Abstract
In previous literatures, we found that similar studies on the short-term prognosis of synchronous brain metastases (S-BM) from other systems are rare. Our aim was to evaluate the early mortality rate of patients with S-BM from the Surveillance, Epidemiology, and End Result (SEER) database and explore the risk factors for early mortality (≤ 1 year). We used Kaplan-Meier (KM) curves to evaluate early mortality in patients with S-BM from the SEER database. Logistic regression analyses were used to identify significant independent prognostic factors in patients with a follow-up time > 12 months. And the meaningful factors were used to construct a nomogram of overall early death. The receiver operating characteristic (ROC) curve was used to test the predictive ability of the model, while the decision curve analysis (DCA) curve was used to validate the clinical application ability of the model. A total of 47,284 patients were used for univariate and multivariate logistic regression analysis to screen variables to constructing a nomogram. In the all-cause early mortality specific model, the area under the ROC (AUC) curve of the training set was 0.764 (95% confidence interval (CI): 0.758–0.769), and the AUC of the validation set was 0.761 (95% CI: 0.752–0.770). The DCA calibration curves of the training set and validation set indicate that the 1-year early mortality rate predicted by this model is consistent with the actual situation. We found that the 1-year early mortality rate was 76.4%. We constructed a validated nomogram using these covariates to effectively predict 1-year early mortality in patients with S-BM. This nomogram can help clinical workers screen high-risk patients to develop more reasonable treatment plans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 10-40% of patients with solid tumors will develop brain metastases (BM) over their clinical course [1]. The most common primary tumors that metastasize to the brain are lung cancer, breast cancer, and melanoma, which account for 67–80% of BM [2]. Synchronous brain metastases (S-BM) and metachronous brain metastases(M-BM) are two distinct clinical conditions. M-BM refer to those that occur during a known underlying cancer disease and have undergone multimodal treatment such as radiotherapy, chemotherapy, immunotherapy, and targeted therapy. Compared with this, S-BM refer to the initial symptoms of previously unknown systemic spreading cancer diseases [3]. S-BM show lower overall survival [4, 5]. Previous studies on the BM of lung adenocarcinoma have also elucidated the differences in tumor microenvironment and mutation profiles between S-BM and M-BM [6]. In this project, we focus on the identification of risk factors in S-BM and the prediction of survival harboring these lesions.
The five-year survival rate is a clinical indicator used to reflect the severity and progression of a certain cancer and also commonly used to evaluate the treatment effect or certain risk factors for malignant tumors [7, 8]. However, the malignancy and progression rate of different tumors vary. This means that the evaluation indicators for different malignant tumors are distinct. Previous studies have also used a three-month survival rate to evaluate the risk factors for malignant tumors and provide some clinical evidence for determining short-term treatment plans for BM [9]. Currently, little is known about the early mortality rate and related risk factors in patients with S-BM. Therefore, identifying the risk factors for early death is of great significance for the prognosis evaluation and clinical treatment guidance of patients with S-BM.
This study focused on patients with S-BM of solid tumors in the SEER database to evaluate the incidence of overall survival rate and explore risk factors for 1-year early mortality. In addition, we developed a nomogram that includes some prognostic factors for predicting 1-year early mortality in S-BM patients.
Materials and methods
Data and study population
We retrospectively analyzed the public data registered in the SEER database from 2000 to 2019, covering 17 registration centers in different regions of the USA. In our study, data from malignant solid tumor patients with S-BM were extracted from the SEER database (2010–2017). The variables included sex, age at diagnosis, race, marital status at diagnosis, primary site, histological type, tumor laterality, T-stage, N-stage, whether or not bone metastasis, whether or not liver metastasis, whether or not lung metastasis, surgery records, chemotherapy records and radiation records. The primary endpoint was all-cause mortality within 12 months.
