Abstract
Electrical stimulation is an important adjuvant therapy for spinal surgery, but whether receiving electrical stimulation can improve the fusion rate after spinal surgery is still controversial. The purpose of this study was to analyse and evaluate the effect of electrical stimulation on the fusion rate after spinal surgery. We systematically searched for related articles published in the PubMed, Embase and Cochrane Library databases on or before September 30, 2023. The odds ratio (OR) with 95% confidence interval (CI) and the fusion rates of the experimental group and the control group were calculated by a random-effects meta-analysis model. The analysis showed that receiving electrical stimulation significantly increased the probability of successful spinal fusion (OR 2.66 [95% CI 1.79–3.97]), and the average fusion rate of the electrical stimulation group (86.8%) was significantly greater than that of the control group (73.7%). The fusion rate in the direct current (DC) stimulation group was 2.33 times greater than that in the control group (OR 2.33 [95% CI 1.37–3.96]), and that in the pulsed electromagnetic field (PEMF) group was 2.60 times greater than that in the control group (OR 2.60 [95% CI 1.29–5.27]). Similarly, the fusion rate in the capacitive coupling (CC) electrical stimulation group was 3.44 times greater than that in the control group (OR 3.44 [95% CI 1.75–6.75]), indicating that regardless of the type of electrical stimulation, the fusion rate after spinal surgery improved to a certain extent. Electrical stimulation as an adjuvant therapy seems to improve the fusion rate after spinal surgery to a certain extent, but the specific effectiveness of this therapy needs to be further studied.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spinal disease is a musculoskeletal disease [1] that includes degenerative diseases, fractures, etc. [2, 3] and often causes neck, back and waist pain. Moreover, excessive strain on the lower limb joints can result in morphological changes in the spine and joint pain, affecting the normal life and labour of patients. By 2016, 32% of European adults had spinal disease [1]. With the increasing prevalence of spinal disease in the population, how to treat spinal diseases and improve patients’ quality of life has become the focus.
At present, Spinal surgery is one of the ways to treat spinal disorders. Although spinal surgery can significantly improve quality of life, complications such as nonunion and pseudarthrosis are generally not conducive to improving patient prognosis, and patients may experience persistent or recurrent pain at the surgical site [4]. The incidence of nonunion is approximately 25–81%, and the incidence of pseudarthrosis is as high as 81% [5]. The success of spinal fusion often determines whether such complications will occur. Spinal fusion is one of the important criteria for of successful spinal surgery [6]. Therefore, researchers have proposed a number of adjuvant therapies, such as biological agents or electrical stimulation therapy (EST), to promote spinal fusion [7, 8]. To date, there are three kinds of electrical stimulation therapy: direct current (DC), pulsed electromagnetic field (PEMF) and capacitive coupling (CC) stimulation. DC stimulation involves the application of a continuous electrical current to promote cellular biological responses. The primary mechanism is through the creation of a stable electric field, which influences the cell membrane potential, thereby regulating cell proliferation, differentiation, and migration. This form of stimulation is widely applied in tissue repair and regeneration. PEMF utilizes short, rapidly changing electromagnetic fields to induce currents, leading to physiological changes both inside and outside cells. PEMF has been demonstrated to have beneficial effects in the repair of bone and soft tissues, primarily by influencing cellular signaling pathways and gene expression. CC stimulation refers to the application of a fixed current intensity to cells or tissues. The goal of CC stimulation is to ensure uniformity and reproducibility of the stimulus by maintaining a consistent current intensity. This method is employed in the recovery of neural and muscular function. PEMF and CC stimulation are noninvasive techniques that do not cause wounds, as only closeness to the skin is needed. DC stimulation requires that an implant be placed in the fusion site and soft tissue to provide continuous stimulation at the fusion site. All three kinds of electrical stimulation fusion therapies have their own advantages [9, 10].
