Abstract
Common voles in western France exhibit three-year population cycles with winter crashes after large outbreaks. During the winter of 2011–2012, we monitored survival, reproduction, recruitment and population growth rate of common voles at different densities (from low to outbreak densities) in natura to better understand density dependence of demographic parameters. Between October and April, the number of animals decreased irrespective of initial density. However, the decline was more pronounced when October density was higher (loss of ≈54 % of individuals at low density and 95 % at high density). Using capture-mark-recapture models with Pradel’s temporal symmetry approach, we found a negative effect of density on recruitment and reproduction. In contrast, density had a slightly positive effect on survival indicating that mortality did not drive the steeper declines in animal numbers at high density. We discuss these results in a population cycle framework, and suggest that crashes after outbreaks could reflect negative effects of density dependence on reproduction rather than changes in mortality rates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Population cycles of voles and lemmings have been studied for nearly a century, but the underlying mechanisms remain quite enigmatic (Krebs 1996, 2013; Stenseth 1999; Lambin et al. 2006). Trophic interactions are generally considered to be important (Berryman 2002; Turchin 2003), although the relative roles of predation, parasitism and herbivory are often unclear and the main agents of cyclicity may actually vary depending on ecological conditions (Krebs 2011). Improved understanding of vole population cycles is critical for management issues such as pest control (Singleton et al. 1999).
Cyclic population dynamics exhibit different phases (e.g., increase, peak, crash, and low phases; Krebs and Myers 1974) and not all phases of the cycle are equally puzzling. For example, fast population increases from low densities in good environmental conditions can be explained by the short juvenile stage and high prolificacy of voles and lemmings (Tkadlec and Zejda 1995) which promote very high population growth rates (e.g., Tkadlec 1997; Turchin and Ostfeld 1997). In contrast, the causes of population crashes and the absence of recovery after the crash (i.e., the low phase) have proven more difficult to explain. This crash phase is critical for understanding cyclic population dynamics (Tkadlec and Zejda 1998), but detailed population monitoring during periods when animals are less numerous and/or more difficult to catch is challenging. The proximate causes of population crashes can be extra mortality and/or reduced reproduction rates, since cyclic populations are often synchronous at a large scale and no large migratory movements are usually observed (except in some cases for lemmings).
In Fennoscandia, cyclic rodent populations’ crashes (e.g., in Microtus agrestis) are often attributed to a high mortality rate due to specialist predators (Hanski et al. 1991), especially small mustelids (Norrdahl and Korpimäki 1995). The predator hypothesis is supported by mathematical models (Hanski et al. 2001) as well as by experimental studies in Finland (Korpimäki 1993; Klemola et al. 1997; Korpimäki and Norrdahl 1998). An alternative view is that a stop of reproduction at high density may be sufficient to provoke a population crash (Łomnicki 1995). This idea is also supported by both empirical evidence in M. agrestis populations in Britain (Ergon et al. 2011) and mathematical modeling (Smith et al. 2006). The Smith et al. (2006) formulation of the theory includes delayed density-dependence of reproductive season length, which eventually results in the decline of the average reproductive rate in spring and summer. This “reproduction stop hypothesis” only assumes that the population loses individuals because reproduction does not compensate for mortality, so that the average age of individuals in the population increases progressively during the crash. Senescence at the individual level, i.e., a decline of performance with age, may further accelerate the crash (Boonstra 1994), but is not a required assumption of the “reproduction stop hypothesis” (Łomnicki 1995).
Support for two contrasting demographic mechanisms (decline of fecundity versus survival) responsible for population cycles in the same species at different locations suggests that the processes involved may depend on local environmental conditions or the animal population considered. Detailed monitoring on specific vole populations is clearly required to assess which demographic rates change during crashes. In this study, we investigated the effect of density on the common vole winter population dynamics in a cyclic population of Western France. The studied common vole population exhibits a 3-year cycle (Turchin 2003; Lambin et al. 2006), with large outbreaks recorded in summer and followed by winter crashes (Barraquand et al. 2014). In Europe, this is one of the most temperate locations where cyclic vole populations are recorded (Lambin et al. 2006); unlike more northern locations where population crashes also occur in winter (e.g., Oksanen and Oksanen 1992; Korpimäki et al. 2002; Jánová et al. 2003) and where snow can be an obstacle to vole monitoring (Krebs 2011), this temperate site allows continuous live-trapping during winter.
In 2012, a massive synchronous outbreak was observed in the common vole populations of Western France, but we recorded an unusual spatial variability in animal density across our study site with some areas at outbreak densities from 2011. We took this opportunity to perform capture-mark-recapture (CMR) in contrasted density conditions. From October to April, eight alfalfa fields in which densities varied by a ≈25-fold factor were surveyed. Our aims were twofold: first, to test whether the population growth rate was affected by density and second, to determine whether demographic parameters (i.e., survival, recruitment and reproduction) were affected by density. Our overall objective was to disentangle alternative hypotheses on population dynamics at field scale, and to determine whether the negative density dependence of population growth rate could be attributed to high mortality rate, to low (or null) reproduction, to low (or null) recruitment, or to a mix between these processes.
