Abstract
Background
The aim of this observational prospective study was to determine the technical feasibility, safety and adequacy of surgical margins for transoral robotic surgery (TORS) in oropharyngeal cancers.
Methods
From March 2013 to May 2014, 60 patients with oropharyngeal lesions underwent TORS with or without neck dissection using the ‘DaVinci’ robot. Patients were observed and data recorded on surgical time, blood loss, complications and functional outcome of patients.
Results
All 60 patients underwent TORS, with neck dissection performed in 45 of the patients. A positive margin was seen in two patients (3.3 %). Intent to treatment was radical in 42 patients and salvage in 18 patients. None of the patients required tracheostomy, and one patient (1.66 %) died postoperatively. Postoperative complications in the form of primary haemorrhage required active intervention in three patients. Average estimated blood loss was 26.5 ± 31.1 ml. Postoperatively, all patients had adequate swallowing and speech function with nasal twang reported in three patients on long-term follow up. Patients started tolerating oral feeds within a week of procedure (mean 3.96 days), with the nasogastric tube removed on the ninth postoperative day (mean 9.19 days). No long-term gastrostomy tube dependency was reported.
Conclusion
TORS is a safe, feasible, minimally invasive procedure in patients with oropharyngeal cancers. It has the least morbidity and offers benefits in terms of avoidance of tracheostomy tube, prolonged Ryle’s tube and gastrostomy dependency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Head and neck cancers are emerging as a major public health problem in India with over 2,00,000 new cases every year [1], with India reported to have the highest incidence of oropharyngeal cancer worldwide [2]. Historically, oropharyngeal cancers were treated by surgical excision and adjuvant therapy. However, increased surgical morbidity and poor cosmesis associated with open surgery led to support for approaches aimed at organ preservation, such as chemoradiation [3]. Chemoradiation in oropharyngeal cancers has achieved excellent local control, but at the cost of greater acute toxicity, with dysphagia being a major concern [4, 5]. Attempts have been made by various researchers to achieve an excellent local control at the cost of minimal functional morbidity. Transoral robotic surgery (TORS) is one such attempt to achieve excellent oncological outcomes with improved speech and swallowing function.
TORS has greatly simplified the surgical approach by providing excellent tumour visualization and magnification. It provides increased maneuverability in a limited field of view. Operative time, hospital stay, percutaneous endoscopic gastrostomy (PEG) tube and Ryle’s tube dependence have significantly decreased with TORS, without compromise of tumour control [4–10]. We designed the prospective study reported here to evaluate the technical feasibility, safety and adequacy of surgical margins with TORS for oropharyngeal lesions.
Materials and methods
Between March 2013 to May 2014, 60 patients with oropharyngeal lesions fulfilling the inclusion and exclusion criteria of our prospective study underwent TORS using the da Vinci robotic surgical system (Fig. 1a, b) for resection of their primary tumour at Rajiv Gandhi Cancer Institute and Research Centre, Delhi. The study was approved by the Institutional Review Board, and written informed consent from the participating patients was obtained. Eligibility criteria included patients aged ≥18 years with oropharyngeal lesions. Contraindications of TORS included decreased mouth opening (maximum mouth opening <1.5 cm) that would prevent adequate exposure of the affected areas, presence of American Journal of Critical Care (AJCC) TNM T4 stage disease, invasion of deep tissue lateral to constrictor muscle or posterior invasion of deep vertebral fascia, unresectability of involved lymph nodes, distant metastasis and medical contraindications for general anaesthesia and surgery. Nodal unresectability was defined as carotid artery encasement with deep neck structure involvement that made complete nodal resection difficult and skin involvement with dermal metastasis. The information collected from each patient included a demographic profile, histology, pathological stage, tissue margin status, operative time, perioperative complications, hospital stay, PEG tube dependency and need for adjuvant therapy.
Neck dissection was performed when indicated as either a staged or concurrent procedure. All 60 patients enrolled in this study underwent ipsilateral selective neck dissection (SND)—level II to IV for node negative neck. Patients underwent bilateral SND for tumours crossing the midline. Modified neck dissection was performed in node-positive necks.
