Abstract
Background
Control of serum uric acid (sUA) levels is very important during chemotherapy in patients with malignant tumors, as the risks of tumor lysis syndrome (TLS) and renal events are increased with increasing levels of sUA. We investigated the efficacy and safety of febuxostat, a potent non-purine xanthine oxidase inhibitor, compared with allopurinol for prevention of hyperuricemia in patients with malignant tumors, including solid tumors, receiving chemotherapy in Japan.
Methods
An allopurinol-controlled multicenter, open-label, randomized, parallel-group comparative study was carried out. Patients with malignant tumors receiving chemotherapy, who had an intermediate risk of TLS or a high risk of TLS and were not scheduled to be treated with rasburicase, were enrolled and then randomized to febuxostat (60 mg/day) or allopurinol (300 or 200 mg/day). All patients started to take the study drug 24 h before chemotherapy. The primary objective was to confirm the non-inferiority of febuxostat to allopurinol based on the area under the curve (AUC) of sUA for a 6-day treatment period.
Results
Forty-nine and 51 patients took febuxostat and allopurinol, respectively. sUA decreased over time after initiation of study treatment. The least squares mean difference of the AUC of sUA between the treatment groups was −33.61 mg h/dL, and the 95 % confidence interval was −70.67 to 3.45, demonstrating the non-inferiority of febuxostat to allopurinol. No differences were noted in safety outcomes between the treatment groups.
Conclusion
Febuxostat demonstrated an efficacy and safety similar to allopurinol in patients with malignant tumors receiving chemotherapy.
Trial registry
http://www.clinicaltrials.jp; Identifier: JapicCTI-132398.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tumor lysis syndrome (TLS) presents with various pathological conditions including hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia, and may lead to life-threatening complications such as acute renal failure [1–3]. TLS is caused by the rapid destruction of tumor cells usually associated with anticancer chemotherapy, followed by accumulation of intracellular components and their metabolites in the bloodstream beyond metabolizing and excreting capacity. TLS occurs most frequently in patients with hematological malignancies, but it may occur in any cancer, including bulky solid tumors which are sensitive to chemotherapy (e.g., neuroblastoma, germ-cell tumors, and small-cell lung cancers) [2].
It is important to provide patients with preventive treatments appropriate to the estimated risk of TLS based on the type of malignant tumor, tumor load, treatment modality, and the patient’s renal function [1–3]. During anticancer chemotherapy, controlling the serum uric acid (sUA) level is very important because acute renal failure due to crystallization of UA in the renal tubules is one of the most serious complications of TLS. The risk of TLS and renal events are increased by 1.75-fold and by 2.21-fold, respectively, with every milligram per deciliter increase in UA levels [1]. In addition to hydration, it is recommended to use allopurinol or rasburicase for prophylaxis of TLS, mainly in patients with an intermediate or high risk of developing TLS [2, 3]. Allopurinol is a purine analogous xanthine oxidase (XO) inhibitor which reduces the production of UA. However, hypersensitivity reactions such as rash, acute hepatitis, and eosinophilia are sometimes experienced. Furthermore, dose reductions are required in patients with renal failure [4]. Rasburicase is a recombinant form of urate oxidase which metabolizes UA to allantoin. Although it acts immediately and is extremely effective, its use is sometimes limited due to immunogenicity and prohibitive cost [5, 6]. Moreover, rasburicase should be avoided in patients with glucose-6-phosphate dehydrogenase deficiency.
Febuxostat is a potent non-purine structured selective XO inhibitor, which prevents the inhibition of other enzymes involved in purine and pyrimidine metabolism. It is known to be well tolerated in patients even with moderate renal impairment, while its effect on lowering sUA remains unaffected.
While allopurinol is administered three times a day, febuxostat shows potent urate-lowering effects as a once-daily treatment for gout or hyperuricemia. Furthermore, once the efficacy and safety of febuxostat has been established to be similar to allopurinol, febuxostat is likely to become a more favorable treatment for TLS prevention. Recent reports showed febuxostat would successfully prevent TLS in patients with malignant tumors [7–9]. However, since the patients enrolled in these studies were limited to those with hematological malignancies, the efficacy and safety of febuxostat remain unclear in patients with solid tumors. In addition, there have been no reports of randomized clinical trials evaluating febuxostat to prevent TLS in Japan. Therefore, we conducted a randomized trial to evaluate the efficacy and safety of febuxostat compared with allopurinol in terms of prevention of TLS in patients with malignant tumors, including solid tumors, receiving chemotherapy.