All patients with S-BM of solid tumor were selected according to the International Classification of Diseases for Oncology, Third Edition (ICD-O-3) standard: head and neck cancer: C0-C14,C76; thyroid: C73,9; lung: C34; breast: C50; colorectal: C18-C21; liver: C22; kidney: C64.9, C65.9; ovary: C56; uterus: C54; prostate: C61.9; melanoma: 872, 873, 874, 875, 876, 877, 878, 879; Other solid tumors. The exclusion criteria were as follows: (a) patients with missing or incomplete information about survival time, survival status, or other important characteristics; (b) preinvasive carcinoma and primary intracranial tumor; and (c) patients with a follow-up time less than 12 months.
Construction and evaluation of the nomogram
Pathological and clinical characteristics such as sex, age at diagnosis, race, marital status at diagnosis, primary site, histological type, tumor laterality, T-stage, N-stage, whether or not bone metastasis, whether or not liver metastasis, whether or not lung metastasis, surgery records, chemotherapy records and radiation records were used for analysis. We randomly selected 70% of the patients included from 2010 to 2017 as the training set and the remaining 30% as the validation set [10]. For the training set, we used univariate and multivariate logistic regression analyses to determine independent risk factors and calculate odds ratio (OR), and 95% confidence intervals (CI). Then, we established a nomogram for predicting the 1-year early mortality of S-BM using these identified independent risk factors.
We evaluated the discrimination of the nomogram by drawing the receiver operating characteristic (ROC) curve [11], drawing a calibration plot to evaluate the calibration of the nomogram, and drawing a decision curve analysis (DCA) curve [12] to evaluate the effectiveness of the nomogram. The value of the area under the ROC curve (AUC) greater than 0.7 indicates a reasonable evaluation. The calibration plot connects the value points of different groups with line segments to form a calibration line in turn and compares it with the standard line of y = x; the closer the calibration curve of the model to the standard line, the better the calibration ability of the model. The nomogram was evaluated by measuring the above curves both internally (training set) and externally (validation set) [13].
Statistical analysis
All statistical analyses were conducted by the R software version 4.3.2 (Institute for Statistics and Mathematics, Vienna, Austria; www.r-project.org). Classified data are described using frequency and percentage (N, %), and continuous data are represented by the mean ± standard deviation (MD ± SD). Early death, defined as death within 1 year after diagnosis, is the endpoint of interest in this study. A two-tailed P value less than 0.05 was considered statistically significant. We used the Kaplan-Meier (KM) method to draw survival curves.
Results
Patient characteristics
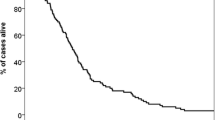
We primarily collected 52,665 S-BM of solid tumor patients from the SEER database from 2010 to 2017 (for longer follow-up time), excluding patients with missing or incomplete important characteristics, leaving 47,572 patients. Then, the KM curve was used to evaluate the overall survival rate of patients, and it was found that the overall survival curve of patients rapidly decreased within 1 year, while the survival rate of patients showed a slow downward trend after 1 year (Fig. 1). Therefore, predicting the 1-year survival rate of patients with S-BM has explicit clinical significance for patient management. Then, patients with a follow-up time of less than 12 months were excluded. Finally, 47,284 patients were included in the research (Fig. 2).
The patients were divided into the training (N = 33,098) and validation (N = 14,186) sets at a ratio of 7:3 using the R function “createDataPartition” to ensure that outcome events were distributed randomly between the two sets [11]. There was no statistically significant difference in baseline data between the training and validation sets. The demographics and tumor characteristics of the patients are summarized in Table 1.
Identifying independent factors
We used univariate and multivariate logistic regression analyses to identify risk factors associated with early mortality in patients with S-BM of the training set. The results of univariate and multivariate factor analyses are shown in Table 2.
