Abstract
Background
The clinical impact of monitoring serum p53 antibodies, carbohydrate antigen19-9, and carcinoembryonic antigen in patients with colorectal cancer has not been fully evaluated.
Methods
A total of 420 surgically treated stage II/III colorectal cancer patients were retrospectively analyzed. Among them, 101 patients developed disease recurrence. The prognostic impact of preoperative and recurrence levels of serum p53 antibodies, carbohydrate antigen19-9, and carcinoembryonic antigen status was evaluated.
Results
Although preoperative carcinoembryonic antigen- and carbohydrate antigen19-9-positive status was significantly associated with recurrence, preoperative serum p53 antibody levels were not. Among two marker combinations, carcinoembryonic antigen + serum p53 antibodies showed the highest positive rate at recurrence. Although carcinoembryonic antigen and carbohydrate antigen19-9 frequently converted from preoperative-negative status to positive status at recurrence, serum p53 antibodies converted to positive status in only one patient. Carcinoembryonic antigen- and carbohydrate antigen19-9-positive status were significant prognostic factors for overall survival after recurrence, but the presence of serum p53 antibodies at recurrence was not.
Conclusions
Postoperative serum p53 antibody status should only be followed in patients with preoperative-positive status. Carcinoembryonic antigen and carbohydrate antigen19-9 should be followed even in preoperative-negative patients. Unlike carcinoembryonic antigen- and carbohydrate antigen19-9-positive status, serum p53 antibody-positive status as recurrence was not a poor prognostic indicator.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) are standard serum markers for colorectal cancer [1, 2]. Recently, a serum autoantibody for p53 (s-p53-Abs) has also been suggested as a useful marker for colorectal cancer [3,4,5,6,7]. The reported s-p53-Abs-positive rate in patients with colorectal cancer is 22–34%. Screening for s-p53-Abs in combination with CEA and CA19-9 yields a colorectal cancer detection rate of about 60% [7]. Because s-p53-Abs-positivity rates are higher than those of CEA and CA19-9 in early stage disease, screening for s-p53-Abs may improve early diagnosis [5,6,7].
S-p53-Abs was previously reported to be useful in predicting tumor recurrence in a patient with rectal cancer, possibly because the antibodies were a response to residual cancer cells [8]. Although CEA or CA19-9 positivity is reported to be a poor prognostic factor, the utility of s-p53-Abs for estimating colorectal cancer prognosis is still considered controversial [3, 4, 6].
CEA- and CA19-9-positivity rates increase with tumor progression [9], with the positivity rates for both markers being highest in patients with stage IV disease. On the other hand, s-p53-Abs-positivity rates do not differ between stage III and IV disease [6, 7]. A previous report demonstrated that s-p53-Abs levels decreased in a patient with liver metastasis in the terminal stage, a seemingly paradoxical finding [7]. Although a few studies have evaluated perioperative changes in CEA, CA19-9, and s-p53-Abs [10], none of these studies have analyzed the levels of these three tumor markers at disease recurrence.
The aim of this study was to analyze the perioperative rates of the tumor markers s-p53-Abs, CA19-9, and CEA in colorectal cancer patients with disease recurrence.
Patients and methods
The records of 420 consecutive patients with stage II or III colorectal cancer surgically treated between January 2010 and December 2014 at Toho University Hospital were evaluated in this retrospective study. Patients selected for the study, including 319 without and 101 with cancer recurrence, are shown (Table 1). Patients treated with neoadjuvant chemotherapy or chemoradiotherapy were excluded. A total of 245 men (58%) and 175 women (42%) with a median age of 69 years (range, 34 to 92 years) were included. Cancer stage was based on TNM classification [11] and determined by pathological evaluation of resected specimens. Stage II was diagnosed in 230 patients, and stage III was diagnosed in 190 patients. All patients were considered cured by primary tumor resection by D2 or more extended lymphadenectomy. Follow-up data were collected until the end of December 2018 or death. The protocol for this retrospective study for medical record review was approved by the institutional review board (M19056 18,002) from Toho University Graduate School of Medicine. Informed consent was obtained from all patients.
S-p53-Abs, CEA, and CA19-9 assays
S-p53-Abs levels were assayed using a highly specific, quantitative enzyme-linked immunosorbent assay kit (MESACUP anti-p53 test; Medical & Biological Laboratories, Nagoya, Japan) [12]. The cutoff value was 1.3 U/mL, which yields a false positive rate in healthy donors of less than 5% [13]. CEA levels were measured using a CEA-2 enzyme immune assay kit (Elecsys CEAII; Roche Diagnostics K.K., Tokyo, Japan) following the manufacturer's instructions. The cutoff value was 5.0 ng/mL. CA19-9 levels were measured using a CA19-9 enzyme immune assay kit (Elecsys CA19-9; Roche Diagnostics K.K., Tokyo, Japan). The cutoff value was 37 U/mL [14].