Materials and methods
Study area and sampling design
The study was conducted in the LTER “Zone Atelier Plaine & Val de Sèvre”, that covers about 430 km2 of an intensive farmland in central-western France (Région Poitou–Charentes, 46°11′N, 0°28′W). Based on preliminary sampling, we selected eight alfalfa fields with contrasted autumn common vole abundance [details in ESM (Electronic Supplementary Material) S1] for winter monitoring. Alfalfa was chosen because it is a perennial crop where common voles can reach high densities, and because no crop management practices occur during autumn and winter. Selected fields were large (average 7.2 ± 3.1 ha, range 2.2–12.2) and were located in open zones in order to buffer demographic stochasticity and to reduce predation risks associated with the presence of hedgerows which favour the presence of opportunistic predators.
Three field plots were selected in high density (HD) zones and five in low density (LD) zones (details in ESM S1). Fields were located in the same climatic area and vegetation cover and height in October were not significantly different between HD and LD plots (ESM S1). In each field plot, a square of 70 m × 70 m was randomly defined.
In LD plots, vole spatial distribution was patchy and colonies were clearly distinct, allowing a comprehensive monitoring of colonies present in the 70 m × 70 m square. In HD plots, the number of burrow openings was considerable (up to 200 per 100 m2) and burrow openings were homogeneously distributed in the field, making individual colonies difficult to distinguish. Trapping all voles in a 70 m × 70 m area was not possible for logistical reasons. Instead we randomly defined three sub-sampling squares of 10 m × 10 m per HD plot in order to estimate the local density based on knowledge of vole burrow systems (Brügger et al. 2010) and home range estimates (Briner et al. 2005). Given that we trapped small areas where voles were known to be present, the local density approximates to the number of voles per unit area with which any given vole frequently interacts, i.e., an index of the perceived vole density by voles. In total, five 70 m × 70 m Capture Mark Recapture (CMR) areas were monitored in LD plots and nine 10 m × 10 m CMR areas were monitored in HD plots. Six sampling sessions were carried out from late October 2011 to early April 2012 (Fig. 1). Trapping sessions were chosen to avoid extreme climatic conditions when voles might die during captures; consequently, the time interval between two successive trapping sessions was not regular (average duration between trapping sessions in the same field: mean 33.0 ± 7.1 days, range 19.0–41.0).
Common vole sampling
We used single-capture INRA traps with plastic boxes in which wooden shavings, wheat grains and carrots were added to maximise vole survival and wellbeing (Le Quilliec and Croci 2006). One trap was placed at a maximum distance of 5 cm from each burrow opening following Gilg (2002). This design, compared to trap lines or grids, maximizes capture rate and avoids trap saturation (daily trap capture rate 0.087 ± 0.043, range 0.0–0.333) without the need for multi-capture traps (that require more time and effort because of the prebaiting session (Brügger et al. 2010). Overall, we trapped with an effort of 10,828 trap-days over the study. Given that peak of vole activity was at dusk and dawn in the monitored field plots, traps were set at the end of the afternoon and collected the next morning to minimize time spent in traps by captured, (maximum of 16 h spent in the trap). Trapping was conducted in conformity with the “Guiding principles in the care and use of animals” approved by the Council of American Physiological Society.
We observed a mortality rate of 11 % on the total number of captures, and most animals found dead in the traps had not eaten the bait. All captured animals were sexed, weighed and checked for reproductive status using standard criteria for vole studies (e.g., Ergon et al. 2001a; Goswami et al. 2011); females were considered sexually active when clearly pregnant (enlarged belly), showing a perforated vagina, and/or lactating (developed nipples) whereas males were considered active if testes were in the scrotal position. Body length from nose to tail was also measured. To minimize potential recording bias, only two field workers were involved in manipulating and measuring voles.
Voles were individually marked with pit tags (Dorset Identification, Trovan® ID100) only when their weight was above 14 grams. Since some trapped voles were untaggable at first capture, we expected to recapture a certain number of untagged voles. To prevent such false zeroes in capture histories and to improve the quality of the data set, a small piece of ear was collected for genetic analyses from each untagged vole. By comparison of vole genotypes at eight microsatellite loci (Gauffre et al. 2008), we found that we re-captured 21 young untagged voles and that 13 tagged voles lost their pit tags between two trapping sessions.
