Abstract
Background
Malignancy is associated with an increased risk of venous thromboembolism. Inferior vena cava filters are a viable alternative when anticoagulation is infeasible because of the risk of bleeding. Although the current guidelines recommend that all patients with a vena cava filter be treated with anticoagulation treatment when the risk of bleeding is reduced, studies concerning the role of concomitant anticoagulation after vena cava filter insertion in high-risk patients are scarce. Since many cancer patients suffer from a high risk of hemorrhagic complications, we aimed to determine the effect of post-filter anticoagulation on mortality in patients with a malignant solid tumor.
Methods
A retrospective cohort study of patients with pulmonary embolism was performed between January 2010 and May 2016. Patients with a solid tumor and vena cava filter inserted because of pulmonary embolism were included. Using Cox proportional hazards model, the prognostic effect of clinical variables was analyzed.
Results
A total of 180 patients were analyzed, with 143 patients receiving and 37 patients not receiving post-filter anticoagulation treatment. Mortality was not significantly different between the two groups. The presence of metastatic cancer and that of pancreatobiliary cancer were significant risk factors for mortality. However, post-filter anticoagulation did not show significant effect on mortality regardless of the stage of cancer.
Conclusion
In patients with cancer-associated pulmonary embolism, the effect of post-filter anticoagulation on mortality may not be critical, especially in patients with a short life expectancy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is well known that malignancy is associated with a high risk of venous thromboembolism (VTE). Previous studies have reported a four–sixfold increased risk of VTE in patients with a cancer compared with those without [1, 2]. Active cancer accounts for almost 20% of new VTE events [3]. For the management of VTE, anticoagulation treatment is essential. In certain cases, however, anticoagulation treatment is often contraindicated or discontinued because of an underlying coagulopathy or hemorrhagic complication. Inferior vena cava (IVC) filters play an important role when complications occur after the initiation of anticoagulation or when anticoagulation is transiently infeasible, as in a case of operation. The use of IVC filters is rapidly increasing, especially with the advent of retrievable IVC filters [4, 5].
The PREPIC (Prévention du Risque d’Embolie Pulmonaire par Interruption Cave) study showed that vena cava filters were associated with an increased risk of deep vein thrombosis (DVT) [6]. Currently, the American College of Chest Physician (ACCP) guidelines recommend that all patients with an IVC filter be treated with anticoagulation treatment when the risk of bleeding is reduced [7]. When considering the hypercoagulable nature of malignancy [8], the additional increment of thromboembolic risk is easily anticipated for IVC filters in patients with cancer. However, such patients are also susceptible to bleeding from the cancer itself or from the adverse effect of chemotherapy [9]; thus, physicians often fall into a difficult situation regarding the decision of anticoagulation after IVC filter insertion.
Although IVC filters are being used more frequently with expanding indications [10], studies concerning the role of concomitant anticoagulation treatment after IVC filter insertion in high-risk patients are scarce [11]. Only a few studies have examined the survival rate depending on whether anticoagulation treatment was given [12,13,14]. Greenfield and Proctor reported improved survival in the anticoagulated group; however, it was attributed to the difference in the underlying diagnosis, with the no-anticoagulation group having more patients with cancer [12]. There has been no research specifically investigating the role of post-filter anticoagulation in patients with malignancy. Knowing whether concomitant anticoagulation is mandatory or not after IVC filter insertion in cancer patients would be valuable, as many of these patients experience hemorrhagic complications and the burden to the patients is not negligible [9]. Thus, we aimed to determine the effect of post-filter anticoagulation on mortality in this population.
Materials and methods
Study design and subjects
We performed a retrospective cohort study of patients with pulmonary embolism (PE) at Asan Medical Center, a 2700-bed tertiary care center in South Korea, between January 2010 and May 2016. Patients aged 18 years or older and with a consultation record for IVC filter insertion were screened for the study. Celect filters (Cook Medical, Bloomington, IN, USA) and Optease filters (Cordis, Milpitas, CA, USA) were used at our institution and all IVC filters used during the study period were retrievable filters that could be left permanently as well. For patients expected to have a short survival duration, filters were inserted for a permanent purpose. Other patients had their filters not retrieved for the following reasons: high risk of recurrence of PE, poor medical condition, or technical difficulties. The decision to retain a filter was made by each attending physician. Patients were included for the analysis if they had a diagnosis of a malignant solid tumor and their filters were not retrieved. Patients with hematologic malignancy were excluded because the concept of metastasis could not be applied.
