period, the number of cases of ARH open surgery at these participating institutions was seven IA2 (2.5%), 231 IB1 (83.4%) and 39 IIA1 (14.1%). The median pathological tumor diameter was 2.0 cm (range 0–7.7). R0 resection was achieved in all patients. The perioperative details are shown in Table 2. Median operating time was 343 min (range 159–742). Median blood loss was 190 ml (range 0–2, 100). Intra- and post-operative complications are shown in Table 3. Main complications encountered following LRH consisted of vascular, bladder, ureteral, and obturator nerve injuries. Similar to what occurs during open laparotomy radical hysterectomy, the perioperative visceral injuries were predominantly related to the urinary tract. In 251 cases, an intraoperative urinary tract injury occurred in three cases [bladder in two (0.8%), ureter in one (0.4%)]. Based on the Clavien–Dindo complication classification system, there were only 0.8% cases of postoperative complications of grade III or higher, with a conversion rate of 0.4%. The median follow-up for this retrospective study was 15.6 months (range 1.0–33.2). During follow-up, 22 patients had a recurrence, of which three patients died of the disease. The location of tumor recurrence was divided amongst the vault, pelvis, abdomen, and distant and/or multiple sites. The distribution of recurrence sites was eight (36%) in the vault, six (27%) in the pelvis, one (5%) in abdomen, two (9%) were distant from the primary tumor, and five (23%) patients had multiple sites of recurrence. The one- and two-year RFS rates were 92.4% (88.0–95.2), and 87.4% (80.0–92.2), respectively (Fig. 1B). The one- and two-year OS was 99.6% (96.9–99.9) and 97.8% (93.1–99.3) (Fig. 1, panels A, C, and E). Among the five pre-specified prognostic factors that we examined for Model 1, pT, tumor diameter and lymphovascular space invasion were found to be significant factors for RFS by univariate analysis (Table 4). The multivariate model with these same five factors revealed that tumor diameter affected recurrence (HR, 3.82 [95% CI: 1.06–13.80]) (Table 5, Model 1). In Model 2, this multivariate model (Table 5) included factors with p < 0.1 in Model 1. The HR [95%CI] of tumor diameter and route of lymph node removal were, respectively, HR, 5.26 [95% CI 1.55–17.77] and 4.43 [1.02-19.20]. Kaplan–Meier curves were drawn for tumor diameter and route of lymph node removal (Fig. 1, panels D and F, respectively).
Discussion
LRH for cervical cancer was found to have such significant advantages over traditional ORH that American, European, and several Asian countries neighboring Japan were quick to adopt LRH when it first appeared. However, LRH’s introduction into Japan was delayed. Gynecological laparoscopic surgery in Japan began for reproductive medicine and benign gynecological diseases. LRH, a technically challenging procedure, was initially received with caution by gynecologic oncologists more comfortable with the traditional ORH approach.
Because of improvements in techniques and equipment, laparoscopic surgery for endometrial cancer was approved for use under insurance in Japan in 2014 and LRH for cervical cancer was simultaneously approved as an ‘advanced medical care’, a designation required before a procedure can receive official insurance coverage. In April of 2018, LRH was approved as a ‘surgery under insurance’. LRH is, thus, relatively new to Japan. The nationwide oncologic outcome for LRH, especially during its critical implementation period, had never before been intensively examined.
The feasibility of a surgical technique can be evaluated by several factors: the operative data and the intraoperative and postoperative morbidities. Given that the retrieval of a minimum of 20 pelvic lymph nodes is the gold standard for adequate lymphadenectomy [9], our patients were generally considered to have received better-than-adequate surgery because the median number of resected lymph nodes was 32 and the positive surgical margin rate was 0% among the 251 cases.
Even in experienced hands, LRH surgery is a relatively time-consuming procedure. From the systematic review by Wang et al., the mean operative time for LRH in other countries is 251.5 min (± 78.3), whereas ORH is 4% (11 min) shorter (240.0 min ± 85.1) [1]. In the present study, during the introduction period in Japan, the median operative time for LRH was 337.5 min. The longer duration is accounted for by the newness of LRH to most surgeons in the 22 facilities reporting here, so there was a cautious and steep learning curve.