Adjuvant therapy was advocated if adverse pathological features were present, such as pT4 disease, pN2 disease, perineural invasion, lymphovascular invasion, extracapsular extension (ECE) and positive/close margins (≤2 mm). Patients received radiotherapy to both sides of the neck and primary site to a dose of 60–66 Gy. Chemotherapy was added to radiotherapy if there was positive margin or ECE. No adjuvant therapy was recommended for previously irradiated patients. Patients were evaluated by means of clinical examination every month for 3 months, followed by a repeat evaluation every 3 months by the surgical team. Follow-up information was also recorded for every patient, including details of recurrence and survival status.
Results
Patients and tumour characteristics
A total of 60 patients with oropharyngeal lesions fulfilling the inclusion criteria participated in the study. Figure 2 shows the flow diagram of treatment for patients enrolled in the study. Intent to TORS treatment was salvage in the 18 previously irradiated patients and curative in the 42 naive patients. TORS was successfully performed in all patients, with no necessity for intraoperative conversion to an open surgical procedure.
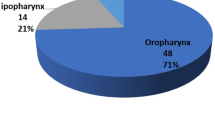
The average age of the patients was 55.8 ± 10.6 (range 31–88) years. The anatomic location of the oropharyngeal lesions are shown in Table 1. The majority of patients had the tumour originating from the tonsil (24/60). The final histopathological study revealed that 58 (96.6 %) patients had malignancy; the exceptions were two patients with biopsy-proven carcinoma who showed no evidence of malignancy on the final histopathological study. The pathological tumor and nodal staging of oropharyngeal carcinoma in both curative and salvage setting are presented in Table 2.
Assessment of surgical outcomes
All patients underwent complete resection of the oropharyngeal lesions with TORS. Frozen sections of tumour margin were examined. Of the 60 patients, positive margin was reported in two patients (3.33 %), while a close margin (<2 mm) was obtained in two (3.33 %) patients. Positive/close margins were reported in previously irradiated patients.
Estimated blood loss for patients undergoing TORS was 26.5 ± 31.1 ml, with no patient requiring blood transfusion. The average hospital stay was 4.15 ± 1.57 days. The main reason for hospital stay was management of neck drain, and patients were discharged after their neck drains had an output of <20 ml/24 h.
The standard setup time was 7.34 ± 8.34 min and robotic setup time was 6.71 ± 5.82 min. Surgical time averaged 49.84 ± 23.93 min. Mean operating time for TORS was significantly more in previously irradiated cases in comparison to previously untreated cases.
No reconstruction was done after primary tumor resection with TORS in any patient.
Adverse events
There were no cases of emergency airway compromise, wound dehiscence or oro-cutaneous fistula during the procedure. Of the 60 patients who underwent TORS, five patients (8.33 %) experienced either intraoperative or postoperative bleeding. Three of these required operative intervention, while bleeding resolved in one patient without any active intervention. Secondary haemorrhage caused one postoperative fatality. Four patients who experienced postoperative bleeding were previously irradiated. All events occurred within the first postoperative week following TORS. None of the patients in our study developed orocervical communication.
Adjuvant therapy
Adjuvant therapy was provided only to those patients who had not previously undergone radiotherapy. Six patients were advocated adjuvant radiotherapy, but only four ultimately underwent the therapy (two defaulted). One patient was incidentally found to have a second malignancy, i.e. mediastinal neuroendocrinal tumor, and hence did not receive adjuvant radiotherapy. Twelve patients underwent concurrent chemoradiation in view of positive neck nodes with ECE, while one patient expired due to secondary haemorrhage. Those patients not receiving adjuvant treatment (i.e. had previously received radiotherapy) were closely monitored during the follow-up. In all patients, the primary site was treated to a smaller radiotherapy dose (60–66 Gy) and volumes than would have been used to primarily treat squamous cell carcinoma (SCC). Overall 51.22 % patients (21/41) treated with radical intent avoided radiotherapy.
