Abstract
Background
The prognosis for locally advanced gastric cancer (AGC) remains unsatisfactory, even with S-1 adjuvant chemotherapy. We investigated the efficacy of neoadjuvant chemotherapy consisting of docetaxel, cisplatin and S-1 (DCS).
Methods
We retrospectively reviewed 59 patients who underwent neoadjuvant DCS therapy for clinical stage III tumors or serosa-positive tumors between January 2009 and December 2013 at Niigata Cancer Center Hospital. The patients received S-1 (40 mg/m2 bid) on days 1–14, and docetaxel (35 mg/m2) and cisplatin (35 mg/m2) on days 1 and 15 every 4 weeks.
Results
Forty-three patients (72.9 %) received two courses of DCS therapy, while 16 patients (27.1 %) received one course of treatment. The clinical response rate of the primary tumor was 74.6 %, and the disease control rate was 100 %. A pathological response, defined as one-third or more of the affected tumor, was observed in 71.2 % of patients. The common grade 3/4 adverse events from chemotherapy were leucopenia (16.9 %), neutropenia (44.1 %), febrile neutropenia (8.5 %), anemia (10.2 %), anorexia (8.5 %) and nausea (6.8 %). Postoperative complications occurred in 11 patients (18.6 %). There was no treatment-related mortality or reoperation. The 3- and 5-year overall survival rates were 88 and 68.6 %, respectively. Clinical responders had a significantly higher survival rate than non-responders. Multivariate analysis identified clinical response as the only independent prognostic factor.
Conclusions
Neoadjuvant DCS therapy demonstrated a very high clinical and pathological response rate with acceptable toxicities. Therefore, this therapy may improve the prognosis of locally AGC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although surgery is the standard treatment for resectable gastric cancer, the prognosis for patients with advanced gastric cancer (AGC) remains poor [1]. Therefore, a multimodal treatment strategy needs to be developed and established to improve patient outcomes.
In Western countries, the survival benefit of perioperative chemotherapy using a combination chemotherapy of ECF therapy [epirubicin, cisplatin and 5-fluorouracil (5-FU)] or CF therapy (cisplatin and 5-FU) has been confirmed by phase III studies, and perioperative chemotherapy has become the standard treatment for AGC [2, 3]. However, the 5-year survival rate remains <40 % in these trials. In Japan, postoperative adjuvant chemotherapy using S-1 for 12 months has been established as the standard treatment after D2 gastrectomy in patients with stage II and III disease based on a large phase III study [4]. However, even with D2 gastrectomy and subsequent adjuvant chemotherapy with S-1, the long-term survival of patients with stage III tumors and serosa-positive tumors remains unsatisfactory, indicating that improved therapeutic strategies are necessary [5]. Recently, a more intensive regimen has been tested as an adjuvant regimen for AGC, i.e., S-1 + cisplatin (SC), which has demonstrated a significantly higher response rate and longer survival than S-1 alone in SPIRITS trials [6]. These studies showed that SC therapy was associated with low compliance and high adverse events in an adjuvant setting [7, 8]. These findings suggested that postoperative intensive chemotherapy might be difficult to complete.
On the other hand, neoadjuvant chemotherapy (NAC) has some theoretical benefits, including downstaging of the tumor, eliminating micrometastasis, evaluating sensitivity of chemotherapy, and improving compliance against more intensive chemotherapy, when compared with postoperative chemotherapy [9]. Indeed, several phase II studies, such as SC, S-1 + docetaxel, paclitaxel + cisplatin, and CPT-11 + cisplatin, were safe and feasible in the neoadjuvant setting [10–15]. Moreover, promising survival results were reported in these trials. Accordingly, NAC is expected to improve long-term prognoses of gastric cancer. Recently, a triplet regimen with docetaxel, cisplatin and S-1 (DCS) has been shown to have a very high response rate and promising median survival time in patients with unresectable AGC [16–18]. Therefore, DCS therapy has been anticipated as a more powerful regimen for the neoadjuvant setting.
From January 2009, we have adopted neoadjuvant DCS therapy for locally AGC. In the present study, we evaluated the efficacy of neoadjuvant DCS therapy in patients with clinically resectable locally AGC.
Patients and methods
Patients
To December 2013, 59 consecutive patients underwent neoadjuvant DCS therapy at Niigata Cancer Center Hospital, and their data were retrospectively analyzed.