Cutoff values of serum antibody titers
Optimized antibody titer cutoff values and a standard cutoff value that was greater than the mean plus 3 standard deviations (SD) of the healthy control cohort were applied to the antibody. Specificity was maintained at over 95%. Details for the 3 SD values of the autoantibody titers are previously described [13]. The assay specificity was calculated as the percentage of the healthy controls from whom a negative result was obtained.
Statistical analysis
Comparisons of paired groups were made using Fisher's exact probability test. Survival probabilities after surgery were calculated by the Kaplan–Meier product limit estimator method. Comparisons of between-group differences were tested using a log-rank test. Statistical analyses were performed using EZR statistical software [15]. Statistical significance was set at p < 0.05.
Results
Clinicopathologic features in patients with and without disease recurrence
Among all 420 patients, 132 (31%) were s-p53-Abs-positive. Although high CEA and CA19-9 levels were associated with recurrence, s-p53-Abs rates did not differ between those with or without disease recurrence (p = 0.90, Table 1). Recurrence was significantly more frequent among patients with T4 than T1, T2, or T3 tumors (p < 0.01) and in those with positive versus negative lymph nodes (p < 0.01). Other clinicopathologic factors were not associated with recurrence.
Among the 101 patients with recurrence, clinicopathologic features were compared between the s-p53-Abs-positive and s-p53-Abs-negative patients (Table 2). There were no significant differences between the two groups (Table 2). The status of CEA and CA19-9 also was not significantly different between groups.
Cumulative recurrence rates according to marker status before surgery in stage II/III
The cumulative recurrence rates were 16% for stage II and 34% for stage III (p < 0.01, Fig. 1a). There was not a significant difference in recurrence rates according to preoperative s-p53-Abs status (Fig. 1b). Conversely, the recurrence rates in preoperative CA19-9-positive patients were significantly higher than in CA19-9-negative patients (p = 0.02, Fig. 1c). Increased recurrence rates were also seen in preoperative CEA-positive patients compared with CEA-negative patients (p < 0.01, Fig. 1d).
Perioperative changing pattern of s-p53-Abs in s-p53-Abs positive patients
Among s-p53-Abs positive patients, the s-p53-Abs was still positive at 1 and 6 months after surgery even in the patients without recurrence (Fig. 2a). The antibody values gradually decreased after surgery. On the other hand, the s-p53-Abs was positive at 1 month after surgery. The antibody values at the recurrence was higher than the antibody values 1 month after surgery. Some patients showed higher values than the values before surgery (Fig. 2b).
Tumor marker positivity rates before surgery and at recurrence
Although there was no significant difference, the preoperative positivity rates of all three tumor markers were higher in patients with stage III than those with stage II disease (Fig. 3a). The CEA- and CA19-9-positivity rates increased with tumor progression and recurrence. In particular, CA19-9-positive rates at recurrence were significantly higher than CA19-9-positive rates before surgery in patients with both stage II and stage III cancer. Although s-p53-Abs-positive rates increased with tumor progression, s-p53-Abs-positive rates at recurrence were not higher than the s-p53-Abs-positive rates before surgery. The CEA- and CA19-9-positive rates at recurrence were significantly higher than their positivity rates before surgery (Fig. 3a, b). But the s-p53-Abs-positive rate at recurrence was not higher than the positivity rate before surgery (Fig. 3a, b).
Positive rates of tumor markers before surgery and at recurrence.
The significant difference between P2 versus R2, P3 versus R3, and P5 versus R5 are indicated. The significant difference between P4 versus P2, P6 versus P3, P7 versus P5, and R4 versus R2 are indicated.
The combined presence of two of three markers showed similar tendencies. Combination of CEA with s-p53-Abs showed the highest positive rate at recurrence (69%) and before surgery (60%). However, there was no significant difference between these two. Combination of CEA and CA19-9 showed significantly higher positivity rates at recurrence (64%) than before surgery (46%, p < 0.01, Fig. 4). Combined with s-p53-Abs showed significant additive effects of perioperative positive rates as follows; CA19-9 vs CA19-9 + s-p53-Abs (p < 0.01), CEA vs CEA + s-p53-Abs (p < 0.01), and CA19-9 + CEA vs CA19-9 + CEA + s-p53-Abs (p < 0.01). Although combined with p53-Abs showed higher sensitivities at recurrence, the differences were not statistically significant.