Another nationwide multicenter study in Japan surveyed perioperative complications occurring after an open radical hysterectomy for clinical stage IB-IIB cervical cancer. Among their 693 patients, an intraoperative urinary tract injury occurred in 22 cases [bladder in 12 (1.7%), ureter injury in 10 (1.4%)], and a postoperative urinary tract fistula occurred in seven cases (1%)] [10]. In our study, an intraoperative urinary tract injury occurred in 1.2% of cases and no postoperative fistula occurred.
In our survey, 82.5% of the patients (207 of 251 patients) were treated by a surgeon certified for conducting minimally invasive surgery and gynecologic oncology (Table 4). This, in addition to the painstaking time taken, would explain why the complication rate for LRH during this break-in period in Japan was so relatively low.
Regarding oncological prognosis, the median follow-up period looking for recurrence was 15.6 months. This observation period is too short yet for a significant statistical analysis. According to our data, 22 patients had a recurrence during their observation period, three of whom died of the disease. Due to the short follow-up period, it is too early to derive an OS with any confidence from the small number of recurrence events thus far.
The one- and two-year RFS rates were 92.4% (88.0–95.2), and 87.4% (80.0–92.2), respectively (Fig. 1b). However, when the RFS rate at two years is compared with patients having larger tumor diameters, < 2 cm versus ≥ 2 cm, the difference was 95.8% versus 80.4%, respectively. Although the patients’ backgrounds might be different, the recurrence rate in our study is higher than that reported in the LACC trial, where the three-year RFS was 91.2%. Also, the 80.5% RFS for cases with a tumor two centimeters or larger was less than ideal when compared to existing ARH data of JCOG0806A (RFS at 5 year was 87.1% with a clinical tumor size of 2 cm <) [11]. Although our institutions performed these operations with varied experience and technique, we analyzed the risk factors for recurrence. Interestingly, from multivariate analysis, tumor size and route of lymph node removal were independent prognostic factors for recurrence.
Tumor diameter has long been known to be a risk factor for recurrence [11]. This is the first report that describes the lymph node removal route through the abdomen as being an independent risk factor for recurrence. Of the 22 recurrent cases, 21 were from cases of lymph node removal through abdomen and only one was through the vagina. Since there was no relationship between the site of recurrence and the lymph node removal route, and there is no case of recurrence at the trocar site, we are as yet unable to explain why the transabdominal removal route increases the risk of recurrence. However, surgical procedures, such as the lymph node removal route, are often consistent among surgeons and facilities, so we plan further investigations to elucidate the reason for this risk factor of recurrence by extending the observation period and examining the details of any surgical procedures which might have a relationship with tumor recurrence. Details of the surgical technique used in the LACC trial were not fully disclosed, so it is impossible for us to compare their results with ours. In our analysis, there is still the possibility that other specific LRH surgical techniques may be playing roles in the risk of recurrence.
The obvious elephant in the room is the fear, among surgeons who undergo MIS RH, that there are risks that an intraoperative manipulation of the tumor or a careless intracorporeal colpotomy might cause an inadvertent dissemination of tumor cells, the recurrence of which could compromise their survival [12].
Using PubMed and the search words “laparoscopic radical hysterectomy”, we reviewed all relevant papers published prior to the 2017 LACC report; the details of this review are shown in Table 6 [references 2, 3, 6, 16-39]. We excluded reports that lacked operative data or details of the procedure, were from the same author or institution, were a multicenter study, used preoperative chemotherapy or radiotherapy, used laparoscopy-assisted radical vaginal hysterectomy, used robot-assisted surgery, or reported on less than 20 cases. In the 27 papers that met our criteria, we also reviewed for the use of a uterine manipulator, the use of a cuff-closure technique, the presence or absence of vaginal manipulation, and details of the lymph node collection route [13].