At present, it is controversial whether electrical stimulation can improve the rate of spinal fusion. Massari, L. et al. [10] explored the effect of DC electrical stimulation on the fusion rate after spinal surgery. The results showed that the postoperative fusion rate of patients in the DC electrical stimulation group was significantly greater than that of patients in the nonelectrostimulation group. However, in a study of 60 patients [11], electrical stimulation did not lead to spinal fusion, so there is no unified conclusion on this issue.
The causes of these disputes may be related to the type of electrical stimulation and the differences in patient characteristics. However, the effect of electrical stimulation as an adjuvant therapy on the fusion rate after spinal surgery is unclear. We reviewed the previous literature on electrical stimulation and spinal fusion surgery and conducted a more comprehensive meta-analysis to evaluate the effect of electrical stimulation on the spinal fusion rate.
Methods
Standard protocol approvals and registrations
The study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines and the Cochrane Handbook. The protocol of the review is registered with PROSPERO and can be accessed on the official website.
Search strategy
From inception to September 30, 2023, two independent investigators systematically searched the PubMed, Embase and Cochrane Library electronic databases without limiting the language or publication date. We used Medical Subject Headings (MeSH) terms to search PubMed and the Cochrane Library; Embase subject headings (Emtree) to search the EMBASE database; and combined free text words (including synonyms and closely related words) related to spinal surgery, electrical stimulation and the postoperative spinal fusion rate. The search terms used included "spinal fusion", "spinal dysraphism", "electric stimulation", "electromagnetic field", "electric stimulation therapy", and "fusion rate". The search strategy used for the databases is presented in eTable 1.
To ensure that no relevant articles were omitted, the researchers also hand searched for recent systematic reviews, meta-analyses, and any articles included in our review for other relevant articles. When articles described the same cohort, we retained only the latest publication or the article with the largest sample size.
Selection criteria
The study was performed in accordance with the PICOS guidelines, and two reviewers independently screened the studies according to the selection criteria. Only studies that met the following criteria were included:
-
(1)
Participants: Patients who had undergone spinal fusion surgery.
-
(2)
Intervention: Patients received any of the three types of electrical stimulation—DC, PEMF or CC stimulation—during the course of treatment.
-
(3)
Control: There must be a control group in the study; that is, a group of patients who were given a placebo.
-
(4)
Results: The main outcome was the fusion rate.
-
(5)
Study type: Randomized controlled trial (RCT) or cohort study.
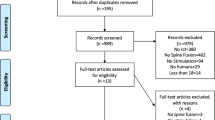
Reviews, letters, case reports, studies of animal models, studies not reporting fusion rates, studies lacking data for the control group and studies whose adjuvant therapy was not electrical stimulation were excluded (Fig. 1).
Data extraction
The two reviewers independently extracted relevant data with the predesigned data extraction table. consulting third party. The following data were extracted: first author, year of publication, geographical area, observation period, sample size, percentage of women, average age, type of electrical stimulation, treatment time, site of spinal surgery, segment and type of spinal fusion, methods for determining the fusion rate, difference in the fusion rate between the experimental group and control group, follow-up date and so on (Table 1).
Quality assessment
Two reviewers used the Risk of Bias 2 tool (ROB 2) and Newcastle‒Ottawa Scale (NOS) to strictly evaluate the quality of the RCTs and cohort studies. The ROB 2 assessment involved five domains: study selection bias, diagnostic bias, reporting bias, integration of bias factors and overall bias risk. Each domain was considered to have a low risk of bias (Fig. 2). The NOS consisted of three parameters of quality, selection, comparability, and outcome, with a total possible score of 9 for each study. A score ≥ 8 indicated high quality (low bias risk) (Table 2).