In univariate analysis, all clinical and pathological features including age, sex, marital status, race, primary tumor location, primary tumor side, T-stage, N-stage, whether or not bone metastasis, whether or not liver metastasis, whether or not lung metastasis, whether or not surgery, whether or not radiotherapy and whether or not chemotherapy were associated with the likelihood of early death. The results of multivariate logistic regression analysis reveal that age (for each year of increase, OR = 1.029, 95% CI: 1.026–1.032, P < 0.001), other marital status (vs. married, OR = 1.272, 95% CI: 1.200-1.348, P < 0.001), primary tumor in the uterus (vs. head&neck, OR = 2.328, 95% CI: 1.082–4.914, P = 0.028), T-2 (vs. T-1, OR = 1.420, 95% CI: 1.292–1.560, P < 0.001), T-3 (vs. T-1, OR = 1.656, 95% CI: 1.499–1.829, P < 0.001), T-4 (vs. T-1, OR = 1.758, 95% CI: 1.596–1.937, P < 0.001), T Other (vs. T-1, OR = 1.543, 95% CI: 1.376–1.731, P < 0.001), N-1 (vs. N-0, OR = 1.272, 95% CI: 1.153–1.403, P < 0.001), N-2 (vs. N-0, OR = 1.542, 95% CI: 1.428–1.665, P < 0.001), N-3 (vs. N-0, OR = 1.654, 95% CI: 1.509–1.814, P < 0.001), N Other (vs. T-1, OR = 1.250, 95% CI: 1.104–1.415, P < 0.001), patients with synchronous bone metastasis (vs. none, OR = 1.229, 95% CI: 1.153–1.310, P < 0.001), synchronous liver metastasis (vs. none, OR = 2.008, 95% CI: 1.854–2.177, P < 0.001) and synchronous lung metastasis (vs. none, OR = 1.101, 95% CI: 1.027–1.181, P = 0.007) are all associated with a higher risk of early mortality. Contrary to above, the results including female (vs. male, OR = 0.691, 95% CI: 0.651–0.733, P < 0.001), API race (vs. NHW, OR = 0.441, 95% CI: 0.401–0.486, P < 0.001), other race (vs. NHW, OR = 0.775, 95% CI: 0.699–0.859, P < 0.001), primary tumor located in the prostate (vs. head&neck, OR = 0.079, 95% CI: 0.040–0.151, P < 0.001), primary tumor located in the breast (vs. head&neck, OR = 0.305, 95% CI: 0.163–0.544, P < 0.001), the primary tumor was melanoma (vs. head&neck, OR = 0.368, 95% CI: 0.195–0.661, P < 0.001), surgery (vs. none, OR = 0.365, 95% CI: 0.324–0.410, P < 0.001), radiation therapy (vs. none, OR = 0.695, 95% CI: 0.647–0.747, p < 0.001) and chemotherapy (vs. none, OR = 0.215, 95% CI: 0.201–0.231, P < 0.001) are associated with a lower risk of early mortality.
Nomogram construction and validation
We successfully constructed a nomogram (Fig. 3) to predict the probability of 1-year early mortality of S-BM patients based on the independent risk factors screened by the multivariate logistic regression in the training set. Each indicator has a specific value. By projecting the values onto the points scale in the first row, the corresponding scores for each indicator can be obtained. The total score is obtained by adding up the scores of all indicators. By calculating the sum of the scores assigned to each variable, the sum can correspond to the probability of early death.
In the training and validation sets, the AUC values for predicting S-BM in patients with solid tumors were 0.764 (95% CI: 0.758–0.769) and 0.761 (95% CI: 0.752–0.770), respectively, indicating that the nomogram has exact discriminative ability (Fig. 4a and b). The calibration curve of the nomogram shows that the predicted and observed early mortality rates are highly consistent (Fig. 5a and b). In addition, DCA analysis indicates that the model has excellent clinical application value (Fig. 6a and b).
Discussion
Brain metastases are the most common cause of malignant brain tumors in adults.