Functional outcomes: swallowing and speech function
Patients tolerated the TORS procedure well, and oral feeding was started as early as the second postoperative day in 20 patients (3.96 ± 2.7 days). The mean duration of Ryle’s tube use in all patients, previously irradiated patients and previously untreated patients was 9.19 ± 5.7, 12.89 ± 8.23 and 7.39 ± 2.81 days, respectively. Only two previously irradiated patients required PEG tube insertion in view of persistent nasal regurgitation, and the tube was removed after 30 days of use. Thus, there was no long-term PEG tube dependency in patients undergoing TORS. Nasal twang following TORS was noted in three (5 %) patients postoperatively on long-term follow up.
Follow-up
The average follow up of the patients was 8 (range 1–18) months. At 18 months of follow-up, the overall survival (OS) of the patients was 93 % and the recurrence-free survival (RFS) rate was 64 %. The OS for patients treated with radical and salvage surgery was 98 % at 17 months and 81 % at 18 months, respectively (p = 0.051). The RFS for patients treated with radical and salvage surgery was 70 % at 17 months and 49 % at 18 months, respectively (p value 0.038).
Discussion
The primary goal of head and neck cancer treatment is local control and organ preservation. Historically, surgical excision followed by adjuvant therapy was the treatment of choice for oropharyngeal cancers. Various studies have shown a 70–84 % local control rate, with 5-year survival ranging from 49–55 % with this treatment option [11]. Based on the results of the PENN (University of Pennsylvania) study (1980–1990s), Machtay et al. [12] recommended that open surgery followed by adjuvant radiotherapy for base of tongue (BOT) lesions achieved excellent local control. However, these open surgical approaches were associated with high rates of surgical morbidity in terms of speech and swallowing function, with 29 % of patients having either long-term PEG tube or tracheostomy tube dependency. Denittis et al. [13] also evaluated surgical and functional outcome in oropharyngeal cancer patients treated with primary surgery followed by adjuvant radiotherapy and concluded that despite excellent locoregional control (73 % at 3 years), patients had a high rate of distant metastasis (29 %) with suboptimal functional outcome. Parsons et al. [11] compared functional outcomes with surgery versus radiotherapy for oropharyngeal cancers and reported higher complication rates in the surgical arm. Despite surgery providing excellent locoregional control, the high morbidity led many surgeons to abandon this approach for this specific site [3].
In view of the high surgical morbidity, the PENN group conducted a subsequent study in which they evaluated the role of chemoradiation in advanced oropharyngeal carcinomas; the results showed a lower rate of distant metastases in patients receiving chemoradiation (15 %) than in the surgical series [14]. However, the PENN chemoradiation study revealed significant acute and chronic toxicity in the patient cohort [15]. In their 1997 study, Machtay et al. reported that chemoradiation was associated with significant speech and swallowing dysfunction, along with varying degrees of xerostomia [12]. Nguyen et al. showed that concurrent chemoradiation had an adverse impact on patients’ quality of life [16]. Taken together, it would appear that although organ-sparing approaches help to preserve vital organs, a large number of patients develop treatment-related toxicity. Numerous reports have shown that a radical dose of radiotherapy (70 Gy) together with concurrent chemotherapy leads to a high rate of acute toxicity [17].
Various attempts have been made to achieve excellent local control at the cost of minimal morbidity. This concerted effort has led to evolution and worldwide acceptance of TORS. However, few centers have committed themselves to TORS to decrease surgical morbidity and maintain the benefits of complete local control [18–20]. Laccourreye et al. [18] demonstrated 89 % local control with a combined transoral surgical resection of tonsillar cancer, neck dissection and adjuvant radiotherapy in 166 patients. Steiner et al. [19] and Grant et al. [20] demonstrated similar rates of preservation of speech and swallowing function without compromising on disease control by using transoral laser microsurgery for BOT lesions. Several other studies have found TORS to be an effective alternative to open surgery for oropharyngeal carcinoma [8–10, 21]. Weinstein et al. [22] demonstrated preservation of oropharyngeal function and adequacy of tumour removal with TORS for tonsillar SCC.
TORS can be challenging in terms of tumour visualization, tissue manipulation in minimal space and functional preservation by securing surrounding structures. The aim of the feasibility part of our study was to investigate whether TORS would meet these challenges. We measured this by recording the ability to expose the tumor and complete the surgery successfully with TORS without conversion to open surgery, as well as by evaluating the safety and adequacy of surgical margins with TORS.