Statistical analyses
A graphical overview summarizing our statistical procedure is provided in Fig. 1. Following Goswami et al. (2011), we used two alternative parameterizations of the temporal symmetry approach of Pradel (Nichols and Hines 2002) to model demographic parameters. This approach assumes that: all individuals (marked and unmarked) have the same capture probability (no trap dependency found; goodness of fit tests made with U-care, Choquet et al. 2009, ESM S2); there is no tag loss (we controlled the low occurrence of tag loss, see above); the sampling periods are instantaneous (12 continuous hours that prevent birth and death processes from occurring during samplings); there is no temporary emigration (with the exception of sessions 3–4 and 4–5 we did not find transience, goodness of fit tests made with U-care, ESM S2) and individuals are independent (Nichols and Hines 2002). The first model estimated apparent survival (Φ, which includes both survival and emigration processes), capture probability (p), population growth rate (λ) - hereafter referred to as pradlambda (all information on software and packages used is available at the end of Materials and methods). The second model estimated recruitment (f, which includes both local reproduction and immigration processes) instead of population growth rate (λ), and is hereafter referred to as pradrec.
All capture histories of dead animals were censored in the analyses of survival (n = 70 on 426 total captures histories; 16 %). Details of the data set are presented in Table 1.
Estimation of density
We used the Petersen method (Nichols and Pollock 1983; Nichols 1992) to estimate the number of animals present in each CMR area following the equation N = c/p (where N is the real population size, c the number of captures and p the probability of capture). The probability of capture p was estimated using the pradlambda (Φ, p, λ) parameterization of Pradel (1996). To account for differences in sampling area, we constructed two separate pradlambda models for the data sets from the HD and LD zones. Despite being fitted separately, both models followed the same model specifications and same covariates (see ESM S3 for variable selection and best models). When p and N were estimated, we finally computed the density (per ha) as D = 10,000 × N/(CMR area size). Given that border effects in the HD areas were expected to be high, we incorporated a buffer zone of 2 m around trapping areas to allow for this (CMR area size in HD: 200 m2, CMR area size in LD: 4900 m2). However, we recognize that our estimates of density may nonetheless be inflated (Nichols and Pollock 1983).
Influence of density on demographic parameters
To test whether population growth rate (λ), survival (Φ) and recruitment (f) were density dependent, data from HD and LD zones were merged into a single dataset and modeled using a single model. Models with both pradrec (estimating Φ, p, f) and pradlambda (estimating Φ, p, λ) formulations were constructed. Capture probability (p) was considered to be zone dependent (i.e., different constants for p in HD and LD zones), and time was parameterized in the model as an additive factorial variable (one level by sessions). We tested the simple hypothesis that demographic parameters (Φ, f and λ) were a function of density (same coefficient from October to April). We also tested whether the effect of density varied with season, between fall (October to December) and winter (December to April). We did not test different effects of density for each session in order to avoid over-parameterized models. Thus, four combinations of parameters were tested in the survival and the population growth rate/recruitment parts of the model (Table 2). This resulted in 16 combinations of parameters tested with the pradrec model (Φ, p, f) as well as the pradlambda model (Φ, p, λ) to estimate density dependence on survival, recruitment and population growth rate (all models tested are presented in Tables S4.2 and S4.4 in ESM S4).
The influence of density on breeding was investigated for female voles at the individual level (n = 281 captures). We built a generalized linear model (binomial family with logit link) to explain the reproductive status of females (0: inactive/1: active) in relation to density (as a continuous variable) and time (as a factorial variable). We applied exactly the same set of model covariates and interactions as above for population growth rate (λ), survival (Φ) and recruitment (f in Table 2, see also ESM S4d).
Correlations between reproduction and recruitment
To investigate the relation between reproduction and local recruitment, we tested whether the fraction of unmarked voles in a CMR area was correlated to the fraction of breeders in the previous trapping session. We used a binomial generalized linear model with logit link (function glm() in R).
Model selection and software
All model selection was performed with Akaike’s Information Criterion (AIC, Burnham and Anderson 2002) where the best model has the smallest AIC. For capture recapture models, the AIC was corrected for small sample sizes (hereafter AICc, Anderson et al. 1998). For each model, we calculated the ΔAICc as the difference in AICc between the focal model and the best model. Thus the best model has a ΔAICc = 0 and models with ΔAICc <2 were considered to be equally supported by the data, Anderson et al. 1998). All selection tables and best model parameters are available in ESM S3 and S4. For the analyses, we did not use forward or backward stepwise selection procedures to avoid bias due to the order of variables in the model (e.g., Whittingham et al. 2006; Mysterud et al. 2007). Instead all models were considered and ordered them with AIC (e.g., Mysterud et al. 2007).
Analyses were performed with R (R Development Core 2009). The capture mark recapture analyses were performed using Program MARK (White and Burnham 1999), implemented in R using the RMark library (Laake and Rexstad 2007). Quantities reported between brackets are 95 % confidence intervals. When parameters estimates are directly reported from the model, they are regression coefficients (transformed by the link function, logit for Φ, reproduction and comparison between reproduction and recruitment; log for f and λ).
