Abstract
Background
We conducted a phase II study to evaluate the efficacy of neoadjuvant chemotherapy with docetaxel and carboplatin followed by radical hysterectomy for patients with non-squamous cell carcinoma of the uterine cervix.
Methods
Sixty-one patients with International Federation of Gynecology and Obstetrics stage IB2, IIA2, or IIB non-squamous cell carcinoma of the uterine cervix were enrolled. The patients were administered docetaxel at a dose of 60 mg/m2, followed by carboplatin at a dose based on an area under the curve of 6. The treatments were repeated every 21 days for one to three cycles. Fifty-two patients were eligible to evaluate the efficacy of neoadjuvant chemotherapy followed by radical hysterectomy. Adverse events were evaluated in 59 patients.
Results
The response rate was 69 % (95 % CI, 57–82 %), with 5 patients achieving complete response, 31 partial response, 15 stable disease, and 1 progressive disease. Median follow-up duration was 1913 days with a range of 145–2632 days. Of 52 patients, 50 underwent radical hysterectomy after neoadjuvant chemotherapy. The 2-year overall survival rate was 81.8 % for stage IB2, 85.7 % for stage IIA2, and 92.6 % for stage IIB. The most frequent grade 3 and 4 hematological toxicity was neutropenia, with 43 patients experiencing grade 4 and 11 with grade 3. The nonhematological toxicities were mainly grade 1 or 2 in severity.
Conclusion
Neoadjuvant chemotherapy with docetaxel and carboplatin followed by radical hysterectomy may be a useful strategy for patients with non-squamous cell carcinoma of uterine cervix.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Uterine cervical non-squamous cell carcinoma (UCNS) cases have gradually increased recently, comprising more than 20 % of all uterine cervical cancer [1]. Several authors reported that UCNS carried a worse prognosis, at a 10–20 % 5-year overall survival rate, than squamous cell carcinoma (SCC) [2–4]. In addition, recent literature has suggested that UCNS is clearly different from SCC based on its molecular pathogenesis [5, 6]. Consequently, it will be necessary to make a different therapeutic strategy for UCNS. However, it remains unknown whether the histological type is an independent prognostic factor in patients with cervical cancer. The National Comprehensive Cancer Network (NCCN) guideline suggested that patients with adenocarcinoma (AC) are typically treated in a similar manner to SCC [7].
The NCCN guidelines also suggested that radical hysterectomy (RH) is limited to stage IB1 and IIA1 patients with cervical cancer. Based on results of five randomized trials, the NCCN guidelines mainly recommend cisplatin (CDDP)-based concurrent chemoradiotherapy (CCRT) as the most appropriate treatment for stage IB2, IIA2, IIB–IVA patients with cervical cancer. However, these five randomized phase III trials did not include a sufficient number of patients with UCNS. A randomized study on radical surgery and/or adjuvant radiotherapy versus radiotherapy for stage IB–II cervical cancer showed that radiotherapy was less effective than surgery in patients with AC [8], suggesting low radiosensitivity for UCNS. In addition, recent literature suggested that CCRT with weekly CDDP was less effective for patients with UCNS than for those with SCC [9, 10]. Thereby, half of all Japanese gynecological oncologists chose radical hysterectomy for stage IIB patients with UCNS, although only a third selected radical hysterectomy for those with SCC [11].
It also remains uncertain whether neoadjuvant chemotherapy (NAC) followed by radical hysterectomy is beneficial for stage IB2, IIA2, and IIB patients with cervical cancer. A Gynecologic Oncology Group (GOG) study (GOG141) revealed that there was no evidence that NAC followed by RH offered any additional benefit to stage IB2 patients with cervical cancer [12]. In contrast, a systematic review of NAC followed by RH suggested that cisplatin-based NAC had a survival advantage for NAC compared to radiotherapy alone [13]. Additionally, NAC showed significantly decreased lymph node involvement and parametrial infiltration, which were independent prognostic factors in patients with locally advanced cervical cancer [14]. An international collaborative meta-analysis of NAC also reported that NAC reduced the need of adjuvant radiotherapy by decreasing pathological risk factors and distant metastasis, but failed to improve survival compared to RH without NAC [15].