The feasibility of TORS was clearly demonstrated in our study, with 100 % of our patients successfully undergoing TORS without the need for intraoperative conversion. In contrast, Moore et al. [23] attempted nonrobotic transoral surgical resection in 102 patients with SCC of tonsil, of whom 5.9 % ultimately required conversion to an open procedure. Preuss et al. [24] reported open conversion in 3.6 % (10/275) of patients who underwent transoral laser resection of oro-laryngeal tumours, and Genden et al. [8] reported achieving adequate surgical exposure in 90 % of cases using TORS.
Operative time, as an indicator of surgical efficacy, may be influenced by multiple factors, including surgeon experience, tumour size, site, previous history of irradiation, among others and is considered to be a cost-effective and safe indicator. Mean operative time for TORS in our study was 49.84 ± 23.93 min, with the operating time decreasing with increasing surgeon experience. TORS may thus allow for shorter operating times than open surgery, which could be beneficial for elderly patients.
The overall incidence of positive and close tumour margins was the same (3.33 %) in our patient cohort, with positive/close margins found in previously irradiated patients. These rates are similar to those reported for other transoral approaches. Moore et al. [23] observed positive surgical margins in 3.9 % tonsillar tumor cases (n = 102) resected by conventional transoral techniques, and Grant et al. [25] reported positive surgical margins in 3.4 % of individuals who underwent transoral laser resection of BOT tumours. Weinstein et al. [26] evaluated the oncological and functional outcomes of TORS in advanced oropharyngeal cancer and reported a 2 % positive margin rate. Thus, in terms of the adequacy of surgical margins, the TORS approach appears to be at least comparable to other transoral minimally invasive approaches, and at par with open surgery.
TORS in combination with neck dissection facilitated the identification of patients with favourable histological features, thereby allowing us to decrease the radiotherapy dose, and even to omit chemotherapy in a few cases, which resulted in deintensification of the overall treatment. Based on pathological reports, postoperative radiotherapy doses are given to a much smaller surgical bed, rather than to a larger original tumour volume. Deintensification of treatment significantly reduces the long-term swallowing dysfunction which has been reported with chemoradiation [27, 28]. Among our 60 patients, 19 were prescribed adjuvant radiotherapy with or without chemotherapy. Overall, 51.22 % of our patients (21/41) were not given radiotherapy, and no radiation was advocated in previously irradiated patients. Similar to the prospective single arm study by Weinstein et al. [29], ipsilateral SND at the time of TORS for primary oropharyngeal carcinoma allowed us also to “deintensify’’ adjuvant therapy in many patients. Weinstein et al. [30] showed that while most patients in their study had advanced clinical stage, almost all were found to have favourable pathological features. These authors also reported pathological downstaging at both the primary site and neck in the majority of the patients. More et al. [31] reported a lower swallowing dysfunction with the lower dose or deintensified adjuvant radiotherapy in patients undergoing TORS followed by adjuvant radiotherapy in comparison to chemoradiation for advanced oropharyngeal malignancies. Thus, while our data supports deintensification of radiation treatment for oropharyngeal carcinomas, future trials would be required for validation of these results.
TORS allows for organ preservation and a better functional rehabilitation with decreased morbidity. We were able to reduce the dose and volume of the radiation field based on the final histopathology tests. The most commonly used indicators of preservation of swallowing function in our study were duration of Ryle’s tube use and PEG tube dependency. PEG tube insertion was performed in two (3.33 %) previously irradiated patients and the tube was removed after a duration of 30 days. Thus, there was no long-term PEG tube dependency in our study, and the PEG tube insertion rate was lower than that reported for other surgical and nonsurgical therapies [28]. The results were similar to those reported by Weinstein et al. [29] and White et al. [32]. In contrast, PEG tube dependency rate was 9.1 % in the PENN chemoradiation series [14, 15]. Greven et al. [33] reported complete dysphagia for solids/liquids and PEG tube dependence in 18 % patients treated with chemoradiotherapy in advanced oropharyngeal cancer. Recent studies evaluating the role of intensity-modulated radiotherapy in oropharyngeal cancer have reported 9–38 % PEG tube dependency rates after treatment [34–36]. The mean duration of Ryle’s tube use for all patients enrolled in our study was 9.19 ± 5.7 days and was significantly longer in previously irradiated patients. Oral feeding was started as early as the second postoperative day in 20 patients (mean 3.96 days). Resumption of normal diet was observed on average on the 14th postoperative day, thereby indicating rapid swallowing rehabilitation.