The decision on whether to give anticoagulants after IVC filter insertion was at the discretion of each physician, considering the medical condition of each patient. The survival time was calculated from the day of IVC filter insertion to death. Patients were followed up for their survival status until December 2016. We determined whether anticoagulation therapy was given after IVC filter insertion and assessed the effect of anticoagulation therapy on mortality. The effect was further analyzed according to cancer stage, survival duration, and type of anticoagulant.
To quantitate the medical comorbidities into a score, we used a modified version of the Charlson Comorbidity Index (CCI). The original CCI is calculated by adding the comorbidity score to the age score [15]. The comorbidity score comprises various medical conditions including cardiac, cerebrovascular, and respiratory disorders, and includes malignancies such as leukemia and solid tumors. Because we intended to use age and malignancy as separate variables in the statistical analysis, we calculated the modified CCI by omitting age and malignancy. Metastatic cancer was defined as stage 4 according to the TNM staging system. Chronic lung disease included asthma, chronic bronchitis, emphysema, and other chronic lung disease causing ongoing symptoms such as dyspnea or cough. The study was approved by the institutional review board (IRB) of Asan Medical Center (IRB no. 2015-0516). The need for informed consent was waived owing to the retrospective nature of the study.
Data collection
The electronic medical records of the study patients were reviewed for age, sex, types and stage of the malignancy, date of IVC filter insertion, medical comorbidities, presence of right ventricular dysfunction, and severity of PE. The severity of PE was calculated using the simplified Pulmonary Embolism Severity Index [16]. The diagnosis of PE was made by each attending physician on the basis of the imaging findings from pulmonary computed tomography (CT) angiography or ventilation/perfusion scan. All images were initially read by radiologists at our institution, and each CT image was checked once more by the authors. The survival status of the patients was judged using data from the electronic medical records or the Korean National Health Insurance.
Statistical analysis
Descriptive statistics are provided for patient demographics. Categorical variables are presented as numbers (%). Age is presented as means and standard deviations, whereas survival time is presented as medians and ranges. The clinical characteristics of the patients were analyzed using the Mann–Whitney U test, χ2 test, and Fisher’s exact test. After assessing the demographic and clinical variables through univariate analysis, statistically significant variables were chosen for multivariate analysis to evaluate their effects on mortality. Further analysis was carried out using a landmark analysis to assess if there was difference in mortality depending on anticoagulation when censored at 90 days. p values < 0.05 were considered statistically significant. Data were analyzed using SPSS version 21 (IBM Corporation, Armonk, NY, USA) analytical software.
Results
Clinical characteristics of patients
Among the 437 patients initially screened, 184 patients were identified as having a diagnosis of active cancer and an IVC filter. Four patients with hematologic malignancy were excluded, leaving 180 patients with solid malignancy for inclusion in the analyses.
A total of 143 patients received post-filter anticoagulation therapy. The remaining 37 patients were not given anticoagulants. Coagulopathy and risk of bleeding were the reasons for no anticoagulation in these 37 patients. The clinical characteristics of the study population are shown in Table 1. The mean age of the anticoagulation group and the no-anticoagulation group was 60.7 and 59.5 years, respectively. Gynecologic cancer was the most common malignancy in the anticoagulation group, followed by pancreatobiliary cancer. The no-anticoagulation group had an almost even distribution of the types of solid tumor. Metastatic cancer accounted for 69.2 and 83.8% in the anticoagulation and no-anticoagulation group, respectively. No significant difference in terms of medical comorbidities was found between the two groups (p = 0.824). Among patients who received anticoagulation treatment, warfarin was most commonly used (53.8%), followed by low-molecular weight heparin (LMWH) (27.3%). About 16.1% of patients were treated with direct oral anticoagulants. Overall, there was no significant difference between anticoagulation and no-anticoagulation group.
During the follow-up period, 114 (79.7%) and 34 (91.9%) patients died in the anticoagulation and no-anticoagulation group, respectively, showing no statistical difference in mortality (p = 0.084). The median survival time was 85 and 78 days in each group. The longest survival time was 2411 days in the anticoagulation group, whereas it was 892 days in the no-anticoagulation group. The most common cause of death was cancer progression in both anticoagulation and no-anticoagulation group, accounting for 63 (55.3%) and 17 (50.0%) patients, respectively. PE-related death was found in 12 (10.5%) and 4 (11.8%) patients in anticoagulation and no-anticoagulation group, respectively. Death caused by bleeding was found in two (1.4%) in anticoagulation group, whereas none in no-anticoagulation group. In each group, 29 (25.4%) and 10 (29.4%) patients were lost to follow-up, and their cause of death could not be identified.
