Abstract
Background
The aim of the present study was to evaluate the prognostic significance of the Glasgow Prognostic Score (GPS) in metastatic renal cell carcinoma (mRCC) patients treated by cytoreductive nephrectomy (CN), and the accuracy of the GPS as a prognostic factor.
Methods
We retrospectively analyzed the data of patients who underwent CN for mRCC between March 1984 and August 2015. In accordance with the GPS criteria, the patients were classified into three groups: GPS 0: C-reactive protein (CRP) ≤ 1.0 mg/dl and albumin ≥ 3.5 g/dl; GPS 1: CRP > 1.0 mg/dl or albumin < 3.5 g/dl; and GPS 2: CRP > 1.0 mg/dl and albumin < 3.5 g/dl.
Results
We enrolled 170 patients (72% male; median age 63.5 years). Fifty-six (33%), 67 (39%), and 47 (28%) patients had a GPS of 0, 1, and 2, respectively. The median overall survivals after CN were 52.4, 19.1, and 8.9 months for patients with a GPS of 0, 1, and 2, respectively (P < 0.0001). In addition to the GPS, Eastern Cooperative Oncology Group performance status (ECOG-PS), Memorial Sloan-Kettering Cancer Center (MSKCC) risk classification, histology, sarcomatoid change, clinical T stage, primary tumor size, number of metastatic organs, non-regional lymph node metastasis, and liver metastasis were included in the Cox hazards regression model. Multivariate analysis of these factors revealed that the GPS was an independent prognostic factor of overall survival (P < 0.0001). Harrell’s concordance index in the multivariate prognostic model based on ECOG-PS, MSKCC risk criteria, histology, sarcomatoid change, clinical T stage, primary tumor size, number of metastatic organs, non-regional lymph node metastasis, and liver metastasis was 0.609, which increased to 0.652 after the inclusion of the GPS.
Conclusions
GPS represents an independent prognostic factor for patients who undergo CN for mRCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cytoreductive nephrectomy (CN) has been shown to result in survival benefits for patients with metastatic renal cell carcinoma (mRCC), as evaluated by two randomized trials and a combined analysis in the cytokine era [1,2,3]. In addition, some retrospective studies have shown similar survival benefits in the molecular-targeted drug era [4,5,6,7], although conflicting opinions exist [8].
These controversial results are currently being evaluated by two ongoing randomized trials (CARMENE and SURTIME). However, it is clear that not all patients with mRCC can receive survival benefits from CN. Thus, the indication for CN should be well-considered to avoid unnecessary invasive surgery. To optimize the benefits of CN, prognostic factors after CN, such as a high serum lactate dehydrogenase level [9,10,11], low albumin level [9, 11], symptoms of metastasis at presentation [9], elevated corrected calcium level [10], low performance status [10], and poor risk according to the Memorial Sloan-Kettering Cancer Center (MSKCC) risk classification [12, 13] have been previously reported.
The Glasgow Prognostic Score (GPS) is a selective combination of the serum levels of C-reactive protein (CRP) and albumin, and is a simpler scoring system than most other prognostic models. This score has been shown to be an independent prognostic factor in a variety of cancers [14,15,16]. In the field of RCC, the GPS has been shown to be an independent prognostic factor in mRCC patients treated with cytokine therapy and in localized RCC patients undergoing potentially curative tumor resection [16, 17]. However, to the best of our knowledge, whether the GPS is also a prognostic factor for mRCC patients treated by CN has not been investigated.
With this in mind, in the present study we evaluated the prognostic significance of the GPS in patients with mRCC treated by CN, with the aim of optimizing the patient selection for CN.
Materials and methods
Patients
After approval by our institutional review board, the medical records of patients treated at our hospital, Tokyo Women’s Medical University, were retrospectively reviewed and 170 patients with mRCC treated by CN between March 1984 and August 2015 were identified. The tumor stage was determined according to the 2009 TNM classification [18]. The pathological diagnoses were made according to the 2016 World Health Organization classification [19]. Stratification of prognostic risk was performed according to the MSKCC risk classification [20].
Measurements and definitions
Clinical, laboratory, and survival data were collected by reviewing the electronic medical records of the patients. Pathological data were obtained from nephrectomy specimens. All surgical specimens were processed according to standard pathological procedures, and all specimens were histologically confirmed to be RCC by an authorized pathologist (YN).