Of the nearly 1.5 million patients in the USA who received a primary diagnosis of cancer in 2007, approximately 70 000 of these primary diagnoses are estimated to eventually relapse in the brain [14, 15]. Patients with solid tumors present with either synchronous, latent, or metachronous brain metastases that exhibit different manifestations [16]. For the large and retrospective cohorts, undertaken in 17 registries in the USA, we found a 12-month early mortality rate of 76.4% in patients with S-BM. To our knowledge, this study represents the largest cohort of patients with a total of 47,284 patients, and it is the first study to use the SEER database to evaluate risk factors associated with early death in the entire population of patients with S-BM.
Most studies focus on the long-term survival of patients with brain metastases [17, 18], and there is a lack of research exploring the early mortality of these patients. Shen et al. investigated the risk factors for early mortality in patients with synchronous brain metastases from lung cancer. They found that the definition of early death varies from study to study and could be defined as 30 days to 3 months after diagnosis typically. In their study, death that occurred within 3 months was chosen as early death [9]. However, in our view, their setting lacks scientific validity. Cagney et al. studied 26,430 patients diagnosed with invasive solid malignant tumors originating outside of the central nervous system (CNS). Patients with prostate cancer (median survival 12.0 months) displayed the longest survival while that of pancreatic cancer patients (median survival period of 2 months) was the shortest. The median survival time for the most patients was between 4 and 10 months [19]. Therefore, setting early death as three months is not reasonable enough. Our K-M curve based on the population of brain tumor patients suggests that the overall survival curve of patients rapidly decreases near 1 year and tends to flatten after 1 year. Thus, setting early death at 12 months is more reasonable for patients with brain metastases, which is also convenient for comparison with other similar studies [20, 21].
The study of early death has been applied to advanced cancers in other systems and has shown important clinical significance. Kouka et al. found that patients who were male, were older, had a larger tumor diameter, had distant metastasis, and had oral, oropharyngeal, and hypopharyngeal tumors had a significantly higher 180-day mortality rate [22]. Li et al. analyzed data from 1140 patients in the SEER database to evaluate the overall survival and early mortality rates of patients with pheochromocytoma and paraganglioma. Simultaneously, logistic regression analysis was used to identify risk factors and construct a nomogram for early mortality [23]. Similarly, our study also employed logistic regression for risk factor analysis and constructed a nomogram for predicting the 1-year early mortality of synchronous brain metastases, which can be helpful for clinical practitioners in patient management. The Cox proportional risk model focuses on the survival time of patients, but when analyzing highly heterogeneous tumors such as brain metastases, there may be significant biases, which would lead to inaccurate predictions and other issues. Therefore, we adopted logistic regression analysis to identify the factors associated with early 1-year mortality, and that makes our analysis results more accurate and practical.
At present, the treatment of brain metastases usually includes a combination of surgery, radiation therapy, and/or systemic drug therapy, with the overall goal of selecting the best treatment plan for individual patients to maximize their quality of life and overall survival [24]. The SEER database does not provide information related to hormone therapy, targeted therapy, and immunotherapy for patients, which is an inherent limitation of the SEER database. However, the SEER database provides an overview of whether patients undergo surgery, radiation therapy, and chemotherapy after their initial diagnosis, and that had been confirmed in the research of Shen. Nevertheless, Shen et al. overlooked the impact of surgery, radiotherapy, and chemotherapy on prognosis and did not provide a reasonable explanation when conducting risk factor analysis and establishing predictive models. We have fully considered the impact of treatment on the prognosis of S-BM and establish a nomogram for predicting early death, which can better assist clinical management decisions and generate high clinical value. That provides a widely recognized method by researchers [25, 26]. The nomogram of our research showed good predictive ability and clinical applicability. Meanwhile internal validation of the nomogram showed good agreement between the predicted early deaths and the actual deaths. DCA curves showed that our nomogram has good clinical value and practicability in predicting the survival rate.