The eligibility criteria were histologically proven gastric adenocarcinoma, the tumor penetrating the serosa (se) or clinical stage III tumor according to the second edition of the Japanese Classification of Gastric Carcinoma [19], Eastern Cooperative Oncology Group performance status 0–1, no previous chemotherapy or radiotherapy, no uncontrolled infection or cardiopulmonary disease, and adequate bone marrow, renal and hepatic function. The initial clinical evaluation was performed by upper gastrointestinal endoscopy, computed tomography (CT) with contrast, and an upper gastrointestinal barium meal study. In principle, a general laparoscopic examination of the abdominal cavity was carried out before starting the treatment. This study protocol was approved by the institutional review board of the hospital, and written informed consent was obtained from all participants.
Neoadjuvant DCS chemotherapy and surgery
S-1 was given orally twice daily for the first 2 weeks of a 4-week cycle, which was calculated according to the patient’s body surface area as <1.25 m2 = 40 mg; 1.25–1.5 m2 = 50 mg; and >1.5 m2 = 60 mg. Docetaxel and cisplatin was given as an intravenous infusion of 35 mg/m2 on days 1 and 15 of each cycle. Two courses of DCS therapy were generally planned; however, it was discontinued if there was documented disease progression, unacceptable toxicity, or withdrawal of consent.
The objective response to chemotherapy was evaluated, and surgical resection was then performed 2–4 weeks after completion of the last course unless curative resection was considered difficult. Depending on the location of the primary tumor, the surgical procedure selected was either total gastrectomy or distal gastrectomy with D2 lymphadenectomy. Splenectomy and/or distal pancreatectomy were not carried out unless there was direct tumor invasion or metastasis to the lymph nodes at the splenic hilum.
Clinical response and histological evaluation of surgical specimen
Adverse events were evaluated by the National Cancer Institute Common Toxicity Criteria version 4.0. Surgical complications were assessed according to the Clavien−Dindo classification [20]. Tumors were staged based on the second edition of the Japanese Classification of Gastric Carcinoma [19]. The objective response to chemotherapy was evaluated as complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD) according to the criteria of Japanese Gastric Cancer Association (JGCA) [21]. CR or PR cases were considered as responders.
The pathological response to chemotherapy was evaluated according to the histological evaluation criteria of the JGCA as grade 0 = no evidence of effect; grade 1a = viable tumor cells occupy more than two-thirds of the tumorous area; grade 1b = viable tumor cells remain in more than one-third but less than two-thirds of the tumorous area; grade 2 = viable tumor cells remain in less than one-third of the tumorous area; and grade 3 = no viable tumor cells remain [22]. Patients with grades 0–1a lesions were regarded as pathological non-responders and those with grades 1b–3 lesions were regarded as pathological responders.
Statistical analysis
Categorical variables are presented as numbers and percentages, and continuous variables are expressed as the median with ranges. Overall survival (OS) was defined as the duration from the date of starting DCS therapy to death. Survival curves were estimated according to the Kaplan–Meier method, and differences between the curves were analyzed using the log-rank test. Univariate and multivariate hazard ratios were calculated using a Cox proportional hazard model. All variables with a p value of <0.1 in the univariate analysis were entered into a multivariate analysis. A p value of <0.05 was considered to be significant, and confidence intervals (CI) were calculated at the 95 % level.
Results
Patient demographics and tumor characteristics are shown in Table 1. There were 12 patients (20.3 %) with stage II disease, 25 (42.4 %) with stage IIIA disease, and 22 (37.3 %) with stage IIIB disease. Before starting DCS therapy, a laparoscopy was performed in 51 patients (86.4 %).
Neoadjuvant chemotherapy and clinical response
Of the 59 patients, 43 patients (72.9 %) received two courses of DCS therapy defined by the protocol, and 16 (27.1 %) underwent only one course of chemotherapy for reasons which included adverse events, gastric outlet obstruction, patient request, etc.
A total of 44 patients were responders (including four with CR and 40 with PR) and 15 patients had SD (Table 2). The clinical response rate (cRR) was 74.6 %, and the disease control rate was 100 %. The cRR according to the number of courses of chemotherapy was 56.3 % (9/16) for one course of chemotherapy and 81.4 % (35/43) for two courses of treatment. The clinical response of lymph node(s) could be evaluated in 41 patients, and the cRR of lymph node(s) was 75.6 %.
Adverse events from neoadjuvant chemotherapy
Grade 3–4 serious toxicities occurred as follows (Table 3)—leucopenia (16.9 %), neutropenia (44.1 %), febrile neutropenia (8.5 %), anemia (10.2 %), thrombocytopenia (1.7 %), anorexia (8.5 %), nausea (6.8 %), vomiting (1.7 %), diarrhea (5.1 %), stomatitis (1.7 %) and pain (1.7 %). The incidence of grade 3 or 4 hematological and non-hematological adverse events was 49.2 and 15.3 %, respectively. Granulocyte colony-stimulating factor (G-CSF) was administered for neutropenia in 27 patients (45.8 %). There were no treatment-related deaths.