The GPS was calculated as previously described [14]. Briefly, patients with an elevated CRP concentration (>1.0 mg/dl) and a decreased albumin concentration (<3.5 g/dl) were assigned a score of 2. Patients with an elevated CRP concentration (>1.0 mg/dl) or a decreased albumin concentration (<3.5 g/dl) were assigned a score of 1, while patients with a CRP concentration of ≤1.0 mg/dl and an albumin concentration of ≥3.5 g/dl were assigned a score of 0 (Table 1). The serum CRP and albumin levels were routinely measured before surgery. The targeted molecular therapy (TMT) era was defined as the period from March 2008, when sorafenib was first introduced in Japan.
Statistical analysis
The clinicopathological variables were compared between the different GPS groups using the χ2 test or analysis of variance, as appropriate. Overall survival (OS) curves were estimated using the Kaplan–Meier method and compared using the log-rank test. Survival analysis was performed using Cox proportional hazards models. Their predictive accuracy was evaluated using Harrell’s concordance index (c-index) [21]. A difference was considered significant at P < 0.05. All statistical analyses were performed using JMP 11.0.0 (SAS Institute, Cary, NC, USA) and SAS v.9.4 (SAS Institute).
Results
Patient characteristics
Table 2 shows the characteristics of the 170 mRCC patients treated with CN. Because all patients had synchronous metastasis at the time of diagnosis of RCC, there were no patients defined as having favorable risk in the MSKCC risk classification. A total of 119 (70%) and 51 (30%) patients were classified as being at intermediate and poor risk, respectively. According to the GPS criteria, 56 (33%), 67 (39%), and 47 (28%) patients were categorized as GPS 0, GPS 1, and GPS 2, respectively. In addition, the treatments used for the metastases existing at the time of CN are described in Table 3.
Association between the GPS and survival
During the follow-up period, 108 patients (62%) died of various causes, including 99 patients (57%) due to RCC. Because the Kaplan–Meier curves for OS were stable after 100 months of follow-up (data not shown), Fig. 1 shows the survival data until 100 months after CN. As a result, a significant difference in the OS rates between patients with GPS 0 (median 52.4 months), GPS 1 (median 19.1 months), and GPS 2 (median 8.9 months) was observed (P < 0.0001).
Differences in clinicopathological features between patients with GPS 0, GPS1, and GPS 2
Table 4 shows the differences in clinicopathological features between the patients with GPS 0, GPS 1, and GPS 2. The MSKCC risk (P = 0.0006), treatment for metastasis existing at the time of CN (P = 0.02), sarcomatoid change (P = 0.0003), primary tumor size (P < 0.0001), and lung metastasis (P = 0.028) significantly differed between the groups. Furthermore, the association between the GPS and another systemic inflammation marker, the neutrophil-to-lymphocyte ratio (NLR) [22], was investigated. We evaluated 152 patients who had complete data of both the GPS and NLR. As a result, the GPS was significantly associated with the NLR (P < 0.0001).
Relationships between clinicopathological factors and OS in mRCC patients treated with CN
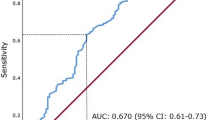
Univariate analysis showed that Eastern Cooperative Oncology Group performance status (ECOG-PS) (P = 0.0006), MSKCC risk (P < 0.0001), histology (P = 0.0077), sarcomatoid change (P = 0.0008), clinical T stage (P = 0.045), primary tumor size (P = 0.0091), number of metastatic organs (P = 0.012), non-regional lymph node metastasis (P = 0.048), liver metastasis (P = 0.0004), and the GPS (P < 0.0001) were significantly associated with OS (Table 5). Multivariate analysis was performed using the factors significantly associated with OS in the univariate analysis, and revealed that the GPS was an independent prognostic factor for OS (P < 0.0001) (Table 6). Furthermore, the c-index was calculated to evaluate the predictive accuracy of the GPS. The c-index of the multivariate prognostic model based on the factors significantly associated with OS in the univariate analysis without GPS (ECOG-PS, MSKCC risk, histology, sarcomatoid change, clinical T stage, primary tumor size, number of metastatic organs, non-regional lymph node metastasis, and liver metastasis) was 0.609, and this value was further enhanced by the inclusion of the GPS (c-index 0.652), as compared to inclusion of CRP alone (c-index 0.631) and albumin alone (c-index 0.649).