Patients and methods
Study design
This was an allopurinol-controlled, multicenter, open-label, randomized, parallel-group comparative phase III study conducted at 24 facilities in Japan from January 2014 to September 2014. The primary objective was to confirm the non-inferiority of febuxostat to allopurinol based on the area under the curve (AUC) of sUA over a 6-day treatment period to evaluate whether these medications have similar efficacy.
Patients
Eligible patients were aged ≥20 years, scheduled to receive a first cycle of chemotherapy (antineoplastic agents including molecular targeted drugs) for malignant tumors, had an intermediate risk of TLS or a high risk of TLS and who were not scheduled to be treated with rasburicase. Necessity of rasburicase treatment was decided at the investigator’s discretion based on contraindications, the patient’s condition (e.g., sUA, renal function, or tumor mass), and planned chemotherapy regimen. TLS risk was assessed according to the recommendations [2]. The main exclusion criteria were sUA ≥10.0 mg/dL, presence of laboratory TLS (LTLS) or clinical TLS (CTLS) before receiving the treatment, a history of allergic reaction to the study drugs, presence of gouty arthritis, Eastern Cooperative Oncology Group performance status of ≥3, and significant renal dysfunction with an estimated glomerular filtration rate (eGFR) of <30 mL/min/1.73 m2. Each patient provided fully informed written consent before enrollment. The study was approved by each site’s institutional review board and was carried out according to the Good Clinical Practice and principles set out in the Declaration of Helsinki 1964 and all subsequent revisions.
Treatments
After enrollment, patients were randomly assigned in a 1:1 ratio to either the febuxostat or allopurinol group by Medidata Balance® (Medidata Solutions, Inc., NY, USA). Randomization was performed with a minimization method [10] to minimize the imbalance of baseline sUA, TLS risk, and primary disease. Febuxostat was administered 60 mg orally once daily (60 mg/day). Allopurinol was administered 100 mg orally 3 times daily (300 mg/day), except in patients with moderate or severe renal impairment (eGFR ≥30 to <45 mL/min/1.73 m2) who received allopurinol 100 mg twice daily (200 mg/day). All patients were started on the study drug at 24 h before chemotherapy and continued for at least 6 days. The study drug could be taken for 14 days maximum according to the investigator’s discretion. Any concomitant medications that might affect sUA, such as rasburicase or benzbromarone, were prohibited during the study, except for fluid replacement.
Efficacy and safety endpoint
The primary endpoint was the AUC of sUA from day −1 to day 6. Day −1 (baseline) was the day of randomization and starting the study drug, day 1 was the day of starting chemotherapy, and day 6 was the 6th day after chemotherapy. Central laboratory evaluation was used for primary efficacy measurements. Secondary endpoints were serum levels of UA, lactate dehydrogenase (LDH), serum creatinine, and serum electrolytes (K, Ca, and P), number of patients whose sUA exceeded the upper limit of normal on ≥2 consecutive days, and number of patients who developed LTLS or CTLS according to the established criteria [2]. Incidence and grade of adverse events (AEs), vital signs, standard 12-lead electrocardiograms, and time courses of laboratory test values were also assessed. The severities of AEs were assessed using Common Terminology Criteria for Adverse Events version 4.0.
Statistical analysis
The primary analysis set for efficacy evaluation was the full analysis set (FAS). For sensitivity analysis, analyses were repeated for the per protocol set (PPS).
For the primary endpoint, the two-sided 95 % confidence interval (CI) of the difference in the AUC of sUA between treatment groups was calculated by adjusting for the baseline sUA. We set the non-inferiority margin at 150 mg h/dL. Since sufficient information regarding the time-course changes of serum UA levels after placebo treatment was not available, we assumed that patients would maintain their baseline serum UA levels as a conservative case. In this instance, 150 mg h/dL is equivalent to half of the difference between assumed sUA AUC after placebo treatment and expected AUC after allopurinol treatment. Furthermore, even if the AUC is elevated to 150 mg h/dL after chemotherapy, persistent hyperuricemia would not be expected. Thus, an AUC of 150 mg h/dL was considered appropriate in this study. For missing data, the last observation carried forward method was applied to patients who discontinued the study, and a linear interpolation method was used to impute the missing data between visits.
Using a sample size of 47 for each group, a two-group t test with a one-sided significance level of 0.025 would have 80 % power to show non-inferiority with a pre-determined non-inferiority margin of 150 mg h/dL, assuming that the expected difference in means was 0 and the common standard deviation was 255 mg h/dL. The sample size of this study was set at 100 patients in 2 groups with 50 patients in each group, including dropouts.
