Abstract
Objective
The article analyzes the clinical features, morphological characteristics, surgical subtleties and long-term outcome of surgery in 89 cases of ‘large’ sized AVMs.
Materials and methods
During the period 2004 to 2022, 89 cases of ‘large’ arteriovenous malformations were operated in the neurosurgery departments of the authors. Large AVMs were defined as those that were more than 4 cm on either lateral or antero-posterior view of digital subtraction angiogram. The factors that determined the extent of surgical difficulties included site and eloquence of the area, number of feeding vascular territories and draining veins, degree and rate of flow, presence of flow-related aneurysms, and the physical nature of the arteriovenous malformation.
Results
There were 59 males and 30 females and the average age was 32 years. Headache, giddiness and convulsions were the common presenting complaints. Six patients were unconscious after surgery. Of these, five patients died in the immediate post-operative period and one patient gradually recovered. Additionally, seven patients developed unilateral limb weakness that included hemiplegia (4 patients) and hemiparesis (3 patients) following surgery. Clinical follow-up ranged from 6 months to 18 years (average 43 months). All surviving patients are leading normal and essentially symptom free life and have recovered from their symptoms of headache, convulsions and giddiness.
Conclusions
Large AVMs are amenable to ‘curative’ surgery with ‘acceptable’ results. The surgery can be challenging and appropriate case selection that is based on the surgeons experience is vital and decisive.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The treatment of arteriovenous malformations (AVM’s) has undergone a wide swing in the last two decades with the emergence and subsequent acceptance of endovascular neuroradiological treatment and the non-invasive method of treatment by radiosurgery [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15]. Despite this, the century old and well established surgical method of treatment constitutes the principal and accepted form of treatment [1, 2, 6, 9]. Large AVM’s are referred to as the ‘last chapter’ in neurosurgery, meaning thereby that all neurosurgical knowledge, philosophy and technique have to be incorporated in the understanding of the pathology, analysis of the patient, taking decision regarding treatment and in conduct of the surgery.
Size, ‘eloquence’ of location and type of feeding vessels and venous drainage have been identified to be defining variables that determine the difficulties that will be encountered during the conduct of surgical procedure. We report our experience with 89 cases of large AVMs wherein the nidus was more than 4 cm in its maximum dimension when measured on lateral profile or anteroposterior imaging of DSA as per the criteria laid down by Luessenhop [16, 17]. Such large AVMs form a discrete sub- group of cases and only a few reports are available in the literature that focus on the surgery related issues and elaborate subtleties of their management [8, 9, 11, 18,19,20].
Materials and methods
During the period 2004 to November 2022, 350 patients with AVMs were surgically treated in the departments of neurosurgery of the authors. For the presented study, 89 cases were selected from this patient cohort wherein on lateral profile or anteroposterior imaging by digital subtraction angiography the maximum dimension of the AVM nidus exceeded 40 mm. Only those cases have been included who underwent surgical treatment. This is a retrospective analysis of these consecutively treated cases. All patients and relatives were duly explained about the various issues that were involved particularly about the potential risk of neurological deficits and even death and a possibility of cure from the otherwise ‘dangerous’ intracranial lesion. A written ‘informed’ consent was taken.
There were 59 males and 30 females. The ages of the patient ranged from 13 to 63 years (average 32 years). Table 1 shows the presenting clinical symptoms. Ninteen patients presented with acute symptoms related to bleed from AVM and in 70 patients symptoms were chronic or long-standing. Ten patients had varying degrees of limb weakness at the time of initial presentation. Apart from MRI and/or CT scan, all patients underwent digital subtraction angiography. Three-dimensional models of the AVM were made in 19 cases [21]. Tables 1 and 2 summarise the sites of the AVM, nature of feeding and draining vessels and the principle characteristics of the AVM nidus. Five patients had undergone preoperative embolization prior to neurosurgery consultation in our own Institution (3 cases) or elsewhere (2 cases). AVMs were classified as per standard and validated Spetzler-Martin classification (Table 3).
Basic surgical steps
The chief operating surgeon himself prepared a road map of surgery. Whenever possible, an attempt was made to witness the procedure of DSA personally, as it gave an idea of the intensity, direction and nature of blood flow in the AVM nidus.