Statistical analysis
Analyses were performed using STATA software (version 16.0; STATA, University Station, Texas, USA). A random-effects meta-analysis was employed because of anticipated between-study heterogeneity, and the average fusion rate of the electrical stimulation group and the control group was determined. In general, fully-adjusted effect estimates (ORs) for the association between electrical stimulation and spinal surgical fusion rates were used to derive pooled risk estimates, depicted graphically with forest plots. Heterogeneity between studies was evaluated using the Cochrane Q test and I2 test, and heterogeneity was judged to be statistically significant at I2 ≥ 50% or P < 0.05. Publication bias was assessed by visually assessing the symmetry of funnel plots. Sensitivity analysis was performed through the sequential omission of individual studies to evaluate the stability of the results.
In addition, we also carried out a subgroup analysis according to smoking status, the number of fused segments, and whether internal fixation was performed.
Results
Literature search
We initially identified a total of 3695 citations through a keyword search, and 2708 citations remained after identifying and deleting duplicate literature. According to the citation titles and abstracts, 57 articles were included for full-text review. After reviewing the full texts, 9 RCTs [9, 10, 12,13,14,15,16,17,18] involving 1261 patients and 7 cohort studies [11, 19,20,21,22,23,24] involving 996 patients were included in the meta-analysis, and 41 additional articles were excluded (Fig. 1). Among the 41 excluded articles, the exclusion criteria were studies focused on pain, studies analysing the function rate rather than fusion rate (n = 12), studies lacking a control group (n = 11), animal model studies (n = 7), review articles (n = 6), and studies not using electrical stimulation (n = 5).
Baseline characteristics
All the included studies were published between 1988 and 2020, and the baseline characteristics of the included studies are shown in Table 1. Among the 9 RCTs included, 4 [9, 12, 17, 18] used DC electrical stimulation, 4 [9, 14,15,16] used PEMF electrical stimulation, and 2 10, 13 used CC electrical stimulation. Among the 7 cohort studies included, 3 [20,21,22] used DC electrical stimulation, and 4 [11, 19, 23, 24] used PEMF electrical stimulation. Among all the studies, thirteen were conducted in the United States, two were conducted in Denmark and one was conducted in Italy. Eleven studies used X-ray imaging to evaluate the success of fusion, five studies used CT, two used both X-ray and CT, one used MRI, and one used VAS, ODI and SF-36 scores.
Effect of EST on spinal fusion
In all the studies, electrical stimulation increased the probability of successful spinal fusion by 2.66 times (OR 2.66 [95% CI 1.79–3.97]) (Fig. 3). There was a significant difference in the fusion rate between the two groups. The average fusion rate of the group treated with electrical stimulation (86.8%) was greater than that of the control group (73.7%) (Table 3). The same results were obtained regardless of which kind of electrical stimulation was used (DC, PEMF or CC). The fusion rate after DC electric stimulation treatment increased 2.33 times (OR 2.33 [95% CI 1.37–3.96]), the fusion rate after PEMF stimulation increased 2.60 times (OR 2.60 [95% CI 1.29–5.27]), and the fusion rate after CC stimulation increased 3.44 times (OR 3.44 [95% CI 1.75–6.75]) (eFigure 1).
In the RCT, the fusion rate of the electrical stimulation group was also significantly higher (OR 2.10 [95% CI 1.35–3.27]) (eFigure 2) than that of the control group (81.2% and 68.2%, respectively) (Table 3), while in the cohort study, the electrical stimulation group also showed a higher fusion rate (OR 3.80 [95% CI 1.93–7.49]) (eFigure 3) than the control group (93.2% and 80.7%, respectively) (Table 3). Sensitivity analysis revealed that after excluding a single study, the combined OR did not significantly change (lowest OR = 2.47, 1.70 to 3.59; highest OR = 2.92, 1.97 to 4.33) (eTable 2). A visual examination of the funnel plot showed that the data were basically symmetrical, indicating that there was no publication bias (eFigure 4).