Nasal twang following TORS was noted in three patients (5 %) in the immediate postoperative period. Moore et al. [13] reported hypernasality in 4/45 patients (8.88 %) after TORS for oropharyngeal cancer, which rapidly resolved in all 45 patients postoperatively by the first follow-up. In a similar study, Leonhardt et al. [37] found speech function to be moderately affected by TORS. Weinstein et al. [22] reported hypernasality in one patient following transoral robotic tonsillectomy, with subsequent transoral scar resection leading to its resolution. Genden et al. [8] however did not notice any significant velopharyngeal insufficiency in his study.
Rapid speech and swallowing rehabilitation in our patients was accompanied by a shorter hospital stay. The mean hospital stay of our patients was shorter than that of patients who would have otherwise undergone an open surgery or would have been admitted for supportive care in view of acute toxicity due to concurrent chemoradiotherapy. In the study by Moore et al., all 35 patients were discharged from the hospital within 6 days [9]; while Boudreaux et al. [10] and Weinstein et al. [7] reported a mean hospital stay of 2.6 and 5–7 days, respectively.
Of the 60 patients who underwent TORS, five experienced postoperative bleeding, among whom three required operative intervention; one patient expired due to secondary haemorrhage. The incidence of postoperative transoral bleeding requiring intervention among our patients is comparable to the range of 0.5–10.4 % reported in patients undergoing transoral surgical resection. None of the patients in our study experienced carotid artery injury; in comparison, the observed rates of carotid artery injury is 1.2–3.0 % after open procedures or radiation treatment [38–42]. Wound dehiscence did not occur in any of our study subjects compared to 2 % reported after transoral surgery and 2–14.3 % following open surgical procedures [42, 43]. No patient in our study developed orocervical communication. Aubry et al. [44] and Moore et al. [45] also reported a low incidence of orocervical communication in their respective studies evaluating the role of TORS in head and neck cancer. Both groups of researchers came to the conclusion that a higher rate of orocervical communication occurred in patients undergoing simultaneous rather than sequential neck dissection. None of the 60 patients in our study required tracheostomy or had airway compromise. In a similar study by Genden et al. [8], tracheostomy tube use or prolonged intubation was not reported in any patient.
Overall, TORS represents a new innovative minimally invasive approach in the treatment of oropharyngeal cancers. It is a safe, feasible and effective alternative to open surgical or standard nonsurgical treatment in patients with oropharyngeal cancer and promotes a decrease in morbidity and achievement of a better functional outcome. The rates of positive surgical margins achieved with TORS in our study are at least equivalent to those reported for other surgical modalities. The limitations of the study include nonrandomization of our patients, small number of patients and a short follow-up period. Additionally, objective assessment of swallowing and speech functions was not done in our study. Instead, we relied on PEG tube dependency, duration of Ryle’s tube use and tracheostomy dependence as indicators of functional outcomes. However, this study represents a unique single institution study experience in India with TORS. We hope that the promising results from our study can serve as a treatment arm for comparisons in future prospective randomized trials examining the role of robotic surgery for treating head and neck cancers.