Prognostic impact of the GPS on mRCC patients treated without CN
It is not clear whether the GPS has prognostic value in mRCC patients treated without CN. Thus, we also assessed the prognostic value of the GPS in mRCC patients treated without CN. Twenty-seven mRCC patients treated without CN were analyzed for OS according to the GPS using Kaplan–Meier curves. As a result, higher GPS tended to be associated with a poor survival rate (median OS: GPS 0: 18 months; GPS 1: 10 months; GPS 2: 6 months), although the difference was not statistically significant (Fig. 2).
Impact of CN on mRCC patients according to the GPS
Finally, we assessed the impact of CN on mRCC patients according to the GPS using Kaplan–Meier curves. Treatment by CN was associated with significantly better survival compared to treatment without CN in mRCC patients classified as GPS 0 and 1, whereas no association was seen in patients classified as GPS 2 (Fig. 3a–c).
Discussion
The present study showed that the GPS was an independent prognostic factor for OS in mRCC patients treated by CN, as determined using multivariate analysis, and that the addition of the GPS to the 9 prognostic factors (ECOG-PS, MSKCC risk, histology, sarcomatoid change, clinical T stage, primary tumor size, number of metastatic organs, non-regional lymph node metastasis, and liver metastasis) improved the predictive accuracy for OS (c-index 0.609 vs. 0.652).
Prognostic factors for mRCC have been investigated in clinical trials and retrospective multivariate analyses. Motzer et al. presented a prognostic model including low Karnofsky performance status, high lactate dehydrogenase, low serum hemoglobin, high corrected serum calcium, and time from initial RCC diagnosis to start of interferon therapy of less than 1 year, using data from the cytokine era [20]. This prognostic model was subsequently validated by Mekhali et al. [23]. In addition, Heng et al. presented the International Metastatic Renal-Cell Carcinoma Database Consortium model, which includes anemia, thrombocytosis, neutrophilia, hypercalcemia, Karnofsky performance status < 80%, and <1 year from diagnosis to treatment, in patients with mRCC treated with first-line vascular endothelial growth factor-targeted treatment [24]. Moreover, CRP and CRP kinetics have been reported as prognostic factors for survival in mRCC patients [25, 26]. However, the prognostic factors for mRCC patients treated by CN have not been sufficiently investigated.
The GPS is calculated based on a combination of the serum CRP and albumin levels and has been shown to be associated with the chronic inflammatory response [14]. This score has been validated as a prognostic marker in a variety of cancers [15]. RCC may induce a systemic inflammatory response, since it has been confirmed that several renal tumors can produce interleukin-6, a pro-inflammatory cytokine [27, 28], resulting in the production of CRP in the liver [29]. Moreover, albumin concentrations can reflect both systemic inflammation and the amount of lean tissue in patients with cancer [15, 30]. Thus, the GPS, a combination of CRP and albumin, has predictive potential in mRCC patients. Lamb et al. prospectively investigated the prognostic value of a modified GPS in 169 patients undergoing curative nephrectomy for clear cell cancer. They concluded that the modified GPS was at least equivalent to, and independent of, other current validated scoring systems [17].
However, as mentioned above, the prognostic value of the GPS has not been examined in mRCC patients undergoing CN thus far. Thus, the present study evaluated the prognostic significance of the GPS in mRCC patients treated by CN and the accuracy of GPS as a prognostic factor. In previous studies, other systemic inflammatory prognostic factors have been examined. For example, the NLR has been reported to be a useful prognostic factor for mRCC patients in some studies [31, 32], and it was confirmed that the GPS was associated with the NLR in the present study (Table 4). Gu et al. indicated that the systemic inflammation response index, based on the pretreatment hemoglobin level and lymphocyte-to-monocyte ratio, was an independent prognostic predictor and was significantly correlated with the tumor behavior in mRCC patients treated by CN [33]. Moreover, Sakai et al. showed that the preoperative CRP level was independently associated with OS in mRCC patients who underwent CN and subsequently received immunotherapy and/or molecular-targeted therapy [34]. Similar to the previous studies for other systemic inflammatory prognostic factors, the prognostic value of the GPS was indicated in the present study using multivariate analysis and the c-index. The results of the present study suggest that the GPS is a potential biomarker and a potential candidate for constructing a nomogram for predicting outcome in mRCC patients treated by CN.