Results
Density trends
We used the estimate of capture probability from the best pradrec model at HD (p = 0.75 [0.64, 0.84]) as well as at LD (p = 0.69 [0.40, 0.88]) to compute the Petersen estimator of density (results of model selection and best models are presented in ESM S3). However, the value estimated by the best pradlambda models was nearly identical (ESM S3).
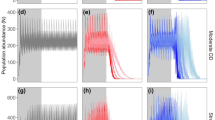
We found a decrease in vole number in all CMR areas during the winter (Fig. 2a, b). However, this decrease was higher in zones where common voles were abundant during the fall (% of density loss [=number of voles at peak/number of voles in April]; HD: mean = 94.6, SD = 5.2, LD: mean = 53.7, SD = 39.4). Furthermore, densities declined until the end of study for all HD CMR areas whereas several CMR quadrats located at LD showed a spring density increase. The decline lasted longer at HD than at LD.
Population dynamics in HD (a) and LD (b) zones. Grey lines represent each trapping area. Black lines represent the average population dynamics. In both a and b, the grey shading represents ±standard deviation from the average value. We used a spline function from a generalized additive model (package mgcv) to smooth the effect of time (represented as Julian day). See ESM S5 for log scale figure
Density dependent models for demographic parameters
In pradrec selection (Φ, p, f) and pralambda selection (Φ, p, λ), one model obtained a Δ AICc <2, respectively (ESM S4). In each case, Φ, f and λ were time and density dependent.
-
1.
Population growth rate
In the best model, population growth rate was time dependent (it decreased during winter, ESM S4a) and was negatively related to density [estimate = −7.62 × 10−6 (−1.28 × 10−5; −2.43 × 10−6)]. The effect of density seemed to be time dependent but models did not converge to generate clear trends (no time dependence in the first best model, dependent of the season in the second best model, Fig. 3a).
Fig. 3 Estimated parameters from CMR (a–c) and binomial (d) models for each field plot and session as a function of density. a Population growth rate, b survival, c recruitment and d reproduction. Symbols in panels a, b and c represent the same periods. In panels b and d, the dark colours of the symbols represent fall (October, November and December) while the light colours represent winter (January, February and March)
-
2.
Survival
Estimations of coefficients were almost identical in the best pradlambda and pradrec models (R 2 = 0.998, ESM S4a, b). Survival decreased until February and was largely influenced by time. Density had a positive effect on survival in fall [estimate = 7 × 10−4 (2.5 × 10−4; 1.31 × 10−3)] but no effect during winter [estimate = −1.5 × 10−6 (−3.3 × 10−3; 3.2 × 10−3), Fig. 3b].
-
3.
Recruitment
Recruitment was time dependent (low from October to February, strong increases in March) and was negatively related to density [estimate = −6.6 × 10−4 (−1.03 × 10−3; −3 × 10−4), Fig. 3c].
-
4.
Reproduction
The best model retained an effect of time (reproduction investment showed small decreases until February) and a seasonal effect of density (ESM S4d). The negative effect of density was higher during winter (−5.48 × 10−3 ± 2.30 × 10−3) than during the fall (−1.43 × 10−3 ± 3.03 × 10−4, Fig. 3d). Differences in breeding were large between LD and HD fields; on average during the whole study, we found that 39 % of females were sexually active in HD zones compared to 75 % of females in LD zones.
Correlations between reproduction and recruitment
The correlation between reproduction at trapping session t and the recruitment at the following trapping session was positive and significant (binomial GLM with logit link, mean ± SE, proportion of breeder at the following trapping session; estimate = 2.7689 ± 0.4794, P value = 7.68 × 10−9). When no reproduction was simulated (probability of population-scale reproduction during the previous month equal to 0), the model predicted a proportion of untagged animals of 0.229 ± 0.043, roughly equivalent to the proportion of voles missed by trapping estimated by CMR models, (1 − p).
Discussion
We thoroughly monitored the population dynamics and demographic parameters of the common vole in winter, taking advantage of spatially heterogeneous densities in autumn. In both low (i.e., 25 voles/ha) and outbreak (up to 1300 voles/ha) densities, local populations decreased strongly during winter. This was expected since winter is a challenging season for voles (Haapakoski et al. 2012). In particular, older and multiparous common voles do not survive the winter (Martinet et al. 1967; Tkadlec 1997) and, during this season, reproductive rate is lower (Pinot et al. 2014). However, we found that the winter population growth rate was negatively related to density (i.e., there was density-dependence of the population growth rate). Moreover, we found coherent effects of density on reproduction, recruitment and survival which explain observed differences of population growth rate.
Why is the population growth rate lower at high density? Survival vs reproduction
Density was found to have opposite effects on survival (positive effect) and recruitment (negative effect). In addition, the fraction of breeders was lower in high density zones (e.g., Fig. 4). Increasing survival with autumn density did not compensate the negative effect of density on recruitment since population declines were higher in high- compared to low-density zones. Altogether our results suggest that stronger declines at high density were due to a lack of recruitment which was, in turn, due to a decrease in reproduction. Cessation of breeding may therefore represent the major proximate cause of population crashes in our study population (Fig. 4).