Positive rates of tumor markers before surgery and at recurrence. White bars (P1 to P7) are preoperative positive rates. Black bars (R1 to R7) are positive rates at recurrence. P1P2P3 are positive rates using single markers. P4P5P6P7 are positive rates using plural markers. The significant difference between P2 versus R2, P3 versus R3, and P5 versus R5 are indicated. The significant difference between P4 versus P2, P6 versus P3, P7 versus P5, and R4 versus R2 are indicated. *p < 0.01, Fischer’s exact probability test
Changes in tumor marker status before surgery to recurrence
Frequency of negative to positive, negative to negative, positive to negative, and positive to positive status for each tumor marker is shown in Fig. 5. The prevalence of “negative to positive” was 1% for s-p53-Abs, 15% for CA19-9, and 20% for CEA. The prevalence of “positive to negative” was 6% for s-p53-Abs, 6% for CA19-9, and 16% for CEA. Therefore, the total prevalence of status changes was 7% for s-p53-Abs, 21% for Ca19-9, and 36% for CEA (Fig. 5).
Comparison of overall survivals after surgery of 4 subgroups according to the tumor marker changes at recurrence
Regarding to s-p53-Abs, there was no differences among the survivals of 4 subgroups (Fig. 6a). Regarding to CA 19-9, the patients with positive status at recurrence showed worse survival than the patients with negative status at recurrence although there was no significant difference (Fig. 6b). Regarding to CEA, the patients with positive status at recurrence showed significantly worse survival than the patients with negative status at recurrence (Fig. 6c).
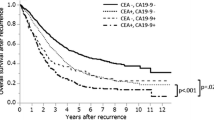
Comparison of overall survival curves after recurrence according to tumor marker status at recurrence
There was no significant difference in survival rate between the s-p53-Abs-positive and s-p53-Abs-negative groups (Fig. 7a). Conversely, the CA19-9-positive group had significantly lower survival rates than the CA19-9-negative group (Fig. 7b, p = 0.02). The CEA-positive group also had significantly lower survival rates than the CEA-negative group (Fig. 7c, p < 0.01).
Univariate and multivariate analyses of risk factors for overall survival after recurrence
Among various clinicopathological factors and tumor markers, CA19-9, CEA, and recurrent site resection were significant prognostic factors in the univariate analysis (Table 3). CEA and recurrent site resection were the only independent risk factors for overall survival in the multivariate analysis.
Discussion
Increased values of all three tumor markers were associated with tumor progression. Although CEA- and CA19-9-positive rates increased at disease recurrence, s-p53-Abs-positive rates did not. There were no significant differences in the recurrent and overall survival rates according to preoperative s-p53-Abs status. Conversely, there were significant differences in the recurrent and overall survival rates according to preoperative CA19-9 and CEA status. CEA-positive status at recurrence was an independent risk factor for poor overall survival after recurrence.
In patients with colorectal cancer, the presence of s-p53-Abs during the perioperative period and recurrence differed from patients with esophageal cancer. Only one out of 75 s-p53-Abs-negative colorectal cancer patients were s-p53-Abs-positive at recurrence. In patients with esophageal cancer, 10% of s-p53-Abs-negative patients were s-p53-Abs positive after surgery [16]. In the present study, the s-p53-Abs-positivity rate did not increase at recurrence in patients with colorectal cancer. This is similar to previous findings that the s-p53-Abs-positivity rate did not increase in stage IV colorectal cancer [7]. Therefore, tracking s-p53-Abs status following surgery may not be useful in s-p53-Abs-negative patients.
Anti-p53-Abs production may decrease in patients with liver metastasis. It is possible that this reflects immunologic suppression because of immune tolerance due to liver metastasis, although no clear evidence for such an effect has been published. Tang et al. reported a similar finding regarding preoperative s-p53-Abs-negative status in patients with colorectal cancer [17]. It has also been demonstrated that the autoantibody profiles in patients with colorectal cancer were consistent from early- to late-stage disease [18].
In contrast to s-p53-Abs, increases in CA19-9- and CEA-positivity rates were associated with tumor progression and recurrence. CEA showed high positive conversion rates at recurrence. However, the negative conversion rate of CEA was also relatively high. The duality of CEA expression should be carefully considered. The negative conversion rate of CA19-9 was relatively low. Therefore, CEA/CA19-9 should be tracked following surgery, even in patients that were double-negative for CEA/CA19-9 before surgery.