Although it has not been determined which mRCC patients can benefit from CN, one retrospective study aimed to determine the OS benefit of CN compared with no CN in mRCC patients treated with targeted therapies using data from 1658 mRCC patients from the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) [5]. This study indicated that mRCC patients with four or more of the IMDC prognostic criteria did not benefit from CN. In the present study, a GPS of 2 (median OS 8.9 months) was associated with poor OS in mRCC patients treated with CN, as compared with a GPS of 0 or 1 (median OS 49.5 and 19.1 months, respectively). In addition, mRCC patients with GPS 0 and 1 benefitted from CN, whereas those with GPS 2 did not in our study (Fig. 3a–c). These results suggest that the GPS may also be useful for careful patient selection for CN.
There are some limitations in the present study, including its retrospective and single-center study design. In addition, we were not able to completely adjust for all potential confounding factors, owing to unknown or uncollected factors. Nevertheless, the present study indicates that the GPS may represent a useful prognostic factor for OS in mRCC patients treated by CN. Future large-scale, prospective, multi-center studies are warranted to confirm our findings.
References
Flanigan RC, Salmon SE, Blumenstein BA et al (2001) Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med 345(23):1655–1659
Mickisch GHJ, Garin A, van Poppel H et al (2001) Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet. https://doi.org/10.1016/s0140-6736(01)06103-7
Flanigan RC, Mickisch G, Sylvester R et al (2004) Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol. https://doi.org/10.1097/01.ju.0000110610.61545.ae
Choueiri TK, Xie W, Kollmannsberger C et al (2011) The impact of cytoreductive nephrectomy on survival of patients with metastatic renal cell carcinoma receiving vascular endothelial growth factor targeted therapy. J Urol. https://doi.org/10.1016/j.juro.2010.09.012
Heng DY, Wells JC, Rini BI et al (2014) Cytoreductive nephrectomy in patients with synchronous metastases from renal cell carcinoma: results from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur Urol. https://doi.org/10.1016/j.eururo.2014.05.034
Mathieu R, Pignot G, Ingles A et al (2015) Nephrectomy improves overall survival in patients with metastatic renal cell carcinoma in cases of favorable MSKCC or ECOG prognostic features. Urol Oncol. https://doi.org/10.1016/j.urolonc.2015.05.014
Petrelli F, Coinu A, Vavassori I et al (2016) Cytoreductive nephrectomy in metastatic renal cell carcinoma treated with targeted therapies: a systematic review with a meta-analysis. Clin Genitourin Cancer. https://doi.org/10.1016/j.clgc.2016.04.001
You D, Jeong IG, Ahn JH et al (2011) The value of cytoreductive nephrectomy for metastatic renal cell carcinoma in the era of targeted therapy. J Urol. https://doi.org/10.1016/j.juro.2010.09.018
Culp SH, Tannir NM, Abel EJ et al (2010) Can we better select patients with metastatic renal cell carcinoma for cytoreductive nephrectomy? Cancer. https://doi.org/10.1002/cncr.25046
Richey SL, Culp SH, Jonasch E et al (2011) Outcome of patients with metastatic renal cell carcinoma treated with targeted therapy without cytoreductive nephrectomy. Ann Oncol Off J Eur Soc Med Oncol ESMO. https://doi.org/10.1093/annonc/mdq563
Margulis V, Shariat SF, Rapoport Y et al (2013) Development of accurate models for individualized prediction of survival after cytoreductive nephrectomy for metastatic renal cell carcinoma. Eur Urol. https://doi.org/10.1016/j.eururo.2012.11.040
Powles T, Blank C, Chowdhury S et al (2011) The outcome of patients treated with sunitinib prior to planned nephrectomy in metastatic clear cell renal cancer. Eur Urol. https://doi.org/10.1016/j.eururo.2011.05.028
Debra J, Thomas EH, Charles LC et al (2011) Efficacy and toxicity of sunitinib in patients with metastatic renal cell carcinoma with severe renal impairment or on heamodialysis. BJU Int 108(8):1279–1283
McMillan DC (2008) An inflammation-based prognostic score and its role in the nutrition-based management of patients with cancer. Proc Nutr Soc. https://doi.org/10.1017/s0029665108007131
McMillan DC (2013) The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev. https://doi.org/10.1016/j.ctrv.2012.08.003
Ramsey S, Lamb GW, Aitchison M et al (2007) Evaluation of an inflammation-based prognostic score in patients with metastatic renal cancer. Cancer. https://doi.org/10.1002/cncr.22400