Monthly population growth rate (a), survival (b), recruitment rate (c) and breeder proportion (d) averaged for one high density field plot (dots, “Les Chirons quadrat B”) and for one low density field plot (triangles, “Terre de Jules”). Estimation of population growth rate, survival and recruitment rate was made with CMR models (temporal symmetry approach of Pradel), estimation of the proportion of breeders was made with a generalized linear model. 95 % confidence intervals are drawn as vertical bars
Previous studies on rodents have highlighted the influence of density on reproduction at the individual level due to increased physiological stress caused by hierarchy or inter-individual aggressive behaviour (for a review, see Marchlewska-Koy 1997). When this stress is transmitted from mothers to their offspring, we observe a so-called maternal effect; these have recently been demonstrated to be influential in shaping the end of the crash phase in snowshoe hares (Sheriff et al. 2009). In mice, a species more closely related to the common vole, it has been demonstrated that chemical components present in urine have a negative effect on reproduction, particularly when density is high (Champlin 1971; Ma et al. 1998). Changes in food resources may modify reproduction rates if high density induces strong competition for food (e.g., Turchin 2003). In addition, interference may induce reproduction loss in subordinate females (e.g., Batzli et al. 1977; Dolby 2009). Thus, intrinsic regulation, through either maternal effects or more direct interference effects, or changes in the environment could help explain the observed decrease in reproduction.
In parallel to the low reproduction rate, we detected a negative effect of density on weight (taking account size and pregnancy, A. Pinot and H. Lisse, unpublished data), consistent with theoretical predictions (Ergon et al. 2004). Lower body weight may not be directly responsible for lower reproduction rates but provides an indication on the general health status of the population; density seems to decrease average individual quality, with cascading effects on reproductive rate in mammals (Sheriff et al. 2009; Pinot et al. 2014). This pattern could also be a symptom of overgrazing.
Could lower recruitment in high density areas reflect shifts in immigration or emigration rather than reproduction?
Several lines of evidence lead us to believe that this is unlikely. Firstly, we found a positive correlation between the fraction of breeders and the fraction of unknown animals (i.e., recruited animals + residents never caught) at the next trapping session in the same trapping areas. Our analyses indicated that the percentage of unknown individuals was only 20 % when reproduction was null during the previous trapping session (i.e., the proportion of immigrants is lower than 20 %). However, because the probability of capture varied between 0.69 and 0.75, some migrants were simply residents that we never caught. Thus immigration processes are probably not quantitatively as important as reproduction for recruitment, and most of the recruitment likely resulted from local reproduction. Secondly, we found no negative effect of density on apparent survival indicating that, if present, emigration was not higher in the HD zone. As movement processes seem to be negligible in winter (Bonnet et al. 2013), we suggest that low reproduction rate at HD is the likely cause of the population crash observed in our study population. Further insights into local immigration and recruitment processes could be obtained by using newly developed, spatially-structured CMR models (reviewed in Borchers 2012).
Link to population cycles
In the high density zone, vole densities were comparable to the densities typically observed in the same area during spatially synchronized peaks. However, unlike the highly synchronous peaks observed previously (Barraquand et al. 2014), the asynchronized peak of 2011 allowed us to set up this quasi-experimental design. It is notable that long-term time series at this site (17 years) indicate that common voles stop breeding at high densities during fall and winter, while they continue to reproduce during winter when density is low (Pinot et al. 2014). Thus changes in reproduction in response to a spatial variation in density reported here are consistent with changes in reproduction in response to temporal variation observed over the 17-year time series. This suggests that our results may apply to a much longer timeframe than simply the year of the study.
In our study, low reproduction and relatively high survival translated into a general ageing of the population at high density. In small mammals, density-dependent population ageing has previously been invoked to explain rodent cycles (Boonstra 1994; Łomnicki 1995; Tkadlec and Zejda 1998). However, this specific hypothesis has been explicitly tested and rejected in common vole populations elsewhere (Jánová et al. 2003). Discrepancy in the results between studies may reflect methodological differences; Jánová et al. (2003) did not consider vole density per se but analysed a phase effect by comparing two consecutive peak-decline years with similar maximal population size (first year: from 600 individuals in fall to 100 individuals next spring, second year from 700 individuals in fall to 100 individuals next spring). More data explicitly considering the effect of density on demographic parameters needs to be collected to conclude about the generality of the “reproduction stop hypothesis” for common voles.
The mechanisms observed locally in this study may provide more insights into population crashes in cyclic species. Population crashes in our common vole system are largely season dependent (i.e., crashes occur systematically in winter) as it is often the case in other cyclic systems (Tkadlec and Zejda 1998; Hansen et al. 1999). However, the environmental factor driving seasonal patterns of mortality rates cannot be snow cover as in Nordic systems since snow is almost inexistent here. Instead, seasonality in vole mortality appears to be related to less productive vegetation in winter in our study area (Barraquand et al. 2014).