From our review of papers published prior to the LACC report, we found that most surgeons were indifferent to the use of a manipulator or the method of vaginal incision—almost all used a uterine manipulator and an intracorporeal colpotomy without creating a vaginal cuff to isolate the cervical tumor. If particular laparoscopic surgical techniques, such as the use of a uterine manipulator and intracorporeal colpotomy, are in fact significantly affecting prognosis, then the use of an improved intracorporeal colpotomy procedure might yet allow the MIS RH procedure to survive and evolve. The results of LACC trial provided a valuable opportunity to reconsider the LRH procedure. After the LACC report, promising results using the vaginal cuff-closure technique to isolate the cervical tumor were reported [14, 15]. An investigation that is now in progress should clarify whether technical issues surrounding LRH, such as the isolation of the cervical tumor during colpotomy, or the use of a uterine manipulator, can improve outcomes.
We acknowledge that our study has several limitations. First, it is a retrospective study and has a non-randomized design. Second, we have not been able to verify the precise surgical techniques used, i.e., the colpotomy method, or as to whether the surgeons choose the cuff-closure technique, or if they performed an intracorporeal colpotomy. Third, the mean of the duration of the follow-up period is thus too short for reaching any definitive conclusions about OS yet.
We believe that the primary strength of this study is that the data are from the early stages of the nation-wide introduction of LRH that have never before been intensively investigated. Previous reports of LRH outcomes in other countries were conducted mostly by seasoned surgeons, so their results are unlikely to reflect the real-world experiences of the average surgeon as they begin their use of LRH. Our report reflects the multicenter, nation-wide experience of the early years of introducing LRH for cervical cancer into Japan. Our data are valuable because it will provide real-world data concerning the real risks and benefits of LRH for other counties, as they begin introducing LRH.
In addition, we have already launched a supplementary survey, as JGOG 1081 s-A1, which includes a survey of details the surgical procedures used, such as the colpotomy technique and the specimen removal method with a longer follow-up period of our patient population. From such studies, we should be able to establish comprehensive guidance for LRH surgical techniques so as to improve outcomes for our patients.
Conclusion
When LRH was first introduced into Japan, we found that the routes of lymph node removal were independent prognostic factors for recurrence in addition to large tumors (≧2 cm); our results suggest that prognosis may be secured by paying attention to the lymph node removal route.
Abbreviations
- LRH:
-
Laparoscopic radical hysterectomy
- ORH:
-
Open radical hysterectomy
- RFS:
-
Recurrence-free survival
- TLRH:
-
Total laparoscopic
- TRRH:
-
Total robotic radical hysterectomy
- MIS RH:
-
Minimally invasive surgery for radical hysterectomy
- OS:
-
Overall survival
References
Wang YZ, Deng L, Xu HC et al (2015) Laparoscopy versus laparotomy for the management of early stage cervical cancer. BMC Cancer 15:928
Nam JH, Park JY, Kim DY et al (2012) Laparoscopic versus open radical hysterectomy in early-stage cervical cancer: long-term survival outcomes in a matched cohort study. Ann Oncol 23(4):903–911
Wang W, Chu HJ, Shang CL et al (2016) Long-term oncological outcomes after laparoscopic versus abdominal radical hysterectomy in stage IA2 to IIA2 cervical cancer. Int J Gynecol Cancer 26(7):1264–1273