If radiotherapy including CCRT with weekly CDDP might be less effective for patients with UCNS, NAC followed by RH might be a useful strategy for patients with UCNS. Our previous study indicated that docetaxel (DTX) and carboplatin (CBDCA) combination chemotherapy (DC chemotherapy) was a well-tolerated and effective chemotherapeutic regimen for cervical cancer, especially UCNS [16]. We conducted this phase II study to evaluate the efficacy and safety of DC chemotherapy followed by RH for stage IB2, IIA2, and IIB cervical cancer patients with UCNS. To our knowledge, this is the first phase II study for which only patients with UCNS are eligible.
Patients and methods
Sixty-one patients with International Federation of Gynecology and Obstetrics (FIGO) stage IB2, IIA with bulky tumor (≥4 cm in tumor diameter) and IIB with ≥2 cm of tumor diameter cervical cancer with non-SCC were enrolled in this study. Patients were accrued between February 2007 and May 2010, mainly at member institutions of the Sankai Gynecologic Study Group (SGSG), and followed up until July 2014. Of these 61 patients, 2 refused the protocol-scheduled treatment after giving written informed consent. Therefore, the adverse events were evaluated in 59 patients. In addition, 2 patients were diagnosed with endometrial cancer after radical hysterectomy: 1 patient underwent four cycles of NAC, and 1 patient received NAC with a violation of the dose reduction criteria. The central pathological review revealed that 1 patient had SCC with non-keratinizing type. As a result, the remaining 52 patients were eligible to evaluate the efficacy of DTX and CBDCA combination NAC followed by type III radical hysterectomy.
The study protocol was approved by the institutional review board at each institution. This study was conducted based on the ethical principles of the Declaration of Helsinki and in compliance with good clinical practice. This trial was also registered with the University Hospital Medical Information Network (UMIN: http://upload.umin.ac.jp/cgi-open-bin/ctr/ctr.cgi) with the No. UMIN000000560.
The patients were administered DTX at a dose of 60 mg/m2 by intravenous infusion for 1 h, followed immediately by CBDCA at a dose based on an area under the curve (AUC) of 6 by intravenous infusion for 2 h. The treatments were repeated every 21 days for one to three cycles as NAC. The timing of radical hysterectomy was decided at each institution. Adjuvant treatment was also decided at each institution by histological findings after radical hysterectomy.
All patients, aged between 20 and 74 years, had histological confirmation of non-SCC of the uterine cervix at each institution. Patients had an Eastern Cooperative Oncology Group performance status of 0 or 1. Additional criteria were as follows: adequate bone marrow function [platelets ≥100,000/mm3, hemoglobin ≥9.0 g/dl, absolute neutrophil count (ANC) ≥2,000/mm3], serum AST and ALT ≤100 U/mL, alkaline phosphatase ≤750 IU/l, total bilirubin ≤1.5 mg/dl, serum creatinine ≤1.5 mg/dl, electrocardiography normal or minor change without symptoms that required any therapeutic intervention, and survival expectancy 3 months or longer. All subjects received an explanation of this study from the physicians with written informed consent forms and other relevant information and freely provided their informed consent before this study.
Patients who met any of the following exclusion criteria were excluded from this study: fever >38.0 °C, typical infection or WBC count >12,000/mm3, severe comorbidities including myocardial infarction or angina within 90 days before this study, uncontrolled diabetes, uncontrolled hypertension, uncontrolled hypercalcemia, having had double invasive cancer within 5 years of the disease-free interval, requiring continuous drainage of pleural effusion, ascites, and pericardial fluid, having interstitial pneumonitis, peripheral neuropathy or edema of grade 2 or more, having received prior chemotherapy including a taxane compound, and being judged by the physician to be ineligible for this study. Treatment was delayed when the neutrophil count was <1,500/mm3 or the platelet count was <100,000/mm3. For cases of febrile neutropenia, the dose of DTX was reduced by 10 mg/m2. Granulocyte colony-stimulating factor (G-CSF) was administered subcutaneously to patients with a white blood cell count <1,000/μl, neutrophil count <500/μl, or febrile neutropenia. Patients did not receive prophylactic G-CSF unless they had a treatment delay or neutropenic complications after treatment. Prophylactic antibiotic cycles were recommended for complicated grade 4 neutropenia. When the platelet count was <75,000/mm3, the dose of CBDCA was reduced by AUC of 1.0 in the subsequent cycle. If the patient needed platelet infusion at the dose of AUC = 5, the dose of CBDCA was reduced to AUC of 4 in the subsequent cycle. In patients with grade 3 or 4 nonhematological toxicity, except for vomiting, anorexia, fatigue, and allergy, the dose of DTX was reduced by 10 mg/m2. The scheduled chemotherapy could be delayed for a maximum of 20 days. The scheduled chemotherapy was also discontinued in the case of disease progression, excessive toxicity, or refusal of scheduled treatment by patients.