Cessation of breeding has previously been suggested to explain M. agrestis population cycles in the Kielder forest (UK) where summer population crashes occur (Ergon et al. 2011). The difference in the season of crash at our study site probably reflects the short lag of density dependence in our case (Barraquand et al. 2014) compared to the Kielder site (Smith et al. 2006). In both systems, reproduction rather than survival underpins most of the density-dependence observed (the proximate cause for cycling is likely the density-dependence of reproduction), but in Kielder density-dependence occurs with a longer delay. The ultimate cause for the longer delay in regulation in Kielder is currently not known (see Reynolds et al. 2013 for some season-based mechanistic modelling involving plant quality). However, it is possible that the different delays in the negative density-dependence of reproduction may be a consequence of differences in life-histories, induced by environmental productivity. Indeed, cyclic populations of M. agrestis in the Kielder forest live in grasslands in wood regeneration areas. Such habitat is more stable than agricultural field plots since grass is not harvested there, but also less productive than fertilized plants selected for high yields in agricultural fields. As a consequence, field voles in Kielder may have lower reproduction rates and higher survival (e.g., Ergon et al. 2001b; Smith et al. 2006) than common voles in our agricultural system in France. Thus if field voles (M. agrestis) stop breeding after a peak but survival is higher than for Microtus arvalis, a population crash could occur much later after the peak than for M. arvalis.
What would be the evolutionary cause for faster life-histories in M. arvalis? Plant productivity is highly seasonal in intensively-cultivated farmlands of western France due to agricultural practices (the production is high in spring and vegetation is scarce during winter due to harvesting). This may select for faster life-histories (Stenseth et al. 1985; high reproduction rate when the environment is productive and low investment in survival during winter). In the French Alps (same latitude but at a higher altitude and in a less productive environment), M. arvalis shows slower life-histories (lower reproduction rate and higher survival) and stable dynamics, consistent with the hypothesis that life history traits are mediated by environmental productivity (Yoccoz and Ims 1999). Further work is required to determine how variation in productivity and length of season affect life-histories within/between species. For instance, it is not entirely clear why the increased seasonality of the Alpine environment (selecting for fast life histories) does not counteract the effects of lower environmental productivity (selecting for slower life histories).
The present study highlights that a halt in reproduction rather than decreased survival may induce vole crashes, and therefore that the proximate cause of vole cycles, in the studied population at least, could be density-dependence of reproduction rates. Whilst this seems particularly likely where there is also a strong seasonality in the food availability (as in intensively-cultivated farmlands), it might be also applicable to other seasonal environments where there is an “adverse” reproductive period (Tkadlec and Zejda 1998; Taylor et al. 2013). It is unclear whether population ageing is enhanced by individual senescence (i.e., a decline of the individual survival and reproductive performance with age, Boonstra 1994), and age-structured CMR are needed to address this question. Studies investigating explicitly how vital rates of different age classes are affected by density factors would be a logical follow-up to the present one, and are likely to provide valuable knowledge on the proximate causes of population cycles of voles.
References
Anderson DR, Burnham KP, White GC (1998) Comparison of Akaike information criterion and consistent Akaike information criterion for model selection and statistical inference from capture-recapture studies. J Appl Stat 25:263–282
Barraquand F, Pinot A, Yoccoz NG, Bretagnolle V (2014) Overcompensation and phase effects in a cyclic common vole population: between first and second order cycles. J Anim Ecol 83:1367–1378
Batzli GO, Getz LL, Hurley SS (1977) Suppression of growth and reproduction of microtine rodents by social factors. J Mammal 58:583–591
Berryman A (2002) Population cycles, cause and analysis. In: Berryman A (ed) Population cycles. Oxford University Press, New York, pp 3–28
Bonnet T, Crespin L, Pinot A, Bruneteau L, Bretagnolle V, Gauffre B (2013) How the common vole copes with modern farming: insights from a capture–mark–recapture experiment. Agric Ecosyst Environ 177:21–27
Boonstra R (1994) Population cycles in microtines: the senescence hypothesis. Evol Ecol 8:126–219