Indications for filter insertion
We categorized the common IVC filter indications into six, and Table 2 shows the frequency of indications in each group. The anticoagulation group and the no-anticoagulation group had different indications for IVC filter insertion. In comparison with the anticoagulation group, in which extensive iliofemoral DVT was the most common cause followed by preoperative/periprocedural insertion, contraindication to and complication of anticoagulation were the frequent causes in the no-anticoagulation group.
Risk factors for mortality
Using Cox proportional hazards model, the prognostic effect of clinical variables was evaluated. The presence of chronic lung disease, metastatic cancer, and a diagnosis of pancreatobiliary cancer were found as statistically significant risk factors for mortality in the univariate analysis (data not shown). In the multivariate analysis, the presence of metastatic cancer and pancreatobiliary cancer remained predictive of mortality, whereas anticoagulation did not (Table 3). We further analyzed the effect of anticoagulation by separately calculating the hazard ratio (HR) according to the stage of cancer (Table 4). In both nonmetastatic and metastatic cancer, anticoagulation treatment was not associated with improved survival (p = 0.567 and 0.799, respectively).
Landmark analysis
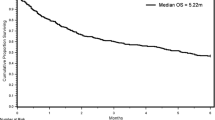
As shown in Fig. 1, there was no statistical significance in survival between the anticoagulation and no-anticoagulation group (p = 0.061), especially for the first few months. We performed a landmark analysis to evaluate if there was a difference in the effect of post-filter anticoagulation between the patients with a short survival (less than 90 days) and a longer survival, and the landmark point was set at 90 days which was close to the median survival time of the anticoagulation group.
When censored at 90 days, anticoagulation was not protective in terms of mortality (adjusted HR 0.894; 95% confidence interval, 0.525–1.523) as shown in Table 5. In patients who survived more than 90 days, there was a trend for reduced mortality with anticoagulation treatment in the univariate analysis (HR 0.602; p = 0.076), but no statistical significance was found in the multivariate analysis.
Effect on mortality depending on the type of anticoagulant
Warfarin was found most commonly used for the patients in this study, accounting for more than 50% (Table 1). The recommended type of anticoagulant in cancer-associated PE, LMWH, was used in only 27.3% of patients. The reasons for the frequent use of warfarin were renal insufficiency, difficulty with subcutaneous injection, and others. To adjust a suboptimal effect of warfarin in cancer-associated PE, we performed further analysis by separating warfarin users from LMWH users. They were compared with the no-anticoagulation group. More patients with pancreatobiliary cancer and metastatic cancer were found in the LMWH group (online supplementary Table E1 and E2). When censored at 90 days, neither the warfarin nor LMWH group showed benefit in terms of mortality as shown in online supplementary Table E3 (adjusted HR 0.663; p = 0.190 and adjusted HR 1.070; p = 0.841, respectively).
Discussion
This is the first study to evaluate the role of post-filter anticoagulation in patients with malignant solid tumors. During the study period, 148 died among the 180 study patients. The presence of metastatic cancer and that of pancreatobiliary cancer were significant predictors of mortality, whereas anticoagulation was not. Importantly, anticoagulation treatment was not associated with a reduced mortality especially in patients with a short survival, regardless of stage and the type of anticoagulant. In addition, two patients died of bleeding in anticoagulation group, whereas none in no-anticoagulation group.
Anticoagulation after IVC filter insertion has several theoretical advantages: it limits the propagation of DVT, prevents recurrent PE, and decreases the risk of caval thrombosis [17]. Likewise, Cugell stated that anticoagulants should always be used in conjunction with an IVC filter to minimize the likelihood of caval thrombosis [18]. However, Ray et al. pointed out the paucity of studies specifically addressing the need for anticoagulation after IVC filter insertion in their meta-analysis [11]. Research so far has shown only limited results about the effect of post-filter anticoagulation on mortality. Chow et al. found a favorable effect on mortality of post-filter anticoagulation; however, the outcome was attributed to a confounding effect of patient selection for anticoagulation [17]. In their small retrospective study, Dovrish et al. showed improved survival when long-term anticoagulation was given after IVC filter insertion; but whether anticoagulation was still beneficial in high-risk patients was not determined [13]. Recently, Falatko et al. reported impact of anticoagulation after IVC filter insertion may be attenuated in elderly patients [19]. They pointed out the importance of identifying the role of post-filter anticoagulation in specific high-risk populations. We attempted to determine the effect of anticoagulation treatment after vena cava filter insertion in patients with solid tumors, who are susceptible to bleeding complications.