The response was evaluated based on the response evaluation criteria in solid tumors (RECIST) guidelines version 1.0. Complete response (CR) was defined as complete disappearance of all target lesions. Partial response (PR) was defined as at least 30 % reduction in the sum of the longest diameters of the target lesions. Progressive disease (PD) was defined as a greater than 20 % increase in the sum of the greatest diameters of the target lesions within 2 months of study entry, or the appearance of any new lesions, and/or unequivocal progression of existing nontarget lesions. Stable disease (SD) was any condition not meeting any of these three criteria. The tumors of all the patients were measured by magnetic resonance imaging by radiologists at each institution. Because the scheduled chemotherapy was performed as NAC in this study, radical hysterectomy was allowed to be performed without confirmation 4 weeks after complete disappearance of the tumor or tumor shrinkage by 30 %. The severity of adverse events was assessed according to the common terminology criteria for adverse events (CTCAE) version 3.0.
The primary endpoint of this study was the response rate. A 95 % confidence interval for the estimate of the response rate was calculated. Assuming a response rate of 70 %, the study was designed with 80 % power such that the lower limit of the 95 % confidence interval (CI) for the estimate of the response rate was higher than 50 %. A sample size of 48 assessable patients was required. Secondary endpoints were to assess adverse events, the 2-year progression-free survival (PFS) rate, and the 2-year overall survival (OS) rate. The Kaplan–Meier method was used to determine patient survival distribution in the assessable population; 95 % confidence intervals of survival distributions were estimated by means of Greenwood’s formula. The significance of the survival distribution in each subgroup was tested by the log-rank and log-rank trend test. A value of p < 0.05 was considered statistically significant. All analysis was performed with SAS system version 9.1.3 (SAS Institute, Cary, NC, USA).
Results
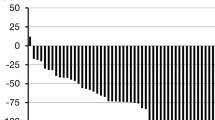
The characteristics of 52 patients who were eligible to be evaluated for the efficacy of DTX/CBDCA combination chemotherapy followed by radical hysterectomy are shown in Table 1. Forty patients (77 %) received two cycles of NAC, 4 had one cycle of NAC, and 8 had three cycles of NAC. The response rate was 69 % (95 % CI, 57–82 %), with 5 patients achieving CR, 31 PR, 15 SD, and 1 PD. Tumor reduction rates are shown in Fig. 1. Only 1 patient had tumor growth in the uterine cervix after NAC. Fifty patients (96 %) could undergo radical hysterectomy after NAC. Two patients in stage IIB could not undergo radical hysterectomy; : patient had PD with a new lesion, and the other showed PR, but peritonitis carcinomatosa was observed at laparotomy, and they received CCRT.
In 50 patients who underwent radical hysterectomy, NAC brought downstage to 9 patients (64.3 %; 9/14) in stage IB2, to 3 (42.9 %; 3/7) in stage IIA2, and to 17 (54.8 %; 17/29) in stage IIB (Table 2). Seventeen (34 %) had only pelvic lymph node involvement; 18 (36 %) had only parametrial infiltration. Ten patients (20 %) had both pelvic lymph node involvement and parametrial infiltration. Twenty-five (50 %) patients had neither pelvic lymph node involvement nor parametrial infiltration. Eleven patients received no adjuvant treatment after radical hysterectomy. Twenty-six patients received adjuvant chemotherapy, including 21 patients with DTX/CBDCA combination chemotherapy, 3 irinotecan/nadaplatin combination chemotherapy, and 2 paclitaxel/CBDCA combination chemotherapy. Nine patients underwent CCRT, and 4 patients underwent radiotherapy alone as adjuvant treatment after radical hysterectomy. In 17 patients with pelvic lymph node involvement, 9 patients received adjuvant chemotherapy after RH, CCRT for 6 patients and RT for 2 patients. There is no difference in recurrence rate between CCRT and chemotherapy as adjuvant treatment [recurrence rate: 44.4 % (4/9) for CCRT vs. 42.3 % (11/26) for chemotherapy].