Malzoni M, Tinelli R, Cosentino F et al (2009) Total laparoscopic radical hysterectomy versus abdominal radical hysterectomy with lymphadenectomy in patients with early cervical cancer: our experience. Ann Surg Oncol 16:1316–1323
Ramirez PT, Flumovitz M, Pareja R et al (2018) Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Eng J Med 379:1895–1904
Puntambekar SP, Palep RJ, Puntambekar SS et al (2007) Laparoscopic total radical hysterectomy by the Pune technique: our experience of 248 cases. J Minim Invasiv Gynecol 14(6):682–689
Xu H, Chen Y, Li Y et al (2007) Complications of laparoscopic radical hysterectomy and lymphadenectomy for invasive cervical cancer: experience based on 317 procedures. Surg Endosc 21:960–964
Pomel C, Atallah D, Le Bouedec G et al (2003) Laparoscopic radical hysterectomy for invasive cervical cancer: 8-year experience of pilot study. Gynecol Oncol 91:534–539
Panici PB, Scambia G, Baiocchi G et al (1991) Technique and feasibility of radical para-aortic and pelvic lymphadenectomy for gynaecologic malignancies: a prospective study. Int J Gynecol Cancer 1:133–140
Machida H, Matsuo K, Furusawa A et al (2019) Profile treatment-related complications in women with clinical stage IB-IIB cervical cancer: a nationwide cohort study in Japan. PLoS ONE 7:1–15
Kato T, Takashima A, Kasamatsu T et al (2015) Clinical tumor diameter and prognosis of patients with FIGO stage IB1 cervical cancer (JCOC0806-A). Gynecol Oncol 137:34–39
Kong TW, Chang SJ, Piao X et al (2016) Pattern of recurrence and survival after abdominal versus laparoscopic/robotic radical hysterectomy in patients with early cervical cancer. J Obstet Gynaecol Res 42:77–86
Gottschalk E, Lanowska M, Chiantera V et al (2011) Vaginal-assisted laparoscopic radical hysterectomy: rationale, technique, results. JSLS 15(4):451–459
Kanao H, Matsuo K, Aoki Y et al (2019) Feasibility and outcome of total laparoscopic radical hysterectomy with no-look no-touch technique for FIGO IB1 cervical cancer. J Gynecol Oncol 30:1–12
Kohler C, Hertel H, Herrmann J et al (2019) Laparoscopic radical hysterectomy with transvaginal closure of vaginal cuff - a multicenter analysis. Int J Gynecol Cancer 29(5):845–850
Spirtos NM, Eisenkop SM, Schlaerth JB et al (2002) Laparoscopic radical hysterectomy (type III) with aortic and pelvic lymphadenectomy in patients with stage I cervical cancer: surgical morbidity and intermediate follow-up. Am J Obstet Gynecol 187(2):340–348
Lee CL, Huang KG, Jain S et al (2002) Comparison of laparoscopic and conventional surgery in the treatment of early cervical cancer. J Am Assoc Gynecol Laparosc 9(4):481–487
Obermair A, Ginbey P, McCartney AJ (2003) Feasibility and safety of total laparoscopic radical hysterectomy. J Am Assoc Gynecol Laparosc 10(3):345–349
Gil-Moreno A, Puig O, Pérez-Benavente MA et al (2005) Total laparoscopic radical hysterectomy (type II-III) with pelvic lymphadenectomy in early invasive cervical cancer. J Minim Invasive Gynecol 12(2):113–120
Ramirez PT, Slomovits BM, Soliman PT et al (2006) Total laparoscopic radical hysterectomy and lymphadenectomy: the M.D. Anderson Cancer Center experience. Gynecol Oncol 102(2):252–255
Malzoni M, Tinelli R, Cosentino F et al (2007) Feasibility, morbidity, and safety of total laparoscopic radical hysterectomy with lymphadenectomy: our experience. J Minim Invasive Gynecol 14(5):584–590
Li Guangyi, Yan X, Shang H et al (2007) A comparison of laparoscopic radical hysterectomy and pelvic lymphadenectomy and laparotomy in the treatment of Ib-IIa cervical cancer. Gynecol Oncol 105(1):176–180
Zakashansky K, Chuang L, Gretz H et al (2007) A case-controlled study of total laparoscopic radical hysterectomy with pelvic lymphadenectomy versus radical abdominal hysterectomy in a fellowship training program. Int J Gynecol Cancer 17(5):1075–1082
Pellegrino A, Villa A, Fruscio R et al (2008) Total laparoscopic radical hysterectomy and pelvic lymphadenectomy in early stage cervical cancer. Surg Laparosc Endosc Percutan Tech 18(5):474–478
