Abstract
Background
The standard regimen of systemic chemotherapy for patients with advanced urothelial cancer (UC) changed from methotrexate, vinblastine, adriamycin, and cisplatin (MVAC) to gemcitabine and cisplatin (GC) in 2008 when the use of gemcitabine for UC began to be reimbursed by public health insurance in Japan. We examined its influence on the chemotherapy trend in elderly patients aged ≥80 years.
Methods
Among 345 patients included in our previous multicenter retrospective cohort study (chemotherapy for urothelial carcinoma: renal function and efficacy study; CURE study), the outcome of 30 patients aged ≥80 years was reviewed before and after 2008 and compared with 315 young patients.
Results
There were only 7 (4.6 %) elderly individuals among all registered patients before 2008, whereas the number increased to 23 (12 %) after 2008. Before 2008, only one elderly patient received MVAC, while GC (whose rate was similar to the rate in young patients) was administered to 13 patients (56.5 %) after 2008. The chemotherapeutic effect and overall survival (OS) rate was not significantly different between young and elderly patients. In the elderly treated with the GC regimen, the renal impairment rate after the first cycle was significantly higher, and the presence of distant metastases and renal impairment were independent prognostic factors in a multivariate analysis.
Conclusion
Since GC was approved as the standard regimen for first-line chemotherapy in UC, selected elderly patients have been able to safely receive systemic chemotherapy like young patients. The clinical response rate and OS rate were similar to the young, but we need to monitor changes in renal function more closely in the elderly treated with GC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer and aging are intimately linked [1], and urothelial cancer (UC), which occurs most commonly in the elderly, illustrates this connection well. Recently, individuals aged 75–84 years old account for the largest percentage (30 %) of new cases of UC [2]. The vast majority of invasive UC occurs in patients aged >65 years. At the time of initial diagnosis, the median age is 71 and rising [3].
The methotrexate, vinblastine, adriamycin and cisplatin (MVAC) regimen was used previously as first-line chemotherapy for metastatic UC [4]. Because the prognosis of advanced UC is relatively poor with an aggressive tumor, active treatment should be considered even in such an elderly population. However, systemic chemotherapy in elderly patients with advanced UC lacks data concerning therapeutic efficacy and toxicity because common clinical trials regarding MVAC, or other multidrug combination chemotherapy, have been performed mostly on younger and healthier individuals due to its severe toxicity. It is difficult to apply those results directly to treatment decisions in the elderly, who often exhibit considerable comorbidity and progressive restriction in the functional reserve of multiple organ systems, which affects the pharmacokinetics and pharmacodynamics of drugs including chemotherapeutic agents [5, 6]. However, gemcitabine and cisplatin (GC) have recently become the new standard chemotherapy regimen based on a large multinational randomized clinical trial (RCT) comparing MVAC and GC which indicated, in 2000, that GC had similar oncological efficacy and a lower toxicity profile in advanced UC [7]. GC has also been used widely in Japan as first-line chemotherapy for UC since the use of gemcitabine for UC began being reimbursed by the government in 2008. This transition may have an impact on clinical decision-making, owing to a lower toxicity profile, indicating that even elderly patients can possibly benefit from systemic chemotherapy.
This study was conducted to clarify the current status of systemic chemotherapy in elderly patients aged ≥80 years with advanced UC based on our previous multicenter retrospective cohort study (chemotherapy for urothelial carcinoma: renal function and efficacy; CURE study) of 345 Japanese UC patients who received systemic chemotherapy for metastatic or unresectable cancer [8, 9]. We think that this retrospective analysis may encourage future progress in finding the optimal strategy for those patients.
Patients and methods
Patients
Basic patient data was provided from a CURE study in which 345 patients with advanced or unresectable UC had received systemic chemotherapy at a total of 17 Japanese institutions between January 2004 and December 2010, after excluding those who received peri-operative chemotherapy (either neoadjuvant or adjuvant chemotherapy) or chemoradiation for bladder preservation. This retrospective study was an Institutional Review Board-approved study with all participating sites providing the necessary institutional data sharing agreements prior to initiation.
Data at the start of chemotherapy included patient age, height, weight, gender, performance status (PS), comorbidity, TNM stage, site of metastases, kidney status, and serum creatinine levels. In addition, the regimen of each patient’s first-line chemotherapy, the planned dose of each drug, and the presence or absence of dose reduction at the start of chemotherapy were recorded and analyzed. The definition of dose reduction depended on each physician. The median follow-up duration was 10.4 months (range 1–97 months). The observed toxicities during induction chemotherapy were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) v4.0.