A limitation of this study is that p53 protein expression levels in tumors were not analyzed because pathology specimens of metastases were collected from only a few patients. Furthermore, there are no data concerning p53 mutations in the metastases.
In conclusion, the s-p53-Abs-positivity rate during disease recurrence was similar to CA19-9 and lower than CEA. Because s-p53-Abs-negative patients rarely converted to positive status at recurrence, s-p53-Abs should be postoperatively followed only in patients that were positive before surgery. Unlike s-p53-Abs-positive status, CEA- and/or CA19-9-positive status at recurrence was a poor prognostic indicator.
Abbreviations
- CEA:
-
Carcinoembryonic antigen
- CA19-9:
-
Carbohydrate antigen 19-9
- s-p53-Abs:
-
Serum p53 antibodies
References
McKeown E, Nelson DW, Johnson EK et al (2014) Current approaches and challenges for monitoring treatment response in colon and rectal cancer. J Cancer 5:31–43
Kahi CJ, Anderson JC, Rex DK (2013) Screening and surveillance for colorectal cancer: state of the art. Gastrointest Endosc 77:335–350
Tokunaga R, Sakamoto Y, Nakagawa S et al (2017) The utility of tumor marker combination, including serum p53 antibody, in colorectal cancer treatment. Surg Today 47:636–642
Kunizaki M, Sawai T, Takeshita H et al (2016) Clinical value of serum p53 antibody in the diagnosis and prognosis of colorectal cancer. Anticancer Res 36:4171–4175
Ochiai H, Ohishi T, Osumi K et al (2012) Reevaluation of serum p53 antibody as a tumor marker in colorectal cancer patients. Surg Today 42:164–168
Yamaguchi T, Takii Y, Maruyama S (2013) Usefulness of serum p53 antibody measurement in colorectal cancer: an examination of 1384 primary colorectal cancer patients. Surg Today 44:1529–1535
Suzuki T, Funahashi K, Ushigome M et al (2017) Diagnostic and prognostic impact of serum p53 anti body titration in colorectal cancer. Toho J Med 11:107–115
Suzuki T, Shimada H, Ushigome M et al (2016) Three-years monitoring of serum p53 antibody during chemotherapy and surgery for stage IV rectal cancer. Clin J Gastroenterol 9(55–58):16
Shibutani M, Maeda K, Nagahara H et al (2014) Hirakawa K Significance of CEA and CA19-9 combination as a prognostic indicator and for recurrence monitoring in patients with stage II colorectal cancer. Anticancer Res 34:3753–3758
Kawahara H, Watanabe K, Enomoto H et al (2013) Normalization of serum p53 antibody levels in patients after curative resection for colorectal cancer. Anticancer Res 33:2221–2225
Sobin LH, Gospodarowicz MK, Wittekind C (2010) TNM classification of malignant tumors, 7th edn. Wiley-Blackwell, Hoboken
Suzuki T, Yajima S, Ishioka N et al (2018) Prognostic significance of high serum p53 antibody titers in patients with esophageal squamous cell carcinoma. Esophagus 15:294–300
Shimada H, Ochiai T, Nomura F (2003) Titration of serum p53 antibodies in 1,085 patients with various types of malignant tumors: a multi-institutional analysis by the Japan p53 Antibody Research Group. Cancer 97:682–689
Ito M, Oshima Y, Yajima S et al (2019) Diagnostic impact of high serum midkine level in patients with gastric cancer. Ann Gastroenterol Surg. 3(2):195–201
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl 48:452–458
Shimada H, Shiratori T, Takeda A et al (2009) Perioperative changes of serum p53 antibody titer is a predictor for survival in patients with esophageal squamous cell carcinoma. World J Surg. 33:272–277
Tang R, Yeh CY, Wang JY et al (2009) Serum p53 antibody as tumor marker for follow-up of colorectal cancer after curative resection. Ann Surg Oncol 16:2516–2523
Ushigome M, Nabeya Y, Soda H et al (2018) Multi-panel assay of serum autoantibodies in colorectal cancer. Int J Clin Oncol 23:917–923
Acknowledgements
This work was partially supported by JSPS KAKENHI (grant number 26462029). The authors would like to thank MARUZEN-YUSHODO Co., Ltd. (https://kw.maruzen.co.jp/kousei-honyaku/) for the English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Ushigome, M., Shimada, H., Miura, Y. et al. Changing pattern of tumor markers in recurrent colorectal cancer patients before surgery to recurrence: serum p53 antibodies, CA19-9 and CEA. Int J Clin Oncol 25, 622–632 (2020). https://doi.org/10.1007/s10147-019-01597-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-019-01597-6