Apart from the limitations mentioned in the above chapters, this study has certain limitations. Due to the lack of data on recurrence, different treatment methods, and hormone status in the SEER database, we were unable to conduct research and calculate progression-free survival. In addition, the SEER database does not provide information on medical complications that are important for evaluating quality of life, mental health, and disease burden. Additionally, our preliminary results and predictive model should be externally validated.
Conclusion
We used the SEER database to evaluate the risk factors for 1-year early mortality in patients with S-BM, and found that the 1-year early mortality rate was 76.4%. Our data show a total of 13 covariates associated with 1-year early mortality. We constructed a validated nomogram using these covariates to effectively predict 1-year early mortality in patients with S-BM. This nomogram can help clinical workers screen high-risk patients to develop more reasonable treatment plans.
Data availability
No datasets were generated or analysed during the current study.
Code availability
Not applicable.
Change history
29 July 2024
A Correction to this paper has been published: https://doi.org/10.1007/s10143-024-02590-y
References
Lamba N, Wen PY, Aizer AA (2021) Epidemiology of brain metastases and leptomeningeal disease. Neuro Oncol 23:1447–1456. https://doi.org/10.1093/neuonc/noab101
Nayak L, Lee EQ, Wen PY (2012) Epidemiology of brain metastases. Curr Oncol Rep 14:48–54. https://doi.org/10.1007/s11912-011-0203-y
Achrol AS, Rennert RC, Anders C et al (2019) Brain metastases. Nat Reviews Disease Primers 5. https://doi.org/10.1038/s41572-018-0055-y
Che W, Liu J, Fu T et al (2022) Recent trends in Synchronous Brain Metastasis incidence and mortality in the United States: ten-year Multicenter Experience. Curr Oncol 29:8374–8389. https://doi.org/10.3390/curroncol29110660
Ruste V, Sunyach MP, Tanguy R et al (2021) Synchronous brain metastases as a poor prognosis factor in clear cell renal carcinoma: a strong argument for systematic brain screening. J Neurooncol 153:133–141. https://doi.org/10.1007/s11060-021-03751-5
Chen J, Yang H, Zhao C et al (2023) Mutational signatures of synchronous and metachronous brain metastases from lung adenocarcinoma. Experimental Hematol Oncol 12. https://doi.org/10.1186/s40164-023-00418-x
Geisler JP, Geisler HE, Wiemann MC et al (1999) p53 expression as a prognostic indicator of 5-year survival in endometrial cancer. Gynecol Oncol 74:468–471. https://doi.org/10.1006/gyno.1999.5482
Cortes JE, Kim DW, Pinilla-Ibarz J et al (2018) Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial. Blood 132:393–404. https://doi.org/10.1182/blood-2016-09-739086
Shen H, Deng G, Chen Q et al (2021) The incidence, risk factors and predictive nomograms for early death of lung cancer with synchronous brain metastasis: a retrospective study in the SEER database. BMC Cancer 21:825. https://doi.org/10.1186/s12885-021-08490-4
Che W-Q, Li Y-J, Tsang C-K et al (2023) How to use the Surveillance, Epidemiology, and end results (SEER) data: research design and methodology. Military Med Res 10. https://doi.org/10.1186/s40779-023-00488-2
Wu J, Zhang H, Li L et al (2020) A nomogram for predicting overall survival in patients with low-grade endometrial stromal sarcoma: a population-based analysis. Cancer Commun (London England) 40:301–312. https://doi.org/10.1002/cac2.12067
Fitzgerald M, Saville BR, Lewis RJ (2015) Decision curve analysis. JAMA 313:409–410. https://doi.org/10.1001/jama.2015.37
Song W, Lv CG, Miao DL et al (2018) Development and validation of a nomogram for predicting survival in patients with gastrointestinal stromal tumours. Eur J Surg Oncol 44:1657–1665. https://doi.org/10.1016/j.ejso.2018.07.004
Lin NU, Lee EQ, Aoyama H et al (2015) Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol 16:e270–e278. https://doi.org/10.1016/s1470-2045(15)70057-4