In this study, pancreatobiliary cancer and the presence of metastasis were important factors for mortality, which makes sense because they are well known predictors for far poorer prognosis. Anticoagulation treatment, however, was not associated with a survival benefit. Although we were not able to detect a statistical significance, the Kaplan–Meier curve showed a trend for a favorable outcome of anticoagulation in long-term survivors (Fig. 1); thus, we performed further analysis with a 90-day survival as a landmark point. In patients who survived > 90 days, we observed a trend for a protective effect of anticoagulation consistent with the previous reports [13, 17], although we were not able to prove the statistical significance due to the small number. In contrast, anticoagulation was not protective in patients with a short life expectancy (< 90 days), suggesting post-filter anticoagulation may not be critical in these patients.
The ACCP guideline and the European Society of Medical Oncology guideline recommend that anticoagulation treatment should be given once the risk of bleeding is reduced in the presence of an IVC filter [7, 20]. However, even in cases in which bleeding discontinue, there is always a risk of rebleeding. Physicians often face difficulties in weighing the risks and benefits of anticoagulation: anticoagulation treatment prevents recurrent VTE but the consequence can sometimes be catastrophic (e.g., cerebral hemorrhage). In our study, two patients died of bleeding while receiving anticoagulation treatment for PE: one from gastrointestinal bleeding and the other from cerebral hemorrhage. In this context, the National Comprehensive Cancer Network guideline states that there are factors to consider before implementing anticoagulation treatment, including the lack of a therapeutic advantage such as limited survival, high risk of anticoagulation therapy, and no planned oncologic intervention [21]. This recommendation is based on common sense, however. The result of our study which showed that anticoagulation did not convey an advantage in terms of reducing mortality in patients with a short life expectancy (< 90 days), can add evidence to such a recommendation.
Our study has several limitations. First, owing to the retrospective nature of the study, there may be biases. Second, we were not able to specify the cause of death in about a quarter of the patients. Some of the patients were lost to follow-up, and only the survival status was obtained from the National Health Insurance database. However, in a previous report involving 9000 patients with malignancy, the cause of death also could not be determined in > 80%. This report demonstrated that among patients in whom the cause of death was determined, about 50% died of disease progression and about 14% died of VTE [22], and the result was similar in our study. Finally, more than a half of the patients in this study were treated with warfarin instead of LMWH, which is the first choice in patients with malignancy, unless otherwise contraindicated. However, when we analyzed the hazard ratios for the mortality in warfarin users and LMWH users, LMWH was not associated with improved survival. Regardless of the type of anticoagulant, statistically significant difference was not found in patients with a short life expectancy (< 90 days).
Despite the limitations, this study has a value in that it is the first study to investigate the effect of post-filter anticoagulation on mortality in patients with solid tumors. No study has evaluated this issue in high-risk patients. Our finding suggests that concomitant anticoagulation after IVC filter insertion in patients with cancer-associated PE may not be required, especially for those with a short life expectancy. Cancer patients are vulnerable to hemorrhagic complications by the tumor itself, or the adverse effects of chemotherapy such as thrombocytopenia. Moreover, given the fact that the currently recommended anticoagulation treatment accompanies the discomfort of subcutaneous injection as well as economic burden, this finding is especially beneficial for patients with a short life expectancy. For patients with a longer life expectancy (> 90 days), we found a tendency of reduced mortality; however, further studies are needed to determine the role of post-filter anticoagulation in those patients.
Conclusion
In patients with cancer-associated pulmonary embolism, the effect of post-filter anticoagulation may not be critical, especially in patients with a short life expectancy.