References
National Cancer Registry Programme (ICMR) (2008). Consolidated report of population based cancer registries: 2004-05. ICMR, Bangalore
Warnakulasuriya S (2009) Global epidemiology of oral and oropharyngeal cancer. Oral Oncol 45:309–316
Gourin CG, Johnson JT (2001) Surgical treatment of squamous cell carcinoma of the base of tongue. Head Neck 23:653–660
Goguen LA, Posner MR, Norris CM et al (2006) Dysphagia after sequential chemoradiation therapy for advanced head and neck cancer. Otolaryngol Head Neck Surg 134:916–922
Gillespie MB, Brodsky MB, Day TA et al (2004) Swallowing-related quality of life after head and neck cancer treatment. Laryngoscope 114:1362–1367
Weinstein GS, O’Malley BW Jr, Hockstein NG (2005) Transoral robotic surgery: supraglottic laryngectomy in a canine model. Laryngoscope 115:1315–1319
Weinstein GS, O’Malley BW Jr, Desai SC et al (2009) Transoral robotic surgery: does the ends justify the means? Curr Opin Otolaryngol Head Neck Surg 17:126–131
Genden EM, Desai S, Sung CK (2009) Transoral robotic surgery for the management of head and neck cancer: a preliminary experience. Head Neck 31:283–289
Moore EJ, Olsen KD, Kasperbauer JL (2009) Transoral robotic surgery for oropharyngeal squamous cell carcinoma: a prospective study of feasibility and functional outcomes. Laryngoscope 119:2156–2164
Boudreaux BA, Rosenthal EL, Magnuson JS et al (2009) Robot-assisted surgery for upper aerodigestive tract neoplasms. Arch Otolaryngol Head Neck Surg 135:397–401
Parsons JT, Mendenhall WM, Stringer SP et al (2002) Squamous cell carcinoma of the oropharynx: surgery, radiation therapy, or both. Cancer 94:2967–2980
Machtay M, Perch S, Markiewicz D et al (1997) Combined surgery and postoperative radiotherapy for carcinoma of the base of radiotherapy for carcinoma of the base of tongue: analysis of treatment outcome and prognostic value of margin status. Head Neck 19:494–499
Denittis AS, Machtay M, Rosenthal DI et al (2001) Advanced oropharyngeal carcinoma treated with surgery and radiotherapy: oncologic outcome and functional assessment. Am J Otolaryngol 22:329–335
Machtay M, Rosenthal DI, Hershock D et al (2002) Organ preservation therapy using induction plus concurrent chemoradiation for advanced resectable oropharyngeal carcinoma: a University of Pennsylvania phase II trial. J Clin Oncol 20:3964–3971
Koch WM (2000) Head and neck surgery in the era of organ preservation therapy. Semin Oncol 27[Suppl 8]:5–12
Nguyen NP, Moltz CC, Frank C et al (2004) Dysphagia following chemoradiation for locally advanced head and neck cancer. Ann Oncol 15(3):383–388
Forastiere AA, Goepfert H, Maor M et al (2003) Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med 349:2091–2098
Laccourreye O, Hans S, Menard M et al (2005) Transoral lateral oropharyngectomy for squamous cell carcinoma of the tonsillar region. Arch Otolaryngol Head Neck Surg 131:592–599
Steiner W, Fierek O, Ambrosch P et al (2003) Transoral laser microsurgery for squamous cell carcinoma of the base of tongue. Arch Otolaryngol Head Neck Surg 129:36–43
Grant DG, Salassa JR, Hinni ML et al (2006) Carcinoma of the tongue base treated by transoral laser microsurgery, Part 1: a prospective analysis of oncologic and functional outcomes. Laryngoscope 116:2150–2155
Iseli TA, Kulbersh BD, Iseli CE et al (2009) Functional outcomes after transoral robotic surgery for head and neck cancer. Otolaryngol Head Neck Surg 141:166–171
Weinstein GS, O’Malley BW, Snyder W et al (2007) Transoral robotic surgery: radical tonsillectomy. Arch Otolaryngol Head Neck Surg 133:1220–1226
Moore EJ, Henstrom DK, Olsen KD et al (2009) Transoral resection of tonsillar squamous cell carcinoma. Laryngoscope 119:508–515
Preuss SF, Cramer K, Klussmann JP et al (2009) Transoral laser surgery for laryngeal cancer: outcome, complications and prognostic factors in 275 patients. Eur J Surg Oncol 35:235–240