Surgical findings and postoperative complications
The surgical data are summarized in Table 4. Twenty-five patients underwent total gastrectomy, while 34 patients underwent distal gastrectomy. Only 3 patients received combined organ resection, and 7 patients received blood transfusion perioperatively. R0 resection was performed in 53 patients (89.8 %), R1 in 5 (positive surgical margin, three; positive peritoneal washing cytology, two) and R2 in one patient with peritoneal dissemination.
Postoperative complications occurred in 11 patients (18.6 %), grade 2 in 5 patients and grade 3a in 6 patients. There was no surgical mortality or reoperation. The median length of postoperative hospital stay was 11 days (range 9–54 days).
Pathological findings
Pathological findings are summarized in Table 5. Pathological response (grade 1b–3) was observed in 42 patients (71.2 %). The pathological response rate (pRR) was 56.3 % for one course of chemotherapy (9/16) and 76.7 % (33/43) for two courses of treatment. Almost 90 % of 44 clinical responders exhibited a pathological response, while 15 clinical non-responders showed a pathological response of only 26.7 %.
Adjuvant chemotherapy
Overall, 54 patients (91.5 %) received adjuvant chemotherapy; these included S-1 in 49 patients, CPT-11 + cisplatin in two patients, and S-1 + docetaxel, capecitabine + cisplatin + trastuzumab and paclitaxel in one patient each.
Postoperative survival
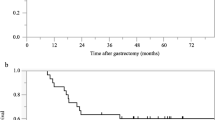
At the time of final follow-up (January 2015), the median follow-up was 40.9 months, and 50 patients (84.7 %) were alive. The 3- and 5-year OS rates were 88 and 68.6 %, respectively (Fig. 1).
Tumor recurrence occurred in seven patients (12.1 %). The site of recurrence was the peritoneum in three patients, liver in two, and lymph node and ovary in one patient each. Of these seven patients, 5 received chemotherapy; these included DCS therapy, S-1 + docetaxel, CPT-11 + cisplatin, paclitaxel and albumin-bounded paclitaxel in one patient each. In one patient with right ovarian metastasis, a right oophorectomy was performed. In another patient with rectal stenosis caused by peritoneal dissemination, a sigmoid colostomy was performed without further chemotherapy.
The 3-year OS rate of the clinical responders was 97.3 %, while that of the non-responders was 54.8 % (p = 0.001; Fig. 2a). The 3-year OS rate of the pathological responders was 92.2 %, while that of the pathological non-responders was 77.6 % (p = 0.075; Fig. 2b).
In survival analysis, univariate analysis identified clinical response as a significant prognostic factor (Table 6). Multivariate analysis demonstrated that clinical response was the only independent predictor of OS (hazard ratio 0.178, 95 % CI 0.04–0.792, p = 0.023).
Discussion
In the present study, we evaluated the efficacy of neoadjuvant DCS therapy in clinically resectable locally AGC. A number of studies have tried various pre- and postoperative chemotherapies for locally AGC. When considering tolerability, intensification of postoperative chemotherapy seems to be limited [7, 8]; therefore, NAC has received increasing attention. Several phase II trials have shown favorable results for NAC using the doublet regimens [10–15]. Although administration of the most effective chemotherapeutic regimens is likely to be essential in the neoadjuvant setting, it remains unknown whether a more intensive regimen is necessary for potentially curative gastric cancer. The present neoadjuvant DCS therapy demonstrated a very high cRR of 76.4 % and pRR of 71.2 %. In addition, the 3-year OS of 88 % in this study is encouraging. Furthermore, chemotherapy-related adverse events and postoperative complications were manageable and acceptable. These results indicate that neoadjuvant DCS therapy will be an effective treatment option for locally AGC.
Previous studies clearly showed that the pathological response separated the survival of gastric cancer patients who received NAC, suggesting that the pRR was one of the best surrogated endpoints of NAC for AGC [23, 24]. Therefore, it is necessary to increase the pRR in order to achieve a further improvement of patient outcomes. Previous studies of NAC using the doublet regimen, which employed the same Japanese criteria that was used in the present study, reported a pRR of 14.5–51 % [10–15], and the efficacy of these treatments is not sufficient from the viewpoint of the pRR. On the other hand, DCS therapy has been associated with a high pRR of 87.5 % [25]. Neoadjuvant DCS therapy demonstrated a higher pRR of 71.2 % in the present study compared with that achieved by the doublet regimen. Furthermore, patients with a high pathological response had improved survival rates compared with patients with a low pathological response, in accordance with previous reports [23, 24]. Therefore, neoadjuvant DCS therapy is a very promising regimen for locally AGC.