References
Blom JW, Doggen CJ, Osanto S, Rosendaal FR (2005) Malignancies, prothrombotic mutations, and the risk of venous thrombosis. Jama 293(6):715–722. https://doi.org/10.1001/jama.293.6.715
Cronin-Fenton DP, Sondergaard F, Pedersen LA et al (2010) Hospitalisation for venous thromboembolism in cancer patients and the general population: a population-based cohort study in Denmark, 1997–2006. Br J Cancer 103(7):947–953. https://doi.org/10.1038/sj.bjc.6605883
Heit JA, O’Fallon WM, Petterson TM et al (2002) Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med 162(11):1245–1248
Stein PD, Kayali F, Olson RE (2004) Twenty-one-year trends in the use of inferior vena cava filters. Arch Intern Med 164(14):1541–1545. https://doi.org/10.1001/archinte.164.14.1541
Kaufman JA, Kinney TB, Streiff MB et al (2006) Guidelines for the use of retrievable and convertible vena cava filters: report from the Society of Interventional Radiology multidisciplinary consensus conference. J Vasc Interv Radiol 17(3):449–459. https://doi.org/10.1097/01.rvi.0000203418.39769.0d
Group PS (2005) Eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC (Prevention du Risque d’Embolie Pulmonaire par Interruption Cave) randomized study. Circulation 112(3):416–422. https://doi.org/10.1161/CIRCULATIONAHA.104.512834
Kearon C, Akl EA, American College of Chest P et al (2012) Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th edn: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141(2 Suppl):e419S-494S. https://doi.org/10.1378/chest.11-2301
Caine GJ, Stonelake PS, Lip GY, Kehoe ST (2002) The hypercoagulable state of malignancy: pathogenesis and current debate. Neoplasia (New York NY) 4(6):465–473. https://doi.org/10.1038/sj.neo.7900263
Kamphuisen PW, Beyer-Westendorf J (2014) Bleeding complications during anticoagulant treatment in patients with cancer. Thromb Res 133(Suppl 2):S49-55. https://doi.org/10.1016/s0049-3848(14)50009-6
Ray CE, Mitchell E, Zipser S et al (2006) Outcomes with retrievable inferior vena cava filters: a multicenter study. J Vasc Interv Radiol 17(10):1595–1604. https://doi.org/10.1097/01.rvi.0000239102.02956.65
Ray CE, Prochazka A (2008) The need for anticoagulation following inferior vena cava filter placement: systematic review. Cardiovasc Intervent Radiol 31(2):316–324. https://doi.org/10.1007/s00270-007-9244-x
Greenfield LJ, Proctor MC (2001) Recurrent thromboembolism in patients with vena cava filters. J Vasc Surg 33(3):510–514. https://doi.org/10.1067/mva.2001.111733
Dovrish Z, Hadary R, Blickstein D, Shilo L, Ellis MH (2006) Retrospective analysis of the use of inferior vena cava filters in routine hospital practice. Postgrad Med J 82(964):150–153. https://doi.org/10.1136/pgmj.2005.037911
Yale SH, Mazza JJ, Glurich I et al (2006) Recurrent venous thromboembolism in patients with and without anticoagulation after inferior vena caval filter placement. Int Angiol 25(1):60–66
Charlson ME, Sax FL, MacKenzie CR et al (1987) Morbidity during hospitalization: can we predict it? J Chronic Dis 40(7):705–712
Jimenez D, Aujesky D, Moores L et al (2010) Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 170(15):1383–1389. https://doi.org/10.1001/archinternmed.2010.199
Chow FC, Chan YC, Cheung GC et al (2015) Mid- and long-term outcome of patients with permanent inferior vena cava filters: a single center review. Ann Vasc Surg 29(5):985–994. https://doi.org/10.1016/j.avsg.2015.01.009
Cugell DW (1993) Vena caval filters and anticoagulants for pulmonary emboli. Jama 270(15):1867–1868
Falatko JM, Dalal B, Qu L (2017) Impact of anticoagulation in elderly patients with pulmonary embolism that undergo IVC filter placement: A Retrospective Cohort Study. Heart Lung Circ. https://doi.org/10.1016/j.hlc.2017.01.011
Mandala M, Falanga A, Roila F et al (2011) Management of venous thromboembolism (VTE) in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol 6(22):vi85-92. https://doi.org/10.1093/annonc/mdr392
Streiff MB, Bockenstedt PL, Cataland SR et al. (2013) Venous thromboembolic disease. JNCCN 11(11):1402–1429
Kroll MH, Pemmaraju N, Oo TH et al (2014) Mortality from cancer-associated venous thromboembolism. Blood 124(21):4829–4829
Acknowledgements
This study was supported by University of Ulsan College of Medicine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Kang, J., Kim, S.O., Oh, YM. et al. Effect of post-filter anticoagulation on mortality in patients with cancer-associated pulmonary embolism. Int J Clin Oncol 23, 1007–1013 (2018). https://doi.org/10.1007/s10147-018-1290-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-018-1290-7