Effect of DC stimulation on spinal fusion
RCT data
A total of 4 RCTs [9, 12, 17, 18] reported the relationship between DC and the postoperative fusion rate after spinal surgery. Posterolateral fusion was used in all the operations studied by an RCT with DC electrical stimulation. The fusion rate of the treatment group was 65.7% (30%-93.8%), while that of the control group was 54.5% (24.0%-83.4%) (Table 3). However, there was no significant difference in the effect of DC electrical stimulation or nonelectrical stimulation (OR 1.50 [95% CI 0.84–2.69]) (Fig. 4) on the fusion rate according to the meta-analysis. Sensitivity analysis revealed that after excluding a single study, the combined odds ratio (OR) did not significantly change (the lowest OR = 1.14, 0.60 to 2.16; the highest OR = 1.86, 0.86 to 4.05) (eTable 2). A visual examination of the funnel plot showed that the data were basically symmetrical, indicating no publication bias (eFigure 5).
Cohort study
Three cohort studies [20,21,22] examined the effect of DC on spinal fusion; only one study [21] used only posterolateral fusion, and the other two studies [20, 22] used both anterior and posterior lumbar interbody fusion. The fusion rate in the treatment group was 94.3% (90.9%-97.0%), while that in the control group was 81.5% (73.3%-86.0%) (Table 3). According to our meta-analysis, the average fusion rate of patients receiving DC stimulation was almost 4 times greater than that of patients not receiving electrical stimulation (OR 4.03 [95% CI 2.12–7.66]) (Fig. 4). Sensitivity analysis revealed that after excluding a single study, the combined OR did not significantly change (the lowest OR = 3.71, 1.30 to 10.59; the highest OR = 4.26, 2.06 to 8.84) (eTable 2). A visual examination of the funnel plot showed that the data were basically symmetrical, indicating no publication bias (eFigure 6).
Effect of PEMF therapy on spinal fusion
RCT data
Four RCTs [9, 14,15,16] reported the effect of PEMF electrical stimulation as an adjuvant therapy on the fusion rate after spinal surgery. All operations were performed with interbody fusion. The fusion rate was 90.5% (85.1%-94.9%) in the treatment group and 83.0% (72.6%-91.4%) in the control group (Table 3). According to the results of the meta-analysis, there was no obvious difference in the average fusion rate between the treatment group and the control group (OR 2.03 [95% CI 0.91–4.54]) (Fig. 4). Sensitivity analysis revealed that after excluding a single study, the combined OR did not significantly change (lowest OR = 1.37, 0.84 to 2.23; highest OR = 2.82, 1.20 to 6.66) (eTable 2). A visual examination of the funnel plot showed that the data were basically symmetrical, indicating no publication bias (eFigure 7).
Cohort study data
A total of 4 cohort studies [11, 19, 23, 24] examined the effect of PEMF stimulation on spinal fusion. The fusion rate was 91.8% (85.8%-96.4%) in the treatment group and 79.6% (59.8%-94.3%) in the control group (Table 3). According to the results of the meta-analysis, there was no significant difference in the effect of PEMF stimulation on the spinal fusion rate between the PEMF stimulation group and the nonstimulation group (OR 3.46 [95% CI 0.84–14.31]) (Fig. 4). Sensitivity analysis revealed that after excluding a single study, the combined OR did not significantly change (lowest OR = 2.11, 0.54 to 8.28; highest OR = 5.67, 1.79 to 17.90) (eTable 2). A visual examination of the funnel plot showed that the data were basically symmetrical, indicating no publication bias (eFigure 8).
Effect of CC stimulation on spinal fusion
Only 2 RCTs [10, 13] described the application of CC electrical stimulation in spinal fusion patients, and both of these studies involved anterior and posterior lumbar interbody fusion. The fusion rate of the treatment group was 85.7% (77.8%-92.3%), which was significantly greater than that of the control group (62.7%) (53.2%-71.9%) (Table 3). According to our meta-analysis, the average fusion rate among patients who received CC stimulation was significantly greater than that of patients who did not receive electrical stimulation (OR 3.44 [95% CI 1.75–6.75]) (Fig. 4). A visual examination of the funnel plot showed that the data were basically symmetrical, indicating no publication bias (eFigure 9).