Two courses of DCS therapy may be required in order to achieve a higher response rate. Two courses of preoperative DCS therapy have revealed an extremely high cRR of the primary tumor (81.3 %) and pRR (87.5 %) [25]. When compared with these results, both the cRR of 74.6 % and pRR of 71.2 % obtained in the present study were relatively lower. This is possibly due to lower response rates observed in patients who received only one course of treatment. In the present study, 27.1 % of patients received one course of DCS therapy because of various reasons. The patients treated with two courses of DCS therapy exhibited an extremely high cRR of 81.4 % and pRR of 76.7 %. Although neutropenia was the major adverse event of DCS therapy, G-CSF administration could allow a certain number of patients to complete the planned two courses of treatment.
In the present study, the clinical response was evaluated using the JGCA criteria. The Response Evaluation Criteria in Solid Tumors (RECIST) is the gold standard in the evaluation of tumor response, but it requires the presence of a target lesion [26]. In the neoadjuvant setting, resectable gastric cancer seldom has target lesions as the primary tumor is regarded as a non-target lesion. Therefore, the evaluation of clinical response using RECIST is quite difficult in such situations. Indeed, only 12 patients (20.3 %) from the present study had target lesions. The JGCA criteria include a response evaluation of the primary tumor using barium X-ray and/or endoscopic examinations [21], and any gastric cancers can be evaluated using these criteria regardless of the presence of target lesions. When evaluating the clinical response of the primary tumor using these criteria, 74.6 % of patients were responders; the clinical responders had a significantly higher survival rate than the non-responders. Furthermore, clinical response was identified as the only independent predictor of OS. These findings indicate that the response of the primary tumor is a valuable preoperative parameter predicting patient outcomes, and its evaluation is therefore considered essential in the neoadjuvant setting. If showing a high response, only one course of DCS therapy may achieve an encouraging result. On the other hand, it is possible to expect poor survival in clinical non-responders, and further DCS therapy or an alternative chemotherapeutic regimen would be performed in such cases.
Postoperative chemotherapy for patients who underwent preoperative chemotherapy remains a concern. In most Japanese trials of NAC, no postoperative chemotherapy or S-1 monotherapy was prescribed [10, 12, 14, 15]. In one phase II trial of neoadjuvant paclitaxel + cisplatin, two courses of the same regimen were postoperatively performed for patients experiencing a pathological tumor response [13]. In the present study, most patients received adjuvant S-1 monotherapy regardless of the degree of response. Although the assessment of pathological response can provide useful information about chemosensitivity, it remains unclear whether additional postoperative DCS therapy contributes to a further improvement in survival of responders. In general, gastrectomy deteriorates tolerability of chemotherapy, and it was observed in the MAGIC trial, in which 86 % of patients completed preoperative ECF therapy, whereas only 55 % of patients started postoperative ECF therapy and 42 % completed the treatment [2]. Further investigations are necessary to determine optimal adjuvant chemotherapy for patients who underwent NAC.
Another concern in the preoperative chemotherapy is the loss of a chance to receive R0 resection as a result of tumor progression during long-term chemotherapy. In the present study, R0 resection rate was 89.8 %, and no patients experienced PD during the NAC. These data also highlighted the effectiveness of DCS therapy.
On the other hand, all patients in the present study were diagnosed initially with stage II–III resectable tumors. However, positive cytology and peritoneal dissemination were observed during surgery in two patients and one patient, respectively, despite the finding that no patient exhibited PD. It is likely that these metastases were already present at the time of diagnosis, when considering that they did not receive a staging laparoscopic examination before starting DCS therapy. A staging laparoscopy should be required for all patients undergoing preoperative chemotherapy in order to evaluate tumor spread precisely.
In conclusion, neoadjuvant DCS therapy is feasible, highly effective and attractive for resectable stage II–III locally AGC. However, the survival and surgical benefit of NAC for AGC still remains controversial, and further large-scale investigations are therefore required to validate them.