The median follow-up duration was 1913 days with a range of 145–2632 days. The 2-year PFS rate and OS rate were 67.9 % (95 % CI, 53.0–78.9 %) and 85.9 % (95 % CI, 72.7–93.0 %), 61.4 % and 76.9 % in stage IB2, 71.4 % and 85.7 % in stage IIA2, and 70.0 % and 90.0 % in stage IIB, respectively (Fig. 2). The 2-year PFS rate and OS rate were not statistically significant by FIGO stage (trend test, p = 0.9953 and p = 0.4782, respectively). Seventeen patients with pelvic lymph node involvement had a significantly worse outcome than those without pelvic lymph node involvement (2-year PFS: 47.1 % vs. 81.1 %; p = 0.0041; 2-year OS: 76.5 % vs. 93.6 %; p < 0.1200). Similarly, 18 patients with parametrial infiltration had a significantly worse outcome than those without parametrial infiltration (2-year PFS: 50.0 % vs. 77.9 %; p = 0.0024, 2-year OS: 72.2 % vs. 93.8 %; p = 0.0165). Patients with adenosquamous cell carcinoma (ASC) had a relatively worse outcome than those with AC, but the difference was not significant (2-year PFS: AC, 73.1%; ASC, 54.2 %; other, 66.7 %; p = 0.4262).
The 2-year progression-free survival (PFS) and overall survival (OS) by FIGO stage. With the median follow-up duration of 1913 days (range, 145–2632 days), the 2-year PFS rate and OS rate were 67.9 % (95 % CI, 53.0–78.9 %) and 85.9 % (95 % CI, 72.7–93.0 %), 61.4 % and 76.9 % in stage IB2, 71.4 % and 85.7 % in stage IIA2, and 70.0 % and 90.0 % in stage IIB, respectively
Twenty patients recurred and 15 patients died. Two patients who were not indicated for radical hysterectomy recurred and died. Five patients who achieved CR were alive without recurrence. Of 46 patients with PR or SD, 19 patients had recurrence (11 for PR and 8 for SD). Of 20 patients with recurrence, 7 patients had both pelvic lymph node involvement and parametrial infiltration, 4 patients had only pelvic lymph node involvement, 4 patients had only parametrial infiltration, 3 patients had neither pelvic lymph node involvement nor parametrial infiltration, and 2 patients were not indicated for radical hysterectomy. Five patients recurred in the vaginal stump. In 10 patients with distant recurrence, 5 patients recurred in the lung, 4 patients had distant lymph node recurrence, and 3 patients had bone metastasis. Four patients recurred in an extrapelvic lymph node, such as paraaortic, two mediastinal, and cervical nodes. Among 3 patients with pelvic recurrence, 2 patients showed peritonitis carcinomatosa.
Adverse events of DTX/CBDCA combination chemotherapy were evaluated in 59 patients. The most frequent grade 3 and grade 4 hematological toxicity was neutropenia, with 43 patients (73 %) having grade 4 neutropenia and 11 patients (19 %) having grade 3 neutropenia (Table 3). Febrile neutropenia was observed in 3 patients, including 2 after the first cycle, and residual neutropenia after the second cycle. Of 59 patients, 34 (58 %) required G-CSF for leukocyte recovery. Five patients (8 %) had grade 3 anemia, including 3 patients after the first cycle and 2 patients after the second cycle. No patients had grade 4 anemia in this study. One patient showed grade 4 thrombocytopenia. The non-hematological toxicities were mainly grade 1 or 2 in severity. The most common grade 1 or 2 adverse event was alopecia. With regard to grade 3 nonhematological toxicities, 5 patients had allergic reaction to DTX or nausea, 3 constipation, and 1 vomiting. Two patients had grade 4 nonhematological toxicities, 1 vomiting, and the other an allergy reaction to DTX.