Subanalysis
Meta-analysis revealed that the success rate of fusion in the group of patients receiving some form of electrical stimulation was almost 118% greater than that in the control group. Considering that smoking status and the degree of fusion may affect the fusion rate among patients, for example, patients who smoke have a high incidence of postoperative complications after spinal surgery, we were interested in determining whether there were differences in treatment among these subgroups. Table 4 summarizes the subgroup meta-analysis of randomized controlled trials and cohort studies (eFigure 10, eFigure 11; eFigure 12, eFigure 13). The variables included smoking history, nonsmoking status, single- and multi-segment fusion status, and whether the patients underwent internal fixation.
Notably, in the RCTs, the fusion rate among patients who received electrical stimulation was greater than that of patients who did not receive electrical stimulation, and the fusion rate of patients who received electrical stimulation without internal fixation was slightly lower than that of patients who did not receive electrical stimulation, which may be due to the decrease in the intensity of electrical stimulation received by patients without internal fixation. Only patients who underwent multi-segment fusion and internal fixation were included in this subgroup, and the difference in the fusion rate between the two groups was significant. In contrast, there was no obvious difference in the fusion rate between patients in the electrical stimulation group and those in the control group in the following subgroups: smokers, nonsmokers, single-segment fusion patients, and patients without internal fixation.
In the cohort studies, the fusion rate of patients who received electrical stimulation was obviously greater than that of patients who did not receive electrical stimulation. In addition, in all the subgroups we investigated, a meta-analysis showed that there was a marked difference in the fusion rate between patients in the electrical stimulation group and patients in the control group.
Discussion
Principal findings
Spinal fusion is one of the most important operations for the treatment of spinal disease, and successful spinal fusion is still a challenge. We performed this meta-analysis to comprehensively evaluate the effect of electrical stimulation on the fusion rate after spinal fusion surgery. The meta-analysis gathered data from 16 studies, 9 RCTs [9, 10, 12,13,14,15,16,17,18] and 7 cohort studies [11, 19,20,21,22,23,24] and revealed that spinal fusion patients who received some kind of electrical stimulation had better surgical fusion rates than did those who did not receive electrical stimulation.
Comparison with other studies
We found that the effect of electrical stimulation on the fusion rate after spinal surgery was similar to that of most previously published RCTs or cohort studies, most of which showed that electrical stimulation could increase the fusion rate after spinal surgery. However, the study of Cheaney, B. et al. [11] involved 72 participants but did not conclude that electrical stimulation was associated with a better fusion rate. This may be because the retrospective study did not consistently obtain patient bone mineral density information or data on patient compliance with electrical stimulation. In a subgroup analysis of smoking and nonsmoking individuals, Jenis, L. G et al. [9] did not find that the postoperative fusion rate in the electrical stimulation group was better than that in the nonelectrical stimulation group. This may be related to the duration and mode of the electrical stimulation intervention and the lack of research participants. This study, the most comprehensive to date, involved 2151 participants and meta-analysed the relationship between different types of electrical stimulation and spinal fusion rates.
Potential mechanisms
The mechanism through which electrical stimulation increases the rate of spinal fusion is complex and multifaceted and involves a variety of biological and physiological mechanisms. Although a large number of studies have been carried out, there is no definite evidence that electrical stimulation directly increases the rate of spinal fusion. However, some studies have suggested possible mechanisms that can explain how electrical stimulation contributes to fracture healing and spinal fusion.
Studies have shown that electrical stimulation can promote the proliferation and differentiation of bone cells (such as osteoblasts and chondrocytes), thus contributing to the formation of new bone tissue [25]. This can be achieved by regulating cellular signalling pathways and gene expression. For example, electrical stimulation can promote the proliferation and differentiation of osteocytes by activating the Wnt/β-catenin signalling pathway, thereby promoting bone formation [26, 27]. Moreover, electrical stimulation can improve blood perfusion in surrounding tissue by increasing capillary volume [28]. Improving blood flow can help eliminate metabolites and provide the necessary growth factors, which are essential for the supply of oxygen and nutrients to support the growth of new bone tissue [29]. Moreover, electrical stimulation can promote collagen deposition by regulating the osteogenesis of MC3T3-E1 cells, which helps to maintain the stability of the fracture or fusion area [30]. Several scholars have also shown that inflammation may have a negative impact on bone fusion. Electrical stimulation can promote bone fusion by reducing the inflammatory response and reducing the interference of inflammation with the healing process [31].