References
Nashimoto A, Akazawa K, Isobe Y et al (2013) Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer 16:1–27
Cunningham D, Allum WH, Stenning SP et al (2006) Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355:11–20
Ychou M, Boige V, Pignon JP et al (2011) Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 29:1715–1721
Sakuramoto S, Sasako M, Yamaguchi T et al (2007) Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357:1810–1820
Sasako M, Sakuramoto S, Katai H et al (2011) Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol 29:4387–4393
Koizumi W, Narahara H, Hara T et al (2008) S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 9:215–221
Kodera Y, Ishiyama A, Yoshikawa T et al (2010) A feasibility study of postoperative chemotherapy with S-1 and cisplatin (CDDP) for gastric carcinoma (CCOG0703). Gastric Cancer 13:197–203
Takahari D, Hamaguchi T, Yoshimura K et al (2011) Feasibility study of adjuvant chemotherapy with S-1 plus cisplatin for gastric cancer. Cancer Chemother Pharmacol 67:1423–1428
Ott K, Lordick F, Herrmann K et al (2008) The new credo: induction chemotherapy in locally advanced gastric cancer: consequences for surgical strategies. Gastric Cancer 11:1–9
Yoshikawa T, Sasako M, Yamamoto S et al (2009) Phase II study of neoadjuvant chemotherapy and extended surgery for locally advanced gastric cancer. Br J Surg 96:1015–1022
Yoshikawa T, Omura K, Kobayashi O et al (2010) A phase II study of preoperative chemotherapy with S-1 plus cisplatin followed by D2/D3 gastrectomy for clinically serosa-positive gastric cancer (JACCRO GC-01 study). Eur J Surg Oncol 36:546–551
Iwasaki Y, Sasako M, Yamamoto S et al (2013) Phase II study of preoperative chemotherapy with S-1 and cisplatin followed by gastrectomy for clinically resectable type 4 and large type 3 gastric cancers (JCOG0210). J Surg Oncol 107:741–745
Tsuburaya A, Nagata N, Cho H et al (2013) Phase II trial of paclitaxel and cisplatin as neoadjuvant chemotherapy for locally advanced gastric cancer. Cancer Chemother Pharmacol 71:1309–1314
Tsuburaya A, Mizusawa J, Tanaka Y et al (2014) Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg 101:653–660
Oki E, Emi Y, Kusumoto T et al (2014) Phase II study of docetaxel and S-1 (DS) as neoadjuvant chemotherapy for clinical stage III resectable gastric cancer. Ann Surg Oncol 21:2340–2346
Takayama T, Sato Y, Sagawa T et al (2007) Phase I study of S-1, docetaxel and cisplatin combination chemotherapy in patients with unresectable metastatic gastric cancer. Br J Cancer 97:851–856
Sato Y, Takayama T, Sagawa T et al (2010) Phase II study of S-1, docetaxel and cisplatin combination chemotherapy in patients with unresectable metastatic gastric cancer. Cancer Chemother Pharmacol 66:721–728
Koizumi W, Nakayama N, Tanabe S et al (2012) A multicenter phase II study of combined chemotherapy with docetaxel, cisplatin, and S-1 in patients with unresectable or recurrent gastric cancer (KDOG 0601). Cancer Chemother Pharmacol 69:407–413
Japanese Gastric Cancer Association (1998) Japanese Classification of Gastric Carcinoma—2nd English Edition. Gastric Cancer 1:10–24
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Japanese Gastric Cancer Association (2001) Japanese classification of gastric carcinoma––2nd English edition––response assessment of chemotherapy and radiotherapy for gastric carcinoma: clinical criteria. Gastric Cancer 4:1–8
Japanese Gastric Cancer Association (2011) Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 14:101–112
Ajani JA, Mansfield PF, Crane CH et al (2005) Paclitaxel-based chemoradiotherapy in localized gastric carcinoma: degree of pathologic response and not clinical parameters dictated patient outcome. J Clin Oncol 23:1237–1244
Kurokawa Y, Shibata T, Sasako M et al (2014) Validity of response assessment criteria in neoadjuvant chemotherapy for gastric cancer (JCOG0507-A). Gastric Cancer 17:514–521
Oyama K, Fushida S, Kinoshita J et al (2012) Efficacy of pre-operative chemotherapy with docetaxel, cisplatin, and S-1 (DCS therapy) and curative resection for gastric cancer with pathologically positive para-aortic lymph nodes. J Surg Oncol 105:535–541
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Conflict of interest
None of the authors has any financial conflicts to disclose in association with this study.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Migita, K., Nashimoto, A., Yabusaki, H. et al. Efficacy of neoadjuvant chemotherapy with docetaxel, cisplatin and S-1 for resectable locally advanced gastric cancer. Int J Clin Oncol 21, 102–109 (2016). https://doi.org/10.1007/s10147-015-0851-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-015-0851-2