Davis FG, Dolecek TA, McCarthy BJ et al (2012) Toward determining the lifetime occurrence of metastatic brain tumors estimated from 2007 United States cancer incidence data. Neuro Oncol 14:1171–1177. https://doi.org/10.1093/neuonc/nos152
Parida PK, Marquez-Palencia M, Nair V et al (2022) Metabolic diversity within breast cancer brain-tropic cells determines metastatic fitness. Cell Metabol 34:90–105e7. https://doi.org/10.1016/j.cmet.2021.12.001
Ascha MS, Ostrom QT, Wright J et al (2019) Lifetime Occurrence of Brain Metastases Arising from Lung, Breast, and Skin Cancers in the Elderly: A SEER-Medicare Study. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 28: 917–925. https://doi.org/10.1158/1055-9965.Epi-18-1116
Wang M, Wu Q, Zhang J et al (2021) Prognostic impacts of extracranial metastasis on non-small cell lung cancer with brain metastasis: a retrospective study based on surveillance, epidemiology, and end results database. Cancer Med 10:471–482. https://doi.org/10.1002/cam4.3562
Cagney DN, Martin AM, Catalano PJ et al (2017) Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: a population-based study. Neuro Oncol 19:1511–1521. https://doi.org/10.1093/neuonc/nox077
Kumar SK, Dispenzieri A, Lacy MQ et al (2014) Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia 28:1122–1128. https://doi.org/10.1038/leu.2013.313
McQuilten Z, Wellard C, Moore E et al (2022) Predictors of early mortality in multiple myeloma: results from the Australian and New Zealand Myeloma and related diseases Registry (MRDR). Br J Haematol 198:830–837. https://doi.org/10.1111/bjh.18324
Kouka M, Buentzel J, Kaftan H et al (2022) Early mortality among patients with Head and Neck Cancer diagnosed in Thuringia, Germany, between 1996 and 2016-A Population-based study. Cancers 14. https://doi.org/10.3390/cancers14133099
Li H, Abbas KS, Abdelazeem B et al (2022) A predictive nomogram for early death in Pheochromocytoma and Paraganglioma. Front Oncol 12:770958. https://doi.org/10.3389/fonc.2022.770958
Suh JH, Kotecha R, Chao ST et al (2020) Current approaches to the management of brain metastases. Nat Rev Clin Oncol 17:279–299. https://doi.org/10.1038/s41571-019-0320-3
Lyu X, Luo B (2021) Prognostic factors and survival prediction in HER2-positive breast cancer with bone metastases: a retrospective cohort study. Cancer Med 10:8114–8126. https://doi.org/10.1002/cam4.4326
Xiong Y, Cao H, Zhang Y et al (2019) Nomogram-predicted survival of breast Cancer brain metastasis: a SEER-Based Population Study. World Neurosurg 128:e823–e834. https://doi.org/10.1016/j.wneu.2019.04.262
Acknowledgements
The authors would like to thank Yahui Lei, Phd. for her help with the preparation and editing of this paper.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
LY. L. and WQ.C. collected data and wrote the main manuscript text; BD.X. and YJ.L. processed data; J.L. revised the manuscript text; YS.Z conceived and revised the manuscript text.All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
All patient data in NCI SEER is anonymized, and the study received a waiver for consent from the ethics committee due to its retrospective nature and guaranteed patient privacy.
Consent to participate
Not applicable as the SEER database was analyzed retrospectively.
Consent for publication
All of the authors consented to have their work published for free.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Article Note
The original online version of this article was revised:
The authors regret that they missed to include the below article note in the original published article.
Leiyuan Liu and Wenqiang Che contributed equally to the work in the article.
The original article has been corrected.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, L., Che, W., Xu, B. et al. Risk factors, prognostic factors, and nomograms for synchronous brain metastases of solid tumors: a population-based study. Neurosurg Rev 47, 296 (2024). https://doi.org/10.1007/s10143-024-02519-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-024-02519-5