Among all patients, 30 individuals were aged ≥80 years. We divided the 30 patients into three groups according to the first-line chemotherapy they received as follows—(1) GC group (3 or 4-week cycle), (2) MVAC/methotrexate, epirubicin, and cisplatin (MEC) group as cisplatin-based treatments, and (3) other/miscellaneous treatment group (including the carboplatin-based [GCarbo/GDCarbo] or non-platinum-based GP group). Tumor responses were assessed based on the Response Evaluation Criteria in Solid Tumors (RECIST v1.1) [10]. A complete response (CR) required the total disappearance of all evidence of UC for at least 4 weeks. A partial response (PR) required a reduction of >30 % in the sum of the longest diameters of the target lesions, without the appearance of any new lesions for at least 4 weeks. Progressive disease (PD) was defined as the appearance of any new lesions, or an increase of >20 % in the size of measurable lesions. No change (NC) was defined as diseases that did not meet any of the above criteria. Renal function was evaluated by the measurement of serum creatinine levels and creatinine clearance (CC) before and after each course of chemotherapy in each institute. CC was measured by 24-h urine collection, or calculated according to the Cockcroft and Gault formula (CG–CCr), where CC = (140 − age) × weight (kg)/0.814 × SC µmol/l, or according to the estimated glomerular filtration rate (eGFR) formula, where eGFR (ml/min/1.73 m2) = 194 × serum Cr − 1.094 × age (years) − 0.287, which was modified for Japanese individuals by adjusting the original Modification of Diet in Renal Disease equation and was recommended by the Japanese Society of Nephrology [11]. For this analysis, we calculated eGFR from patient data in order to apply it to methods to evaluate renal function for the whole population.
Statistical analysis
The variables of the different groups were compared using the Chi-squared test or Mann–Whitney U test. The Kaplan–Meier method was used to estimate the overall survival rate (OS), which was compared using the log-rank test. OS was measured from the start of systemic chemotherapy until the date of death or until the last follow-up. The cause of death was determined by the attending physicians at each institution. Univariate and multivariate analyses for OS were performed using Cox proportional hazards regression models. Differences among groups were regarded as significant when p < 0.05. These analyses were performed with the Statview version 5.0 statistical software package (SAS Institute, Cary, NC, USA).
Results
Characteristics of 30 octogenarians with advanced urothelial cancer treated with systemic chemotherapy
Table 1 shows the clinical characteristics of 345 UC patients included in the CURE study according to age, i.e., <80 years (young patients; n = 315) or ≥80 years (elderly patients; n = 30). No significant difference was observed in clinical parameters such as gender, PS, or initial tumor location among the groups. The percentage of locally advanced disease was significantly higher in the elderly group (5.1 vs 16.7 %, p = 0.0394). Regarding initial renal function, the mean serum creatinine level (± SD) was 1.06 ± 0.36 mg/dl in young patients and 1.13 ± 1.04 mg/dl in elderly patients (p = 0.7482). Nine of the 30 elderly patients had a single functioning kidney because of previous surgery or severe hydronephrosis. There was no significant difference in pre-treatment eGFR between young and elderly patients, although young patients showed significantly higher CG–CCR values (59.4 ± 23.1 vs 43.4 ± 11.7 mg/dl, p < 0.0001).
Among all 30 elderly patients, 13 received GC, one received MVAC, and 16 received other regimens (GCarbo in 1, GDCarbo in 6, GP in 6, and miscellaneous regimens in 3, respectively) as first-line chemotherapy. Compared with young patients, the frequency of MVAC/MEC was significantly lower (p < 0.005). The mean administered cycles of all regimens was 3.1 in the young and 2.9 cycles in the elderly patients (p = 0.8711), while the mean for the GC regimen was 3.8 in the young and 3.2 cycles in the elderly patients. The changes in the patient population were evaluated and divided into before and after 2008 groups, i.e., when the use of gemcitabine for UC began to be reimbursed by public health insurance in Japan. There were only 7 elderly patients (4.6 %) among all registered patients before 2008, whereas the number increased to 23 (12 %) after 2008 (Table 2). When the selection pattern of first-line chemotherapy in the elderly before 2008 was examined, only one patient received MVAC chemotherapy, which was the standard regimen for UC, while the other 6 patients received different regimens. On the other hand, after 2008, GC regimens, which have become the new standard for chemotherapy, were administered to 13 of the elderly patients (56.5 %). This rate was similar to that among young patients in the same era (90/169 patients [53.3 %] received GC chemotherapy).