Discussion
Because UCNS is a relatively rare tumor, no prospective or randomized studies focusing on this rare disease have concluded whether CCRT was effective for locally advanced UCNS as well as SCC. The retrospective analysis of GOG trials reported that patients with adenocarcinoma and adenosquamous carcinomas showed similar outcome compared to SCC when treated with CDDP-based CCRT [17]. However, of 1671 patients, this retrospectively analysis included only 112 patients (6.7 %) with adenocarcinoma and adenosquamous carcinomas who were treated with CDDP-based CCRT. Furthermore, CDDP-based CCRT in this analysis included various chemotherapeutic regimens, such as CDDP alone, CDDP/5-fluorouracil, and CDDP/5-fluorouracil/hydroxyurea. In contrast, recent reports have suggested that CCRT with weekly CDDP was less effective for patients with AC than for those with SCC [9, 10, 18]. In fact, the 5-year OS for patients with cervical AC was reported to be 65.3 % in stage IB2, 66.4 % in stage IIA, and 55.9 % in stage IIB [4], carrying a worse prognosis of about 10 % 5-year OS than SCC. Accordingly, to improve the outcome for stage IB2–IIB cervical cancer patients with UCNS, it is worth reevaluating the efficacy of NAC followed by RH.
A high response rate of NAC is necessary to prove the efficacy of NAC followed by RH. However, the response rate of patients with AC to NAC ranged from 50 % to 67 % [19–21], suggesting that AC might be less sensitive to chemotherapy. We previously reported that the overall response rate to DC chemotherapy was 69 % for patients with UCNS [16]. The present study also showed a similar response rate as NAC for stage IB2, IIA2, and IIB patients with UCNS. Additionally, of 52 patients, DC chemotherapy suppressed tumor growth of the uterine cervix in 51 patients before RH. An international collaborative meta-analysis of NAC also reported that NAC reduced the need of adjuvant radiotherapy by decreasing pathological risk factors and distant metastasis. However, when RH is used to treat FIGO IB2, IIA2, and IIB disease, the majority of patients needed multimodality treatment, including NAC, RH, and adjuvant radiotherapy including CDDP-based CCRT [22]. The multimodality strategy consisting of RH followed by adjuvant radiotherapy is associated with not only impaired quality of life, but also with conflicting cost-effectiveness. Adjuvant radiotherapy sometimes induced severe postoperative complications, such as lower-limb lymphedema, bowel obstruction, urinary disturbance, and sexual dysfunction [22–24]. Consequently, adjuvant radiotherapy should be limited as possible for patients who underwent RH [25].
Recent literature reported adjuvant chemotherapy as useful treatment for patients with uterine cervical cancer after RH [26, 27]. In the near future, we are also going to report a pilot study that evaluates the efficacy and safety of RH followed by adjuvant chemotherapy consisting of taxane/CBDCA combination chemotherapy for high-risk patients with UCNS. Thereby, we can provide a more efficient and less toxic treatment option for patients with lower radiosensitive UCNS, avoiding sequential incorporation with adjuvant radiotherapy. The multimodality strategy, consisting of RH and chemotherapy, as primary treatment for patients with UCNS also provides a chance to control locoregional recurrence by radiotherapy.
Pelvic lymph node involvement is one of the most important prognostic factors in patients with cervical cancer. Recently, based on the recommendation of the NCCN guideline, gynecological oncologists have tended to choose CCRT, not RH, as the primary treatment for stage IB2, IIA2, and IIB patients with cervical cancer. As a result, the accurate incidence of pelvic lymph node involvement by histological type is unknown in stage IB2, IIA2, and IIB patients with cervical cancer. It remains unclear whether patients with UCNS had a higher incidence of pelvic lymph node involvement than those with SCC. Irie et al. showed that a significantly higher incidence of pelvic lymph node involvement was observed in stage II patients with AC than in those with SCC [2]. In contrast, other authors reported that there was no significant difference in pelvic lymph node involvement between SCC and AC in patients with stage IB–IIB cervical cancer [28–30]. In our previous survey of 825 patients with stage IB1–IIB cervical cancer, including 282 patients with cervical AC, the histological type did not affect the incidence of pelvic lymph node involvement [31]. There might be no significant difference in pelvic lymph node involvement between patients with SCC and AC of stage IB–IIB; however, our previous survey suggested that patients with AC having pelvic lymph node involvement showed a significantly poorer outcome than those with SCC [31]. Meta-analysis suggested that NAC showed significantly decreased lymph node involvement and parametrial infiltration in patients with locally advanced cervical cancer [14, 15]. Because this study was not comparative, it could not be evaluated whether NAC decreased pelvic lymph node involvement in stage IB2, IIA2, and IIB patients with UCNS. In this study, the incidence of pelvic lymph node involvement was 43 % (6/14) in stage IB2, 57 % (4/7) in stage IIA2, and 24 % (7/29) in stage IIB, suggesting that NAC might induce a relatively low incidence and a good outcome, especially in stage IIB patients with UCNS. Usually, stage IIB patients with UCNS tend to have more PLN involvement and worse outcome. In the current study, the incidence of PLN involvement of stage IIB seemed to be lower than those with stage IB2 and IIA2, but it was unknown why stage IIB patients with UCNS showed lower incidence of PLN involvement than others. Parametrial infiltration is also an important prognostic factor in patients with cervical cancer. Kasamastu et al. reported that patients with AC showed a relatively poorer outcome than those with SCC and parametrial infiltration after RH [30]. In this study, 4 patients had parametrial infiltration in stage IB2, 3 in stage IIA2, and 13 in stage IIB after RH. Consequently, NAC caused 17 (59 %) of 29 stage IIB patients to downstage, indicating that NAC followed by RH produced a good outcome, especially in stage IIB.