Results
Patients
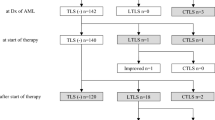
A total of 104 patients were screened and 100 patients were enrolled from January 2014 to September 2014. Forty-nine and 51 patients were randomized to the febuxostat group and the allopurinol group, respectively, and all of the patients took the study drug (Fig. 1). One patient in the allopurinol group was excluded from all analyses due to a lack of written consent. Thus, data from 99 patients were available for FAS analysis, although two patients in the allopurinol group discontinued the study soon after initiation of study treatment. For PPS analysis, data from 91 patients were available. Eight patients (4 patients in each group) were excluded from PPS due to protocol violations which included concomitant use of a prohibited drug, low drug adherence rate, and missing important data.
Demographic and other baseline characteristics for the FAS are presented in Table 1. No remarkable differences were observed between the two groups. The median age was 69.0 (range 22–87) years, median sUA was 5.40 mg/dL, and approximately 20 % of patients had solid tumors. About 30 % of patients were classified as being at a high risk of TLS, and all other patients were at an intermediate risk. In the allopurinol group, three of 50 patients were administered 200 mg/day due to renal impairment.
sUA
The level of sUA decreased over time after initiation of the study treatment (day −1) and became constant from day 4 for each group (Fig. 2). For the febuxostat group, sUA (mean ± standard deviation) was 5.65 ± 1.35 mg h/dL at baseline, 3.08 ± 1.34 mg h/dL at day 3, and 2.55 ± 1.20 mg h/dL at day 6. For the allopurinol group, sUA was 5.52 ± 1.76 mg h/dL at baseline, 3.12 ± 1.29 mg h/dL at day 3, and 2.60 ± 0.94 mg h/dL at day 6. sUA exceeding the upper limit of normal (7.0 mg/dL) on ≥2 consecutive days was noted in one patient in the febuxostat group (2.0 %) and three patients in the allopurinol group (6.0 %); this difference was not statistically significant (Fisher’s exact test; p = 0.617). None of the patients with sUA of ≤7.0 mg/dL at baseline had an increase in sUA exceeding the upper limit of normal after initiation of the study treatment.
The AUC of sUA (least squares mean ± standard error) was 479.82 ± 13.26 mg h/dL in the febuxostat group and 513.44 ± 13.13 mg h/dL in the allopurinol group (Table 2). The least squares mean difference between the two groups was −33.61 mg h/dL, with a 95 % CI of −70.67 to 3.45. The upper bound was well below the non-inferiority margin of 150 mg h/dL, demonstrating the non-inferiority of febuxostat against allopurinol. The results were fully confirmed in the PPS and subgroups of patients with different baseline characteristics in terms of sUA, risk of TLS, primary disease, serum creatinine, and eGFR (Table 3).
TLS
Development of LTLS or CTLS during the period from the initiation of the study drug until day 6 was noted in one patient (2.0 %) in the febuxostat group and two patients (4.0 %) in the allopurinol group; this difference was not statistically significant (Fisher’s exact test; p = 1.000). Of the three patients with LTLS, only one patient (allopurinol 200 mg/day) met the criteria for evaluation of LTLS due to sUA exceeding the upper limit of normal. The maximum sUA in this patient was 9.7 mg/dL at baseline, followed by a decrease over time; however, it remained above the upper limit of normal (7.0 mg/dL) up to day 3. This patient met the criteria for LTLS since phosphorus also exceeded the upper limit of normal on day 2 and day 3. The other two patients met the criteria of LTLS since the potassium and phosphorus exceeded the upper limit of normal. No patients developed CTLS.
Other laboratory values
LDH decreased over time after initiation of chemotherapy. Serum creatinine and electrolytes (K, Ca, and P) remained within normal limits in most patients. No significant differences were noted in these laboratory tests results between treatment groups.
Safety
The safety analysis included 99 patients (49 in the febuxostat group, 50 in the allopurinol group). AEs occurred in 46 patients in the febuxostat group (93.9 %; 252 events) and 48 patients in the allopurinol group (96.0 %; 304 events). AEs which were considered to be related to the study drug occurred in one patient in each of both groups, and were associated with elevated serum transaminase levels. The AEs were Grade 1 in severity. One patient recovered after treatment, and the other had no follow-up results. No differences were noted in the incidence of AEs between the treatment groups.