Borchers D (2012) A non-technical overview of spatially explicit capture–recapture models. J Ornithol 152–2:435–444
Briner T, Nentwig W, Airoldi JP (2005) Habitat quality of wildflower strips for common voles Microtus arvalis and its relevance for agriculture. Agric Ecosyst Environ 105:173–179
Brügger A, Netwig W, Airoldi JP (2010) The burrow system of the common vole. Mammalia 74:311–315
Burnham KP, Anderson DR (2002) Model selection and multimodel inference, 2nd edn. Springer, New York
Champlin AK (1971) Suppression of estrus in grouped mice: the effects of various densities and the possible nature of the stimulus. J Reprod Fertil 27:233–241
Choquet R, Lebreton JD, Gimenez O, Reboulet AM, Pradel R (2009) UCARE: utilities for performing goodness of fit tests and manipulating capture-recapture data. Ecography 32:1071–1074
Dolby A (2009) Breeding suppression between two unrelated and initially unfamiliar females occurs with or without social tolerance in common voles (Microtus arvalis). J Ethol 27:299–306
Ergon T, Lambin X, Stenseth NC (2001a) Life-history traits of voles in a fluctuating population respond to the immediate environment. Nature 411:1043–1045
Ergon T, MacKinnon JL, Stenseth NC, Boonstra R, Lambin X (2001b) Mechanisms for delayed density-dependent reproductive trait in field voles, Microtus agrestis: the importance of inherited environmental effects. Oikos 95:185–197
Ergon T, Speakman JR, Scantlebury M, Cavanagh R, Lambin X (2004) Optimal body size and energy expenditure during winter: why are voles smaller in declining populations? Am Nat 163:442–457
Ergon T, Ergon R, Begon M, Telfer S, Lambin X (2011) Delayed density-dependent onset of spring reproduction in a fluctuating population of field voles. Oikos 120:934–940
Gauffre B, Estoup A, Bretagnolle V, Cosson JF (2008) Spatial genetic structure of a small rodent in a heterogeneous landscape. Mol Ecol 17:4619–4629
Gilg O (2002) The summer decline of the collared lemming, Dicrostonyx groenlandicus, in high arctic Greenland. Oikos 99:499–510
Goswami VR, Getz LL, Hostetler JA, Ozgul A, Oli MK (2011) Synergistic influences of phase, density, and climatic variation on the dynamics of fluctuating populations. Ecology 92:1680–1690
Haapakoski M, Sundell J, Ylönen H (2012) Predation risk and food: opposite effects on overwintering survival and onset of breeding in a boreal rodent. J Anim Ecol 81:1183–1192
Hansen T, Stenseth NC, Henttonen H (1999) Multiannual vole cycles and population regulation during long winters: an analysis of seasonal density dependence. Am Nat 154:129–139
Hanski I, Hansson L, Henttonen H (1991) Specialist predators, generalist predators and the microtine rodent cycle. J Anim Ecol 60:353–367
Hanski I, Henttonen H, Korpimäki E, Oksanen L, Turchin P (2001) Small-rodent dynamics and predation. Ecology 82:1505–1520
Jánová E, Heroldova M, Nesvadbova J, Bryja J, Tkadlec E (2003) Age variation in fluctuating population of the common vole. Oecologia 137:527–532
Klemola T, Koivula M, Korpimäki E, Norrdahl K (1997) Small mustelid predation slows growth of Microtus voles: a predator reduction experiment. J Anim Ecol 66:607–614
Korpimäki E (1993) Regulation of multiannual vole cycles by density-dependent avian and mammalian predation. Oikos 66:359–363
Korpimäki E, Norrdahl K (1998) Experimental reduction of predator reverses the crash phase of the small-rodent cycles. Ecology 79:2448–2455
Korpimäki E, Norrdahl K, Klemola T, Pettersen P, Stenseth NC (2002) Dynamic effects of predators on cyclic voles: field experimentation and model extrapolation. Proc R Soc B 269:991–997
Krebs CJ (1996) Population cycles revisited. J Mammal 77:8–24
Krebs CJ (2011) Of lemmings and snowshoe hares: the ecology of northern Canada. Proc R Soc B 278:481–489
Krebs CJ (2013) Population fluctuations in rodents. University of Chicago Press, Chicago
Krebs CJ, Myers JH (1974) Population cycles in small mammals. Adv Ecol Res 8:268–400
Laake JL, Rexstad EA (2007) RMark. Appendix C. In: Cooch EG, White GC (eds) Program MARK: a gentle introduction, 14th edn. http://www.phidot.org/software/mark/docs/book/. Accessed 1 Oct 2011
Lambin X, Bretagnolle V, Yoccoz NG (2006) Vole population cycles in northern and southern Europe: is there a need for different explanations for single pattern? J Anim Ecol 75:340–349
Le Quilliec P, Croci S (2006) Piégeage de micromammifères: une nouvelle boîte-dortoir pour le piège non vulnérant INRA. Le cahier des Techniques INRA, Numéro Spécial sur les Méthodes et outils pour l’observation et l’évaluation des milieux forestiers, prairiaux et aquatiques (in French)
Łomnicki A (1995) Why do populations of small rodents cycle? A new hypothesis with numerical model. Evol Ecol 9:64–81
Ma W, Miao Z, Novotny MV (1998) Role of the adrenal gland and adrenal-mediated chemosignals in suppression of estrus in the house mouse: the Lee-Boot effect revisited. Biol Reprod 59:1317–1320