While 79 of 315 young patients (25.1 %) received reduced-dose chemotherapy, the rate of reduced-dose chemotherapy tended to increase in the elderly (13/30, 43.3 %, p = 0.0518). Among 13 patients with reduced-dose chemotherapy, 7 (53.8 %) received the GC regimen and the other 6 (35.2 %) received non-GC regimens (p = 0.5193). The dose reduction rate was higher in the patients with an eGFR <60 ml/min/1.73 m2 compared with the patients with an eGFR ≥60 ml/min/1.73 m2, but it was not significant (52.3 and 22.2 %, respectively; p = 0.2603). The reasons for dose reduction were renal impairment in 5 patients, high age or low PS in 4 patients, both in 3 patients, and unknown in one patient. Average relative dose intensity (RDI) was 87.9, 78.3 and 85.1 % for young patients with GC chemotherapy, elderly patients with GC chemotherapy and young patients with MVAC/MEC, respectively. While no significant difference was found between young patients receiving MVAC/MEC and GC chemotherapy, there was a significant difference in average RDI between young and elderly patients receiving GC chemotherapy (p = 0.0077).
Clinical outcomes of 30 octogenarians with advanced urothelial cancer treated with systemic chemotherapy
As shown in Table 3, the first-line chemotherapeutic effect was not significantly different between young and elderly patients. Among the elderly, the CR rate was 13.3 %, while it was 7.0 % in young patients. The response rate (CR + PR rates) in the GC group was 69.2 % in the elderly, which was the highest among all regimens. In the elderly treated with the GC regimen, dose reduction did not have an impact on the chemotherapeutic effect. Four of 6 patients with full-dose GC showed responses, while 5 of 7 patients with reduced-dose GC also showed responses, in contrast to young patients in whom the response rate in the low-dose group was significantly lower than the full-dose group (3/20, 15 % vs 43/72, 59.7 %), respectively; p < 0.001].
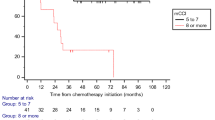
Regarding the adverse effects of GC chemotherapy, there was no significant difference between young and elderly patients according to the National Cancer Institute Common Terminology Criteria (NCI-CTC) classification (Table 4). However, when we analyzed the changes in serum creatinine levels before and after the first cycle of GC chemotherapy [(final creatinine level − initial creatinine level)/initial creatinine level × 100 (%)] to evaluate the influence of the GC regimen on renal function, it was significantly higher in the elderly (1.5 % in young vs 20 % increase in elderly patients; p = 0.0018), indicating that renal damage, which did not emerge according to CTCAE evaluation, may be more severe in elderly patients (Fig. 1). In contrast, there was no remarkable change in serum creatinine level among patients receiving MVAC/MEC or other regimens regardless of their age (data not shown).
As a clinical course, five of 30 elderly patients (16.7 %) received second-line chemotherapy; three of the 5 patients received the GP regimen and the other two patients received the MVAC or GDCarbo regimen. The 1- and 2-year OS rates in the elderly were 54.1 and 29.9 %, respectively, which were not significantly different from those of young patients (46.3 and 24.6 %, respectively).
Prognostic factors in elderly patients with advanced urothelial cancer treated with systemic chemotherapy
To identify the prognostic factors in elderly patients receiving systemic chemotherapy, the seven prognostic variables listed in Table 5 were examined. When we examined the data in univariate analyses, we found that distant metastases, eGFR (>60 vs <60 ml/min/1.73 m2), the chemotherapy regimens (GC vs other), and the effect of first-line chemotherapy (CR + PR vs NC + PD) were significant prognostic factors for OS. On a multivariate analysis using those four variables, the presence of distant metastases and eGFR (>60 vs <60 ml/min/1.73 m2) were independent prognostic factors (p = 0.0285 and 0.0346, respectively). Treatment with GC was not found to be a significant prognostic factor but had a marginal effect on the prognosis (p = 0.0545). On the other hand, the presence of dose reduction, visceral metastasis and poor PS ≥2 were independently poor prognostic factors in young patients and were the same as those in the whole study population [8].
Discussion
In this study, we retrospectively reviewed the current status of systemic chemotherapy in Japanese octogenarians with advanced UC. Among all 345 registered patients in the CURE study, 30 (9 %) were aged ≥80 years. When changes in the percentage of the elderly were evaluated before and after 2008, it increased from 4.6 to 12 % after the use of gemcitabine for UC began to be reimbursed by public health insurance in Japan. Our results showed that the selection pattern of first-line chemotherapy in the elderly also changed after 2008. Only 14.3 % received MVAC chemotherapy, which was the standard regimen for UC before 2008, while 6 patients received other regimens. On the other hand, 56.5 % of the elderly received GC regimens, which have become the new standard in chemotherapy after 2008. This rate was similar to that of young patients in the same era, indicating that there was a trend change in which selected elderly patients have been able to safely receive standard systemic chemotherapy like young patients in the GC era.