The AEs in both groups were generally similar to those expected during chemotherapy. Grade ≥3 AEs occurred in 38 patients (77.6 %; 83 events) in the febuxostat group and 38 patients (76.0 %; 108 events) in the allopurinol group. In both groups, leukopenia, neutropenia, and febrile neutropenia were commonly seen due to bone marrow toxicity from chemotherapy not related to the study drugs. One patient in the allopurinol group experienced attacks of gout. This complication was not thought to be a direct effect of allopurinol. No remarkable differences between the treatment groups were observed when the subgroup analyses for AEs were stratified by demographic and other baseline characteristics. There was no mortality in this study. One patient in the allopurinol group discontinued treatment due to diarrhea. Two patients in the allopurinol group suffered sepsis and cryptococcal fungemia, respectively. A causal relationship to the study drug was not considered applicable.
Discussion
We evaluated the efficacy and safety of febuxostat for prevention of hyperuricemia as one of the important laboratory findings in TLS when patients with malignant tumors, including solid tumors, are to receive chemotherapy. Febuxostat demonstrated similar efficacy and safety to allopurinol in the present study. With regard to efficacy, febuxostat 60 mg/day resulted in decreased sUA and maintained low levels of sUA up to day 6. None of the patients developed TLS, which is caused partly by a high blood concentration of UA after initiation of study treatment in the febuxostat group. In addition, non-inferiority of febuxostat to allopurinol (the primary endpoint) was confirmed based on the AUC of sUA from day −1 to day 6. It is important to note that the efficacy was the result of prevention rather than treatment, as the study drugs were administered 24 h before chemotherapy. Treatment efficacy for TLS needs to be investigated in further studies. From a clinical point of view, control of electrolytes is also important for TLS prevention. High sUA can cause renal dysfunction which leads to electrolyte imbalance. It is important to note that no renal dysfunction occurred and electrolyte levels were well controlled in both the febuxostat and allopurinol groups in this study. With regard to safety, the AEs in this study were similar to the complications which are commonly observed in patients receiving chemotherapy. Since the dose of allopurinol needs to be adjusted according to kidney function, and has to be administered 2–3 times daily, a once-daily fixed dose of febuxostat appears to be more useful and advantageous.
This study has some limitations—it enrolled a relatively small number of patients, was an open-label study, and was limited only to sites in Japan. However, recently, Spina et al. showed that febuxostat (120 mg/day) achieved statistically significant superiority in reduction of sUA over allopurinol (200–600 mg/day) in patients with hematologic malignancies in a large randomized, double-blind phase III trial (FLORENCE study) [9] conducted in a total of 346 patients across 11 European countries and Brazil. Takai et al. also evaluated the hypouricemic effect of febuxostat (40–60 mg/day) prospectively in patients undergoing chemotherapy in Japan, although the number of patients was small and the study was not randomized [7]. Both reports also revealed that febuxostat sufficiently decreased sUA and successfully prevented development of TLS. Regarding kidney function, Takai et al. showed a simultaneous decrease in serum creatinine and increase in eGFR, while Spina et al. reported no change in serum creatinine or creatinine clearance. In our study, creatinine remained constant for both treatment groups. Thus, further studies are needed to evaluate the effect of febuxostat administration on the relationship between kidney function and sUA levels.
Although these two earlier studies were conducted in patients with hematological malignancies only, the primary disease was not limited in our study. As a result, 23 patients (approximately 20 %) with solid tumors were enrolled (11 patients in the febuxostat group and 12 in the allopurinol group). There was no statistically significant difference in the AUC of sUA for underlying malignancies between the study drugs. No TLS occurred in patients with solid tumors in this study, supporting the conclusion that both febuxostat and allopurinol are equally effective for prevention of TLS in patients with solid tumors, as in hematological malignancies.
Febuxostat is approved in Japan to treat hyperuricemia or gout with a dose escalation method employed to reduce the risk of gout attacks associated with a rapid decrease in sUA. By contrast, in patients with malignant tumors scheduled to receive chemotherapy, sUA needs to be lowered quickly and sufficiently enough to prevent the development of TLS. Thus, febuxostat was given up to the maximum dose approved for hyperuricemia and gout in Japan without using a dose escalation method. No patients experienced gouty attacks in the febuxostat group, and there were no other safety concerns, indicating that a fixed dose of 60 mg/day is appropriate to prevent TLS in patients undergoing chemotherapy in Japan.
In conclusion, our study showed that febuxostat is similar to allopurinol in terms of controlling sUA regardless of TLS risk, baseline sUA before chemotherapy, or primary disease, including solid tumors. In addition, both study drugs are generally tolerable. Therefore, this study suggests that febuxostat is effective and safe for the prevention of TLS in patients with malignant tumors who are to receive chemotherapy.