Lamb GW, Aitchison M, Ramsey S et al (2012) Clinical utility of the Glasgow Prognostic Score in patients undergoing curative nephrectomy for renal clear cell cancer: basis of new prognostic scoring systems. Br J Cancer. https://doi.org/10.1038/bjc.2011.556
Sobin LH, Gospodarowicz M, Wittekind CH (2009) Kidney (ICD-O C64). TNM classification of malignant tumors, 7th edn. Wiley-Liss, New York, pp 255–257
Moch H, Cubilla AL, Humphrey PA et al (2016) The 2016 WHO classification of tumours of the urinary system and male genital organs-part a: renal, penile, and testicular tumours. Eur Urol. https://doi.org/10.1016/j.eururo.2016.02.029
Motzer RJ, Bacik J, Murphy BA et al (2002) Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol Off J Am Soc Clin Oncol. https://doi.org/10.1200/jco.2002.20.1.289
Harrell FE Jr, Califf RM, Pryor DB et al (1982) Evaluating the yield of medical tests. JAMA 247(18):2543–2546
Ohno Y, Nakashima J, Ohori M et al (2014) Clinical variables for predicting metastatic renal cell carcinoma patients who might not benefit from cytoreductive nephrectomy: neutrophil-to-lymphocyte ratio and performance status. Int J Clin Oncol. https://doi.org/10.1007/s10147-012-0514-5
Mekhail TM, Abou-Jawde RM, Boumerhi G et al (2005) Validation and extension of the Memorial Sloan-Kettering prognostic factors model for survival in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol Off J Am Soc Clin Oncol. https://doi.org/10.1200/JCO.2005.05.179
Heng DY, Xie W, Regan MM et al (2013) External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol. https://doi.org/10.1016/S1470-2045(12)70559-4
Saito K, Tatokoro M, Fujii Y et al (2009) Impact of C-reactive protein kinetics on survival of patients with metastatic renal cell carcinoma. Eur Urol. https://doi.org/10.1016/j.eururo.2008.10.012
Ito H, Shioi K, Murakami T et al (2012) C-reactive protein in patients with advanced metastatic renal cell carcinoma: usefulness in identifying patients most likely to benefit from initial nephrectomy. BMC Cancer. https://doi.org/10.1186/1471-2407-12-337
Koo AS, Armstrong C, Bochner B et al (1992) Interleukin-6 and renal cell cancer: production, regulation, and growth effects. Cancer Immunol Immunother CII 35(2):97–105
Miki S, Iwano M, Miki Y et al (1989) Interleukin-6 (IL-6) functions as an in vitro autocrine growth factor in renal cell carcinomas. FEBS Lett 250(2):607–610
Gauldie J, Richards C, Harnish D et al (1987) Interferon β2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci USA 84(20):7251–7255
McMillan DC, Watson WS, O’Gorman P et al (2001) Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr Cancer. https://doi.org/10.1207/S15327914nc392_8
Day D, Kanjanapan Y, Kwan E et al (2016) Benefit from cytoreductive nephrectomy and the prognostic role of neutrophil-to-lymphocyte ratio in patients with metastatic renal cell carcinoma. Intern Med J. https://doi.org/10.1111/imj.13202
Keizman D, Ish-Shalom M, Huang P et al (2012) The association of pre-treatment neutrophil to lymphocyte ratio with response rate, progression free survival and overall survival of patients treated with sunitinib for metastatic renal cell carcinoma. Eur J Cancer. https://doi.org/10.1016/j.ejca.2011.09.001
Gu L, Ma X, Wang L et al (2016) Prognostic value of a systemic inflammatory response index in metastatic renal cell carcinoma and construction of a predictive model. Oncotarget. https://doi.org/10.18632/oncotarget.10626
Sakai I, Miyake H, Hinata N et al (2013) Improved survival in patients with metastatic renal cell carcinoma undergoing cytoreductive nephrectomy in the era of targeted therapy. Int J Clin Oncol. https://doi.org/10.1007/s10147-013-0612-z
Acknowledgements
This study was supported in part by JSPS KAKENHI Grant numbers 26460456 and 17K11162 (to Yoji Nagashima). The authors thank Nobuko Hata for secretarial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Tsunenori Kondo received remuneration for a lecture from Pfizer Japan (Tokyo, Japan) and Novartis Japan (Tokyo, Japan).
About this article
Cite this article
Fukuda, H., Takagi, T., Kondo, T. et al. Prognostic value of the Glasgow Prognostic Score for patients with metastatic renal cell carcinoma treated by cytoreductive nephrectomy. Int J Clin Oncol 23, 539–546 (2018). https://doi.org/10.1007/s10147-017-1221-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-017-1221-z