Furthermore, the dose reduction rate also increased in the elderly. We speculate that this observation is due to clinicians paying more attention to renal impairment or low PS in the elderly. In other words, the clinicians give weight to the prolongation of active life expectancy, the compression of morbidity, and functional preservation, in addition to the prolongation of survival and symptom management in chemotherapy in the elderly. We speculate that another reason for dose reduction is that the various methods of evaluating renal function among each institute may have influenced the selection of patients who are unfit for cisplatin [12–14]. A future study to determine which method is appropriate for the evaluation of renal function in elderly patients receiving chemotherapy must be conducted.
There is no significant difference in the efficacy of chemotherapy or post-chemotherapeutic OS rates between young and elderly patients. Interestingly, among the GC-treated population, dose reduction had no influence on the efficacy in elderly patients, unlike young patients. Inappropriate dose reduction commonly leads to under-treatment, but the National Comprehensive Cancer Network guidelines suggested that the first dose of chemotherapy should be adjusted to the GFR in individuals aged ≥65, and in the absence of serious toxicity, successive chemotherapy doses should be escalated to prevent the risk of under-treatment [15]. Although the sample size was small, our result may also reflect that it is most important to control adverse effects in the chemotherapy of elderly patients even if the dose is reduced. In this regard, we found that when we compared the serum creatinine changes in young and elderly GC-treated patients during the first cycle, creatinine levels were significantly increased only in the elderly even if the dose reduction rate was higher. Thyss et al. [16] reported that cisplatin at moderate doses can be administered reasonably to patients aged >80 years, but our results indicate that dose adjustment is strongly recommended in the elderly for agents whose parent compounds are completely or partially excreted by the kidneys and also for agents that give origin to renally excreted active or toxic metabolites. Recently, EORTC 30986 revealed that GCarbo is equivalent to carboplatin/methotrexate/vinblastine (M-CAVI) in efficacy with less adverse effects in cisplatin-unfit patients [17, 18]. In this study, the number of those receiving the GCarbo regimen was very low, but whether we should select lower-dose GC or GCarbo regimens for elderly patients should be investigated in the future.
Our multivariate analysis of survival revealed that distant metastasis and renal impairment (eGFR <60 ml/min/1.73 m2) were independent unfavorable prognostic factors in the elderly, while dose reduction, visceral metastases, and lower PS were independent unfavorable prognostic factors in young patients and were the same as those in the whole study population [8]. Our results indicate that the status of the disease and the physiological condition of patients become more important as the patient ages. In particular, the maintenance of renal function, as it would be more crucial to administer systemic chemotherapy in the elderly compared to the young. Taking into account comorbidities, PS, geriatric functional status including renal function, and the status of the disease, we need to develop ways to guide our decision-making for optimal therapy in the elderly with advanced UC.
Our analysis has several limitations. Many potential biases resulting from the retrospective design of the analysis must be taken into account. Although we examined a relatively large population of metastatic UC patients treated with systemic chemotherapy, the sample size of the elderly was still small. We were not able to estimate the ratio of elderly patients who could receive systemic chemotherapy to the total number of elderly patients with advanced or unresectable UC. Furthermore, we did not investigate which treatment options were performed for patients uninvolved in this study. There may also have been differences in therapeutic flow such as decision-making by physicians, the protocol of the chemotherapy regimen, and the follow-up protocol among the institutions that collaborated with us. Information about supportive care for chemotherapy such as anti-emesis drugs, fluid infusion and hospitalization were lacking. Furthermore, our evaluation of older individuals based on geriatric assessment and other parameters reflecting physiologic age (such as inflammatory cytokines) was not uniform, which may allow for the investigation of prognostic factors in clinical practice. Today, all patients aged ≥70 years are recommended to undergo some form of geriatric assessment such as the Comprehensive Geriatric Assessment [19, 20]. However, we believe that this retrospective data analysis could help in conducting prospective clinical trials for the management of advanced UC in the future. In this study, we recognized that GC regimens can be safely administered and are beneficial for selected elderly patients with advanced UC; however, dose reduction is necessary because of renal impairment, a physiologic change appearing commonly with advanced age. If we could make an individualized assessment of the condition of elderly patients, there is the possibility that advanced age would not be an exclusion criterion in clinical trials of advanced UC.