It should be noted that although some studies support the positive effect of electrical stimulation on spinal fusion, there is no clear evidence that electrical stimulation directly increases the rate of spinal fusion. Therefore, more studies are needed to further verify the effectiveness and safety of electrical stimulation as a method for promoting spinal fusion.
Implications
Our study has several implications for the clinical practice of spinal fusion surgery. We evaluated the success rate of spinal fusion in patients who did and did not receive electrical stimulation. In the early stage before surgery, different treatment methods can be chosen according to the different conditions of patients, which has important clinical significance for improving the success rate of fusion after spinal surgery. At present, there is still some controversy about whether spinal surgery should be assisted by electrical stimulation and which kind of electrical stimulation should be used, and improving the spinal fusion rate is still a difficult problem. This study may provide new reference value for clinicians when dealing with these patients, which is helpful for improving the fusion rate after spinal surgery.
Strengths and limitations
The main strengths of our meta-analysis are as follows. First, the relevant articles included in this study were determined to be the most comprehensive in the meta-analysis of this topic. Other articles included original articles without comparison, while the original articles included in this meta-analysis included control groups, which provided the latest evidence that electrical stimulation increases the fusion rate after spinal surgery. Second, our meta-analysis included a wider range of RCTs and cohort studies. Third, subject words and free words were used to comprehensively search the literature in the PubMed, Embase and Cochrane Library databases, and a retrieval strategy with no language or date restriction was used. In this way, more original articles that met the inclusion criteria could be found, thus avoiding the influence of publication bias on the final results.
There are still some limitations in the existing research. First, we included two articles that showed that electrical stimulation did not increase the fusion rate after spinal surgery, which had a certain impact on our results. Second, we found that regarding single-segment fusion and multi-segment fusion, most of the electrical stimulation methods were meaningless and had high heterogeneity, which may be due to the small sample size. In view of these limitations, additional studies including additional subjects are needed in the future. Thirdly, our results were not confirmed to the same degree in the cohort studies as in the RCTs. This discrepancy may be attributed to differences in study design, patient populations, and methods used to assess fusion outcomes. Cohort studies may be more susceptible to selection bias, potentially inflating the perceived effects of electrical stimulation, while RCTs, with their randomized design, provide a more rigorous evaluation but may include factors that diminish the observed effect size. Finally, Conducting a subgroup analysis based on underlying condition (traumatic fracture, pathologic fracture, degenerative disease, or spinal dysraphism as mentioned above) or presence of osteoporosis is indeed important. Unfortunately, in our current dataset, the information required to consistently categorize patients according to these specific underlying conditions or osteoporosis status was not uniformly reported across all studies.
Conclusions
The present meta-analysis of the effect of electrical stimulation on the fusion rate after spinal surgery showed that electrical stimulation, as an adjuvant therapy, can improve the fusion rate after spinal surgery to some extent. However, the effectiveness of electrical stimulation in improving the fusion rate after spinal surgery needs to be further evaluated in large studies.
Data availability
No datasets were generated or analysed during the current study.