Eifel et al. suggested that AC had statistically significantly increased distant relapse rates compared with SCC regardless of tumor size in stage IB patients receiving radiotherapy [32]. Our previous report, which included 3471 surgically treated cases of stage IB–IIB cervical cancer, suggested that AC predominantly recurred hematogenously, whereas SCC recurred lymphatically [33]. In this study, 4 patients (20 %) recurred only in the vaginal stump, of which 4 patients could be controlled by radiotherapy or surgical resection. In contrast, 10 patients (50 %) had distant metastasis, including 5 with lung metastasis and 3 with bone metastasis, and 9 patients died. Accordingly, a new adjuvant strategy, including molecular targeting therapy, is necessary for high-risk patients with UCNS to reduce hematogenous distant metastasis to as low as possible. The RTOG/GOG724 study evaluates the efficacy of CCRT with weekly CDDP followed by four cycles of paclitaxel/CBDCA combination chemotherapy for high-risk cervical cancer after RH.
DC chemotherapy was generally well tolerated, as demonstrated by the mild to moderate toxicity. Neutropenia was the most frequent hematological toxicity. Grade 4 neutropenia was observed in 41 patients (69 %), but was rapidly reversible with G-CSF administration. In 41 patients receiving two cycles of NAC, no patients had a scheduled treatment delay of more than 8 days. Three patients with febrile neutropenia required hospitalization for the administration of antibiotics and G-CSF, but no patients developed any infection or sepsis. Five patients had grade 3 anemia and 1 patient had grade 4 thrombocytopenia, but no patients required transfusion before radical hysterectomy.
The current study has several limitations. First, this study only evaluated the response rate, adverse events of NAC, and survival, as this study is the phase II study. However, it is also necessary to evaluate the completeness of RH and the short-term/long-term complications of RH. Unfortunately, we could not evaluate the completeness of RH and perioperative adverse events, because it was not scheduled to evaluate these factors at the start of this study. We should conduct the next study of NAC followed by RH to evaluate not only the efficacy of NAC, and adverse events of NAC, but also the completeness of RH and perioperative adverse events, such as lower limb lymphedema and gastrointestinal obstructions. Second, it is difficult to evaluate the long term usefulness of NAC followed by RH, because the criteria of adjuvant treatment and adjuvant treatment were decided at each institution by histological findings after RH in this study. Consequently, we have to decide the protocol-scheduled treatment, such as NAC followed by RH and adjuvant chemotherapy for high-risk UCNS, to evaluate the multidisciplinary strategy with chemotherapy and surgery for UCNS in the near future. In addition, because this phase II study involved a small number of patients, we could not evaluate the difference of recurrent rate by adjuvant treatment sufficiently and discuss which adjuvant treatment is more appropriate for patients with and without PLN involvement.
In conclusion, DC chemotherapy was a well-tolerated NAC regimen. Neoadjuvant DC chemotherapy followed by RH might be a useful strategy for stage IB2, IIA2, and IIB patients with UCNS.