In conclusion, this retrospective multi-institutional study showed that current GC chemotherapy with supportive care has enabled selected elderly patients to receive systemic multidrug chemotherapy like young patients, and the clinical response rate and OS rate were similar between the two populations. Our results may support future prospective clinical trials to determine the population that can receive the benefits of chemotherapy, and the best regimen or appropriate dosage for elderly patients.
Abbreviations
- UC:
-
Urothelial carcinoma
- GC:
-
Gemcitabine and cisplatin
- MVAC/MEC:
-
Methotrexate, vinblastine, adriamycin, and cisplatin/methotrexate, epirubicin, and cisplatin
- GP:
-
Gemcitabine and paclitaxel
- CR:
-
Complete response
- PR:
-
Partial response
- PD:
-
Progressive disease
- NC:
-
No change
- MDRD:
-
Modification of Diet in Renal Disease
- PS:
-
Performance status
References
Serrano M, Blasco MA (2007) Cancer and ageing: convergent and divergent mechanisms. Nat Rev Mol Cell Biol 8:715–722
Surveillance Research Program SEER stat fact sheets: Bladder Cancer. National Cancer Institute. http://seer.cancer.gov/statfacts/html/urinb.html
Rose TL, Milowsky MI (2015) Management of muscle-invasive bladder cancer in the elderly. Curr Opin Urol 25:459–467
Sternberg CN, Yagoda A, Scher HI et al (1988) M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) for advanced transitional cell carcinoma of the urothelium. J Urol 139:461–469
Duthie E (2004) Physiology of age: relevance to symptoms, perceptions, and treatment tolerance. In: Balducci L, Lyman GH, Ershler WB et al (eds) Comprehensive geriatric oncology. Taylor and Francis, London, pp 207–223
Balducci L (2008) Cancer chemotherapy in the older person. In: Balducci L, Ershler B, DeGaetano G (eds) Blood disorders in the elderly. University Press, Cambridge, pp 225–236
von der Maase H, Hansen SW, Roberts JT et al (2000) Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 18:3068–3077
Ichioka D, Miyazaki J, Inoue T et al (2015) Impact of renal function of patients with advanced urothelial cancer on eligibility for first-line chemotherapy and treatment outcomes. Jpn J Clin Oncol 45:867–873
Kikuchi E, Miyazaki J, Yuge K et al (2016) Do metastatic upper tract urothelial carcinoma and bladder carcinoma have similar clinical responses to systemic chemotherapy? A Japanese multi-institutional experience. Jpn J Clin Oncol 46:163–169
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Matsuo S, Imai E, Horio M et al (2009) Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53:982–992
Goto T, Yoshimura K, Matsui Y et al (2011) Impact of different methods of estimating renal function on determining eligibility for cisplatin (CDDP)-based chemotherapy in patients with invasive urothelial carcinoma. Hinyokika Kiyo 57:671–676
Kaag MG, O’Malley RL, O’Malley P et al (2010) Changes in renal function following nephroureterectomy may affect the use of perioperative chemotherapy. Eur Urol 58:581–587
Lane BR, Smith AK, Larson BT et al (2010) Chronic kidney disease after nephroureterectomy for upper tract urothelial carcinoma and implications for the administration of perioperative chemotherapy. Cancer 116:2967–2973
Balducci L, Cohen HJ, Engstrom PF et al (2005) Senior adult oncology clinical practice guidelines in oncology. JNCCN 3:572–590
Thyss A, Saudes L, Otto J et al (1994) Renal tolerance of cisplatin in patients more than 80 years old. J Clin Oncol 12:2121–2125
De Santis M, Bellmunt J, Mead G et al (2009) Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer “unfit” for cisplatin-based chemotherapy: phase II—results of EORTC study 30986. J Clin Oncol 27:5634–5639
De Santis M, Bellmunt J, Mead G et al (2012) Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol 30:191–199
Carreca I, Balducci L (2009) Cancer chemotherapy in the older cancer patient. Urol Oncol 27:633–642
Guancial EA, Roussel B, Bergsma DP et al (2015) Bladder cancer in the elderly patient: challenges and solutions. Clin Interv Aging 10:939–949
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
About this article
Cite this article
Matsui, Y., Ogawa, O., Ishitsuka, R. et al. Current status of systemic chemotherapy for octogenarians with advanced urothelial cancer in Japan: a Japanese multi-institutional study (CURE study) . Int J Clin Oncol 21, 1142–1149 (2016). https://doi.org/10.1007/s10147-016-1007-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-016-1007-8