References
Raciborski F, Gasik R, Kłak A (2016) Disorders of the spine. A major health and social problem. Reumatologia 54:196–200. https://doi.org/10.5114/reum.2016.62474
Bartleson JD (2006) Spine disorder case studies. Neurol Clin 24:309–330. https://doi.org/10.1016/j.ncl.2006.01.004
Sąsiadek M, Jacków-Nowicka J (2024) Degenerative disease of the spine: How to relate clinical symptoms to radiological findings. Adv Clin Exp Med 33:91–98. https://doi.org/10.17219/acem/163357
Preston G, Hoffmann J, Satin A, Derman PB, Khalil JG (2023) Preservation of Motion in Spine Surgery. J Am Acad Orthop Surg 31:e356–e365. https://doi.org/10.5435/jaaos-d-22-00956
Blumenthal SL, Baker J, Dossett A, Selby DK (1988) The role of anterior lumbar fusion for internal disc disruption. Spine (Phila Pa 1976) 13:566–569. https://doi.org/10.1097/00007632-198805000-00023
Morone MA, Feuer H (2002) The use of electrical stimulation to enhance spinal fusion. Neurosurg Focus 13:e5
Eck JC, Hodges SD, Humphreys SC (2001) Techniques for stimulating spinal fusion: efficacy of electricity, ultrasound, and biologic factors in achieving fusion. Am J Orthop (Belle Mead NJ) 30:535–541
Hara S et al (2022) Effect of Spinal Cord Burst Stimulation vs Placebo Stimulation on Disability in Patients With Chronic Radicular Pain After Lumbar Spine Surgery: A Randomized Clinical Trial. JAMA 328:1506–1514. https://doi.org/10.1001/jama.2022.18231
Jenis LG, An HS, Stein R, Young B (2000) Prospective comparison of the effect of direct current electrical stimulation and pulsed electromagnetic fields on instrumented posterolateral lumbar arthrodesis. J Spinal Disord 13:290–296. https://doi.org/10.1097/00002517-200008000-00004
Massari L et al (2020) Does capacitively coupled electric fields stimulation improve clinical outcomes after instrumented spinal fusion? A multicentered randomized, prospective, double-blind, placebo-controlled trial. Int J Spine Surg 14:936–943. https://doi.org/10.14444/7142
Cheaney B 2nd, El Hashemi M, Obayashi J, Than KD (2020) Combined magnetic field results in higher fusion rates than pulsed electromagnetic field bone stimulation after thoracolumbar fusion surgery. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia 74:115–119. https://doi.org/10.1016/j.jocn.2020.02.012
Kane WJ (1988) Direct current electrical bone growth stimulation for spinal fusion. Spine (Phila Pa 1976) 13:363–365. https://doi.org/10.1097/00007632-198803000-00026
Goodwin CB et al (1999) A double-blind study of capacitively coupled electrical stimulation as an adjunct to lumbar spinal fusions. Spine (Phila Pa 1976) 24:1349–1356. https://doi.org/10.1097/00007632-199907010-00013. discussion 1357
Mooney V, McDermott KL, Song J (1999) Effects of smoking and maturation on long-term maintenance of lumbar spinal fusion success. J Spinal Disord 12:380–385
Linovitz RJ et al (2002) Combined magnetic fields accelerate and increase spine fusion: a double-blind, randomized, placebo controlled study. Spine 27:1383–1389. https://doi.org/10.1097/00007632-200207010-00002. discussion 1389
Foley KT et al (2008) Randomized, prospective, and controlled clinical trial of pulsed electromagnetic field stimulation for cervical fusion. Spine journal 8:436–442. https://doi.org/10.1016/j.spinee.2007.06.006
Andersen T et al (2009) The effect of electrical stimulation on lumbar spinal fusion in older patients: a randomized, controlled, multi-center trial: part 2: fusion rates. Spine 34:2248–2253. https://doi.org/10.1097/BRS.0b013e3181b02c59
Andersen T et al (2010) Fusion mass bone quality after uninstrumented spinal fusion in older patients. Eur Spine J 19:2200–2208. https://doi.org/10.1007/s00586-010-1373-2
Mooney V (1990) A randomized double-blind prospective study of the efficacy of pulsed electromagnetic fields for interbody lumbar fusions. Spine 15:708–712. https://doi.org/10.1097/00007632-199007000-00016