References
Smith HO, Tiffany MF, Qualls CR et al (2000) The rising incidence of adenocarcinoma relative to squamous cell carcinoma of the uterine cervix in the United States—a 24-year population-based study. Gynecol Oncol 78:97–105
Irie T, Kigawa J, Minagawa Y et al (2000) Prognosis and clinicopathological characteristics of Ib-IIb adenocarcinoma of the uterine cervix in patients who have had radical hysterectomy. Eur J Surg Oncol 26:464–467
Galic V, Herzog TJ, Lewin SN et al (2012) Prognostic significance of adenocarcinoma histology in women with cervical cancer. Gynecol Oncol 125:287–291
Quinn MA, Benedet JL, Odicino F et al (2006) Carcinoma of the cervix uteri. FIGO 26th annual report on the results of treatment in gynecological cancer. Int J Gynaecol Obstet 95:S43–S103
Wright AA, Howitt BE, Myers AP et al (2013) Oncogenic mutations in cervical cancer: genomic differences between adenocarcinoma and squamous cell carcinoma of the cervix. Cancer (Phila) 119:3776–3783
Tewari KS, Sill MW, Long HJ 3rd et al (2014) Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med 370:734–743
NCCN Clinical Practice Guideline in Oncology (NCCN Guidelines) Version 2 (2015) Cervical cancer. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed 1 Mar 2015
Landoni F, Maneo A, Colombo A et al (1997) Randomised study of radical surgery versus radiotherapy for stage Ib–IIa cervical cancer. Lancet 350:535–540
Niibe Y, Kenjo M, Onishi H et al (2010) High-dose-rate intracavitary brachytherapy combined with external beam radiotherapy for stage IIIb adenocarcinoma of the uterine cervix in Japan: a multi-institutional study of Japanese Society of Therapeutic Radiology and Oncology 2006–2007 (study of JASTRO 2006–2007). Jpn J Clin Oncol 40:795–799
Huang YT, Wang CC, Tsai CS et al (2011) Long-term outcome and prognostic factors for adenocarcinoma/adenosquamous carcinoma of cervix after definitive radiotherapy. Int J Radiat Oncol Biol Phys 80:429–436
Mikami M, Aoki Y, Sakamoto M et al (2014) Disease Committee of Uterine Cervical and Vulvar Cancer, Japanese Gynecologic Oncology Group. Surgical principles for managing stage IB2, IIA2, and IIB uterine cervical cancer (bulky tumors) in Japan: a survey of the Japanese Gynecologic Oncology Group. Int J Gynecol Cancer 24:1333–1340
Eddy GL, Bundy BN, Creasman WT et al (2007) Treatment of (“bulky”) stage IB cervical cancer with or without neoadjuvant vincristine and cisplatin prior to radical hysterectomy and pelvic/para-aortic lymphadenectomy: a phase III trial of the Gynecologic Oncology Group. Gynecol Oncol 106:362–369
Neoadjuvant Chemotherapy for Locally Advanced Cervical Cancer Meta-analysis Collaboration (2003) Neoadjuvant chemotherapy for locally advanced cervical cancer: a systematic review and meta-analysis of individual patient data from 21 randomised trials. Eur J Cancer 39:2470–2486
Rydzewska L, Tierney J, Vale CL et al (2012) Neoadjuvant chemotherapy plus surgery versus surgery for cervical cancer. Cochrane Database Syst Rev 12:CD007406
Kim HS, Sardi JE, Katsumata N et al (2013) Efficacy of neoadjuvant chemotherapy in patients with FIGO stage IB1 to IIA cervical cancer: an international collaborative meta-analysis. Eur J Surg Oncol 39:115–124
Takekida S, Fujiwara K, Nagao S et al (2010) Phase II study of combination chemotherapy with docetaxel and carboplatin for locally advanced or recurrent cervical cancer. Int J Gynecol Cancer 20:1563–1568
Rose PG, Java JJ, Whitney CW et al (2014) Locally advanced adenocarcinoma and adenosquamous carcinomas of the cervix compared to squamous cell carcinomas of the cervix in Gynecologic Oncology Group trials of cisplatin-based chemoradiation. Gynecol Oncol 135:208–212
Lee JY, Kim YT, Kim S et al (2015) Prognosis of cervical cancer in the era of concurrent chemoradiation from national database in Korea: a comparison between squamous cell carcinoma and adenocarcinoma. PLoS One doi:10.1371/journal.pone.0144887
Iwasaka T, Fukuda K, Hara K et al (1998) Neoadjuvant chemotherapy with mitomycin C, etoposide, and cisplatin for adenocarcinoma of the cervix. Gynecol Oncol 70:236–420