Marchlewska-Koy A (1997) Sociogenic stress and rodent reproduction. Neurosci Biobehav Rev 21:699–703
Martinet L, Meunier M, Bureau P (1967) Cycle saisonnier de reproduction du campagnol des champs (Microtus arvalis). Annales de biologie animale, biochimie, biophysique 7:245–259 (in French)
Mysterud A, Barton KA, Jedrzejewska B, Krasinski ZA, Niedzialkowska M, Kamler JK, Yoccoz NG, Stenseth NC (2007) Population ecology and conservation of endangered megafauna: the case of European bison in Białowieża primeval forest, Poland. Anim Conserv 10:77–87
Nichols JD (1992) Capture–recapture models: using marked animals to study population dynamics. Bioscience 42:94–102
Nichols JD, Hines EJ (2002) Approaches for the direct estimation of lambda, and demographic contributions to lambda, using capture-recapture data. J Appl Stat 29:539–568
Nichols JD, Pollock KH (1983) Estimation methodology in contemporary small mammal capture-recapture. J Mammal 64:253–260
Norrdahl K, Korpimäki E (1995) Mortality factors in a cyclic vole population. Proc R Soc B 261:49–53
Oksanen L, Oksanen T (1992) Long-term microtine dynamics in north Fennoscandian tundra: the vole cycle and the lemming chaos. Ecography 15:226–236
Pinot A, Gauffre B, Bretagnolle V (2014) The interplay between seasonality and density: consequences for female breeding decisions in a small cyclic herbivore. BMC Ecol 14:17
Pradel R (1996) Utilization of capture–mark–recapture for the study of recruitment and population growth rate. Biometrics 52:703–709
R Development Core Team (2009) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. http://www.R-project.org
Reynolds JJ, Sherratt JA, White A, Lambin X (2013) A comparison of the dynamical impact of seasonal mechanisms in a herbivore–plant defense system. Theor Ecol 6:225–239
Sheriff MJ, Krebs CJ, Boonstra R (2009) The sensitive hare: sublethal effects of predator stress on reproduction in snowshoe hares. J Anim Ecol 78:1249–1258
Singleton GR, Leirs H, Hinds LA, Zhang Z (1999) Ecologically-based management of rodent pests—re-evaluating our approach to an old problem. Ecologically-based Management of Rodent Pests. Australian Centre for International Agricultural Research (ACIAR), Canberra, pp 17–29
Smith M, White A, Lambin X, Sherratt J, Begon M (2006) Delayed density-dependent season length alone can lead to rodent population cycles. Am Nat 167:695–704
Stenseth NC (1999) Population cycles in voles and lemmings: density dependence and phase dependence in a stochastic world. Oikos 87:427–461
Stenseth NC, Gustafsson TO, Hansson L, Ugland KI (1985) On the evolution of reproductive rates in microtine rodents. Ecology 66:1795–1808
Taylor RA, White A, Sherratt JA (2013) How do variations in seasonality affect population cycles? Proc R Soc B 280:20122714
Tkadlec E (1997) Early age of vaginal opening in common vole (Microtus arvalis). Folia Zool 46:1–7
Tkadlec E, Zejda J (1995) Precocious breeding in female common voles and its relevance for rodent fluctuations. Oikos 73:231–236
Tkadlec E, Zejda J (1998) Small rodent population fluctuations: the effects of age structure and seasonality. Evol Ecol 12:191–210
Turchin P (2003) Complex population dynamics: a theoretical/empirical synthesis. Princeton University Press, Princeton
Turchin P, Ostfeld RS (1997) Effects of density and season on the population rate of change in the meadow vole. Oikos 78:355–361
White GC, Burnham PK (1999) Program MARK: survival estimation from populations of marked animals. Bird Study 46:120–139
Whittingham MJ, Stephens PA, Bradbury RB, Freckleton RP (2006) Why do we still use stepwise modeling in ecology and behaviour? J Anim Ecol 75:1182–1189
Yoccoz NG, Ims RA (1999) Demography of small mammals in cold regions: the importance of environmental variability. Ecol Bull 47:137–144
Acknowledgments
We are especially grateful for the help in the field provided by: Helene Lisse, Ronan Marrec, Marilyn Roncoroni, Mathieu Liaigre, Catherine Michel, Pomme Pinot, Alexandra Scohier, Jean-François Blanc and Samantha Yeo. We thank Laurent Crespin for constructive criticism, and Juliette Bloor for insightful editing and numerous suggestions. We also thank the anonymous reviewers and the editorial board of Population Ecology for their extensive and constructive comments that improved the manuscript. Finally, we thank the nine farmers that agreed to host the study in their fields. Partial funding was supported by EU BiodivERsA project “Ecocycles”. AP was supported by a Ph.D. grant from University Pierre & Marie Curie, Paris, France.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pinot, A., Barraquand, F., Tedesco, E. et al. Density-dependent reproduction causes winter crashes in a common vole population. Popul Ecol 58, 395–405 (2016). https://doi.org/10.1007/s10144-016-0552-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10144-016-0552-3