Meril AJ (1994) Direct current stimulation of allograft in anterior and posterior lumbar interbody fusions. Spine (Phila Pa 1976) 19:2393–2398. https://doi.org/10.1097/00007632-199411000-00004
Rogozinski A, Rogozinski C (1996) Efficacy of implanted bone growth stimulation in instrumented lumbosacral spinal fusion. Spine (Phila Pa 1976) 21:2479–2483. https://doi.org/10.1097/00007632-199611010-00014
Kucharzyk DW (1999) A controlled prospective outcome study of implantable electrical stimulation with spinal instrumentation in a high-risk spinal fusion population. Spine (Phila Pa 1976) 24:465–468. https://doi.org/10.1097/00007632-199903010-00012. discussion 469
Marks RA (2000) Spine fusion for discogenic low back pain: outcomes in patients treated with or without pulsed electromagnetic field stimulation. Adv Ther 17:57–67. https://doi.org/10.1007/bf02854838
Coric D et al (2018) Pulsed electromagnetic field stimulation may improve fusion rates in cervical arthrodesis in high-risk populations. Bone & joint research 7:124–130. https://doi.org/10.1302/2046-3758.72.Bjr-2017-0221.R1
Li P et al (2019) Promoting Proliferation and Differentiation of Pre-Osteoblasts MC3T3-E1 Cells Under Combined Mechanical and Electrical Stimulation. J Biomed Nanotechnol 15:921–929. https://doi.org/10.1166/jbn.2019.2749
Lee PS et al (2022) The interplay of collagen/bioactive glass nanoparticle coatings and electrical stimulation regimes distinctly enhanced osteogenic differentiation of human mesenchymal stem cells. Acta Biomater 149:373–386. https://doi.org/10.1016/j.actbio.2022.06.045
Keum BR, Kim HJ, Kim GH, Chang DG (2023) Osteobiologies for Spinal Fusion from Biological Mechanisms to Clinical Applications: A Narrative Review. Int J Mol Sci 24(24):17365. https://doi.org/10.3390/ijms242417365
Mathieu-Costello O, Agey PJ, Wu L, Hang J, Adair TH (1996) Capillary-to-fiber surface ratio in rat fast-twitch hindlimb muscles after chronic electrical stimulation. J Appl Physiol 80:904–909. https://doi.org/10.1152/jappl.1996.80.3.904. Bethesda, Md. : 1985
Nannmark U, Buch F, Albrektsson T (1988) Influence of direct currents on bone vascular supply. Scand J Plast Reconstr Surg Hand Surg 22:113–115. https://doi.org/10.3109/02844318809072380
Wang Y et al (2016) Modulation of Osteogenesis in MC3T3-E1 Cells by Different Frequency Electrical Stimulation. PLoS ONE 11:e0154924. https://doi.org/10.1371/journal.pone.0154924
Massari L et al (2019) Biophysical stimulation of bone and cartilage: state of the art and future perspectives. Int Orthop 43:539–551. https://doi.org/10.1007/s00264-018-4274-3
Funding
This work was supported by the Health and Family Planning Commission Program of Hunan Province (no. 202204074707), and the Health and Family Planning Commission Program of Wuhan City (no. WX18C29).
Author information
Authors and Affiliations
Contributions
ML and XZ contributed to the study conception and design. Data collection and analysis were performed by XZ, LJ and HW. The first draft of the manuscript was written by ML and XZ, and all authors commented on previous versions of the manuscript. Final version of the article reviewed by ST and ZX.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Ethical assessment and informed consent were not required since primary data collection was not undertaken.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mingjiang Luo and Xin Zeng co-first authors.
Guarantor: Siliang Tang and Zhihong Xiao.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luo, M., Zeng, X., Jiang, L. et al. Effect of electrical stimulation on the fusion rate after spinal surgery: a systematic review and meta-analysis. Neurosurg Rev 47, 618 (2024). https://doi.org/10.1007/s10143-024-02874-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-024-02874-3