Lissoni A, Gabriele A, Gorga G et al (1997) Cisplatin-, epirubicin- and paclitaxel-containing chemotherapy in uterine adenocarcinoma. Ann Oncol 8:969–972
Saito T, Takehara M, Lee R et al (2004) Neoadjuvant chemotherapy with cisplatin, aclacinomycin A, and mitomycin C for cervical adenocarcinoma—a preliminary study. Int J Gynecol Cancer 14:483–490
Yessaian A, Magistris A, Burger RA et al (2004) Radical hysterectomy followed by tailored postoperative therapy in the treatment of stage IB2 cervical cancer: feasibility and indications for adjuvant therapy. Gynecol Oncol 94:61–66
Deura I, Shimada M, Hirashita K et al (2015) Incidence and risk factors for lower limb lymphedema after gynecologic cancer surgery with initiation of periodic complex decongestive physiotherapy. Int J Clin Oncol 20:556–560
Hosaka M, Watari H, Takeda M et al (2008) Treatment of cervical cancer with adjuvant chemotherapy versus adjuvant radiotherapy after radical hysterectomy and systematic lymphadenectomy. J Obstet Gynecol Res 34:552–556
Shimada M, Kigawa J, Takahashi M et al (2004) Stromal invasion of the cervix can be excluded from the criteria using adjuvant radiotherapy following radical surgery for patients with cervical cancer. Gynecol Oncol 93:628–631
Matsumura M, Takeshima N, Ota T et al (2010) Neoadjuvant chemotherapy followed by radical hysterectomy plus postoperative chemotherapy but no radiotherapy for stage IB2–IIB cervical cancer: irinotecan and platinum chemotherapy. Gynecol Oncol 119:212–216
Hosaka M, Watari H, Kato T et al (2012) Clinical efficacy of paclitaxel/cisplatin as an adjuvant chemotherapy for patients with cervical cancer who underwent radical hysterectomy and systematic lymphadenectomy. J Surg Oncol 105:612–616
Nakanishi T, Ishikawa H, Suzuki Y et al (2000) A comparison of prognoses of pathologic stage Ib adenocarcinoma and squamous cell carcinoma of the uterine cervix. Gynecol Oncol 79:289–293
Park JY, Kim DY, Kim JH et al (2010) Outcomes after radical hysterectomy in patients with early-stage adenocarcinoma of uterine cervix. Br J Cancer 102:1692–1698
Kasamatsu T, Onda T, Sawada M et al (2009) Radical hysterectomy for FIGO stage I–IIB adenocarcinoma of the uterine cervix. Br J Cancer 100:1400–1405
Shimada M, Nishimura R, Nogawa T et al (2013) Comparison of the outcome between cervical adenocarcinoma and squamous cell carcinoma patients with adjuvant radiotherapy following radical surgery: SGSG/TGCU intergroup surveillance. Mol Clin Oncol 1:780–784
Eifel PJ, Burke TW, Morris M et al (1995) Adenocarcinoma as an independent risk factor for disease recurrence in patients with stage IB cervical carcinoma. Gynecol Oncol 59:38–44
Shimada M, Kigawa J, Nishimura R et al (2006) Ovarian metastasis in carcinoma of the uterine cervix. Gynecol Oncol 101:234–237
Acknowledgments
The authors extend their gratitude to the investigators at the participating institutions: Eiji Hirata (Hiroshima University Hospital), Kazuhiro Takehara (National Hospital Organization Kure Medical Center), Ryoji Hayase (Fukuyama Medical Center), Yasunobu Kanamori (Yamaguchi Red Cross Hospital), Akihiro Murakami (Yamaguchi University Hospital), and Hideo Fujimoto (JA Hiroshima Kouseiren Hospital), and Shinya Sato (Tottori University Hospital). The authors also thank the statistician, Tetsutaro Hamano, for his support during the independent statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Keiichi Fujiwara received research funding from Sanofi-aventis K.K. Toru Sugiyama received honoraria and research funding from Chugai Pharmaceutical Co., Ltd. The other authors have no conflict of interest.
About this article
Cite this article
Shimada, M., Nagao, S., Fujiwara, K. et al. Neoadjuvant chemotherapy with docetaxel and carboplatin followed by radical hysterectomy for stage IB2, IIA2, and IIB patients with non-squamous cell carcinoma of the uterine cervix. Int J Clin Oncol 21, 1128–1135 (2016). https://doi.org/10.1007/s10147-016-1010-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-016-1010-0