Grant DG, Salassa JR, Hinni ML et al (2006) Carcinoma of the tongue base treated by transoral laser microsurgery, part 2: persistent, recurrent and second primary tumors. Laryngoscope 116:2156–2161
Weinstein GS, O’Malley BW, Cohen MA et al (2010) Transoral robotic surgery for advanced oropharyngeal carcinoma. Arch Otolaryngol Head Neck Surg 136(11):1079–1085
Langendijk JA, Doornaert P, Rietveld DH et al (2009) A predictive model for swallowing dysfunction after curative radiotherapy in head and neck cancer. Radiother Oncol 90(2):189–195
Machtay M, Moughan J, Trotti A et al (2008) Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol 26:3582–3589
Weinstein GS, Quon H, O’Malley BW Jr et al (2010) Selective neck dissection and deintensified postoperative radiation and chemotherapy for oropharyngeal cancer: a subset analysis of the University of Pennsylvania transoral robotic surgery trial. Laryngoscope 120:1749–1755
Weinstein GS, Quon H, Newman HJ et al (2012) Transoral robotic surgery alone for oropharyngeal cancer: an analysis of local control. Arch Otolaryngol Head Neck Surg 138(7):628–634
More YI, Tsue TT, Girod DA et al (2013) Functional swallowing outcome following transoral robotic surgery vs primary chemoradiotherapy in patients with advanced-stage oropharynx and supraglottic cancers. JAMA Otolaryngol Head Neck Surg 139(1):43–48
White HN, Moore EJ, Rosenthal EL et al (2010) Transoral robotic-assisted surgery for head and neck squamous cell carcinoma: one- and 2-year survival analysis. Arch Otolaryngol Head Neck Surg 136(12):1248–1252
Greven KM, White DR, Browne JD et al (2008) Swallowing dysfunction is a common sequelae after chemoradiation therapy for oropharynx carcinoma. Am J Clin Oncol 31:209–212
Lawson JD, Otto K, Chen A et al (2008) Concurrent platinum based chemotherapy and simultaneous modulated accelerated radiation therapy for locally advanced squamous cell carcinoma of the tongue base. Head Neck 30(3):327–335
De Arruda FF, Puri DR, Zhung J et al (2006) Intensity-modulated radiation therapy for the treatment of oropharyngeal carcinoma: the Memorial Sloan-Kettering Cancer Center experience. Int J Radiat Oncol Biol Phys 64(2):363–373
Rusthoven KE, Raben D, Ballonoff A et al (2008) Effect of radiation techniques in treatment of oropharynx cancer. Laryngoscope 118(4):635–639
Leonhardt FD, Quon H, Abrahão M et al (2012) Transoral robotic surgery for oropharyngeal carcinoma and its impact on patient-reported quality of life and function. Head Neck 34(2):146–154
Sobol SM, Freeman R, Thawley S et al (1982) Management of inadvertent injury to the carotid artery during head and neck surgery. Head Neck Surg 4:475–482
Ketcham AS, Hoye RC (1965) Spontaneous carotid artery hemorrhage after head and neck surgery. Am J Surg 110:649–655
Shumrick DA (1973) Carotid artery rupture. Laryngoscope 83:1051–1061
Joseph DL, Shumrick DL (1973) Risks of head and neck surgery in previously irradiated patients. Arch Otolaryngol 97:381–384
O’Brien CJ, Nettle WJ, Lee KK (1993) Changing trends in the management of carcinoma of the oral cavity and oropharynx. Aust N Z J Surg 63:270–274
Weber RS, Berkey BA, Forastiere A et al (2003) Outcome of salvage total laryngectomy following organ preservation therapy: the Radiation Therapy Oncology Group trial 91-11. Arch Otolaryngol Head Neck Surg 129:44–49
Aubry K, Yachine M, Perez AF et al (2011) Transoral robotic surgery for head and neck cancer: a series of 17 cases. Eur Ann Otorhinolaryngol Head Neck Dis 128(6):290–296
Moore EJ, Olsen KD, Martin EJ (2011) Concurrent neck dissection and transoral robotic surgery. Laryngoscope 121:541–544
Acknowledgements
None.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Dabas, S., Dewan, A., Ranjan, R. et al. Transoral robotic surgery in management of oropharyngeal cancers: a preliminary experience at a tertiary cancer centre in India. Int J Clin Oncol 20, 693–700 (2015). https://doi.org/10.1007/s10147-014-0774-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-014-0774-3