References
Coiffier B, Altman A, Pui CH et al (2008) Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J Clin Oncol 26:2767–2778
Cairo MS, Coiffier B, Reiter A et al (2010) TLS Expert Panel. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol 149:578–586
Jones GL, Will A, Jackson GH et al (2015) Guidelines for the management of tumour lysis syndrome in adults and children with haematological malignancies on behalf of the British Committee for Standards in Haematology. Br J Haematol 169:661–671
Ramasamy SN, Korb-Wells CS, Kannangara DR et al (2013) Allopurinol hypersensitivity: a systematic review of all published cases, 1950-2012. Drug Saf 36:953–980
Allen KC, Champlain AH, Cotliar JA et al (2015) Risk of anaphylaxis with repeated courses of rasburicase: a Research on Adverse Drug Events and Reports (RADAR) project. Drug Saf 38:183–187
Lopez-Olivo MA, Pratt G, Palla SL et al (2013) Rasburicase in tumor lysis syndrome of the adult: a systematic review and meta-analysis. Am J Kidney Dis 62:481–492
Takai M, Yamauchi T, Ookura M et al (2014) Febuxostat for management of tumor lysis syndrome including its effects on levels of purine metabolites in patients with hematological malignancies - a single institution’s, pharmacokinetic and pilot prospective study. Anticancer Res 34:7287–7296
Maie K, Yokoyama Y, Kurita N et al (2014) Hypouricemic effect and safety of febuxostat used for prevention of tumor lysis syndrome. SpringerPlus 3:501
Spina M, Nagy Z, Ribera JM et al (2015) FLORENCE: a randomized, double blind, phase III pivotal study of febuxostat versus allopurinol for the prevention of tumor lysis syndrome (TLS) in patients with hematologic malignancies at intermediate to high TLS risk. Ann Oncol 26:2155–2161
Lebowitsch J, Ge Y, Young B et al (2012) Generalized multidimensional dynamic allocation method. Stat Med 31:3537–3544
Acknowledgments
This study was conducted by the following physicians and study centers—Kenichi Ishizawa and Yoko Okitsu (Tohoku University Hospital); Osamu Sasaki (Miyagi Cancer Center); Makoto Maemondo (Miyagi Cancer Center); Masahiro Kizaki (Saitama Medical Center); Noriko Doki (Komagome Hospital); Hiroki Yamaguchi (Nippon Medical School Hospital); Kaoru Kubota (Nippon Medical School Hospital); Kentaro Watanabe (Tokyo Saiseikai Central Hospital); Hironori Ueno (Tokyo Medical Center); Akira Fujita (Showa General Hospital); Katsumichi Fujimaki (Fujisawa City Hospital); Yasukazu Kawai (Fukui Prefectural Hospital); Takashi Nakayama (Fukui-ken Saiseikai Hospital); Keita Kirito (University of Yamanashi); Masataka Okamoto (Fujita Health University Hospital); Toshiki Uchida (Japanese Red Cross Nagoya Daini Hospital); Hirokazu Nagai and Hiroatsu Iida (Nagoya Medical Center); Mitsuru Itoh (Kyoto City Hospital); Masaya Okada (Hyogo College of Medicine); Junji Suzumiya (Shimane University Faculty of Medicine); Kenichi Gemba (Fukuyama Medical Center); Toru Kiguchi (Chugoku Central Hospital); Tetsuya Goto (Tokushima Red Cross Hospital); Masahiko Kaneko (Uwajima City Hospital); Tomoaki Fujisaki (Matsuyama Red Cross Hospital); and Kazuo Tamura (Fukuoka University Hospital). This study was funded by Teijin Pharma Ltd., Tokyo, Japan. Teijin Pharma was involved in the design, conduct, and analysis of the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study was funded by Teijin Pharma Ltd., Tokyo, Japan. Kazuo Tamura was the Coordinating Investigator and Takanori Ueda was the Medical Expert for this study. In addition, Takanori Ueda received honoraria, fees for promotional materials, and research funding from Teijin Pharma Ltd. Toru Kiguchi received honoraria from Teijin Pharma Ltd. Kenji Takeda and Akihiro Nakajima are employees of Teijin Pharma Ltd. The other authors have no conflict of interest to declare.
About this article
Cite this article
Tamura, K., Kawai, Y., Kiguchi, T. et al. Efficacy and safety of febuxostat for prevention of tumor lysis syndrome in patients with malignant tumors receiving chemotherapy: a phase III, randomized, multi-center trial comparing febuxostat and allopurinol . Int J Clin Oncol 21, 996–1003 (2016). https://doi.org/10.1007/s10147-016-0971-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-016-0971-3