Sobiczewski P, Bidzinski M, Derlatka P et al (2009) Early cervical cancer managed by laparoscopy and conventional surgery: comparison of treatment results. Int J Gynecol Cancer 19(8):1390–1395
Shen Y, Wang Z (2010) Total laparoscopic radical hysterectomy for treatment of uterine malignant tumors: analysis of short-term therapeutic efficacy. J Huazhong Univ Sci Technolog Med Sci 30(3):375–378
Canton Romero JC, Anaya-Prado R, Rodriguez-Garcia HA et al (2010) Laparoscopic radical hysterectomy with the use of a modified uterine manipulator for the management of stage IB1 cervix cancer. J Obstet Gynecol 30(1):49–52
Simsek T, Ozekinci M, Saruhan Z et al (2012) Laparoscopic surgery compared to traditional abdominal surgery in the management of early stage cervical cancer. Eur J Gynecol Oncol 33(4):395–398
Hwan JH, Yoo HJ, Joo J et al (2012) Learning curve analysis of laparoscopic radical hysterectomy and lymph node dissection in early cervical cancer. Eur J Obstet Reprod Biol 163(2):219–223
Hong JH, Choi JS, Lee JH et al (2012) Can laparoscopic radical hysterectomy be a standard surgical modality in stage IA2-IIA cervical cancer? Gynecol Oncol 127(1):102–106
Choi CH, Lee JW, Lee YY et al (2012) Comparison of laparoscopic-assisted radical vaginal hysterectomy and laparoscopic radical hysterectomy in the treatment of cervical cancer. Ann Surg Oncol 19(12):3839–3848
Chong GO, Lee YH, Hong DG et al (2013) Robot versus laparoscopic nerve-sparing radical hysterectomy for cervical cancer: a comparison of the intraoperative and perioperative results of a single surgeon’s initial experience. Int J Gynecol Cancer 23(6):1145–1149
Kong TW, Chang SJ, Lee J et al (2014) Comparison of laparoscopic versus abdominal radical hysterectomy for FIGO stage IB and IIA cervical cancer with tumor diameter of 3 cm or greater. Int J Gynecol Cancer 24(2):280–288
Ditto A, Martinelli F, Bogani G et al (2015) Implementation of laparoscopic approach for type B radical hysterectomy: a comparison with open surgical operations. Eur J Surg Oncol 41(1):34–39
Angelopoulos G, Etman A, Cruickshank DJ et al (2015) Total laparoscopic radical hysterectomy: a change in practice for the management of early stage cervical cancer in a U.K cancer center. Eur J Gynaecol Oncol 36(6):711–715
Laterza RM, Uccella S, Casarin J et al (2016) Recurrence of early stage cervical cancer after laparoscopic versus open radical surgery. Int J Gynecol Cancer 26(3):547
Rendon GJ, Echeverri L, Echeverri F et al (2016) Outpatient laparoscopic nerve-sparing radical hysterectomy: a feasibility study and analysis of perioperative outcomes. Gynecol Oncol 143(2):352–356
Zhu T, Chen X, Zhu J et al (2017) Surgical and pathological outcomes of laparoscopic versus abdominal radical hysterectomy with pelvic lymphadenectomy and/or para-aortic lymph node sampling for bulky early-stage cervical cancer. Int J Gynecol Cancer 27(6):1222–1227
Nie JC, Yan AQ, Liu XS (2017) Robotic-assisted radical hysterectomy results in better surgical outcomes compared with the traditional laparoscopic radical hysterectomy for the treatment of cervical cancer. Int J Gynecol Cancer 27(9):1990–1999
Acknowledgements
We would like to thank Dr. G. S. Buzard for the editing of our manuscript, and Ms. H Abe for contributing to data collection.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Kobayashi, E., Kanao, H., Takekuma, M. et al. A retrospective assessment of the safety and efficacy of laparoscopic radical hysterectomy in Japan during the early years following its introduction: a Japanese Gynecologic Oncology Group study (JGOG1081S). Int J Clin Oncol 26, 417–428 (2021). https://doi.org/10.1007/s10147-020-01799-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-020-01799-3