Acute surgery that involved removal of the hematoma and resection of the AVM was done in 2 patients. Both these patients developed hemorrhage during the procedure of endovascular embolization. In all other patients, the surgery was done as a planned procedure. Decompressive craniectomy was not done in any patient. Standard surgical procedures and microdissection techniques described in the literature for surgery on AVMs were employed and are summarized [1, 12, 15, 21]. Large sized craniotomy was done that delineated the AVM nidus circumferentially and additionally exposed a significant part of the brain to allow dissection of the nidus and isolation and handling of the feeding and draining vessels. In cases where the feeding artery had an aneurysm, the surgical exposure generally included access to the aneurysm. In 51 cases, an interhemispheric exposure was included to expose the feeding vessels from anterior cerebral artery (25 cases), from posterior cerebral artery (10 cases) or from both anterior and posterior cerebral arteries (16 cases). For interhemispheric exposure, the craniotomy extended just across the midline and it exposed a part of the superior sagittal sinus [15]. The craniotomy was done after careful protection of the superior sagital sinus and the veins draining into it. The AVM was dissected circumferentially, coagulating and then sectioning the feeding vessels. Differentiation of normal blood vessels from AVM vessels formed the key issue during the entire surgical procedure. The feeding arteries were first identified and dissected off their arachnoid cover, coagulated and then cut. Whenever necessary or when it is felt that the flow in the vessel is significant and the vessel is large and can open up despite coagulation, liga or silver hemoclips was applied on both sides of the cut vessel. Whenever, there was any doubt about the vessel being a normal transit or en-passage vessels, a temporary clip was applied on it. The vessel was then dissected towards the nidus to confirm its nature. Control of moderate to severe bleeding and tackling bleeding from more than one site at the same time was the principle surgical issue. Whilst the steps of surgically dealing with large and middle size vessels is standard and described, ‘small’ vessel bleeding was dealt with by following the vessel even when necessary through the cerebral parenchyma towards its feeding ‘mother’ vessel/s that was first exposed widely and was then coagulated. Use of hemostatic agents or even direct coagulation of the ‘small’ vessels was identified to be ineffective and it was apparent that if the proximal part of the blood vessel that fed the bleeding ‘small’ vessel was not appropriately dissected an opportunity to isolate the feeding vessel and part of the AVM nidus is lost. Such identification of the feeding vessel by tracing bleeding small vessel at the terminal part of the operation facilitated identification of residual AVM and complete resection of the nidus. In 48 cases, the AVM was additionally fed by vessels originating from choroidal arteries in the ventricles, even when in at-least 19 cases no definite choroidal feeding vessel was identified on preoperative angiography. Bleeding from choroidal vessels was significantly brisk in most cases. Identification, dissection, coagulation and sectioning of the feeding arteries and saving the venous tributary/ies and ligation/ clipping/ coagulation towards the end of AVM dissection are standard surgical procedures. Dissection is necessarily executed in a circumferential manner around the AVM nidus and surgical entry into the confines of nidus is avoided. Intraoperative ICG was identified to be useful in the initial part of surgery immediately after the exposure of the AVM in the surgical field. It particularly helped in matching the configuration of AVM during surgery with preoperative angiogram and to an extent in differentiating arteries from veins. In general, it was observed that the aneurysm developed in majority of cases in the feeding arterial channel that was a part of the AVM complex. Resection of the AVM resulted in spontaneous thrombosis of the aneurysm and no direct surgical or endovascular treatment was necessary in any case.
In general, the patients were reversed from anesthesia immediately after surgery. The patient was placed on conventional postoperative drugs. No special drugs were given that increase or decrease blood pressure during or after surgery. No decongestive drugs or steroids were given. Postoperative angiography was generally done after about 2 weeks of surgery.
Results
The clinical and radiological follow-up of all the surviving 84 patients ranged from 6 months to 18 years (average 43 months).
Clinical outcome: (Figures 1, 2, 3, 4, 5, 6 and 7)
There was profuse bleeding in several cases but in none of the cases the wound was closed without achieving satisfactory hemostasis. Five patients died in the perioperative phase. Out of these one patient bled during the procedure of embolization and became deeply unconscious. The patient was operated as an emergency and the hematoma was removed and the AVM was excised however the patient never regained consciousness and finally succumbed. One patient was unconscious in the immediate postoperative phase. This adult male patient with posterior fossa AVM who was deeply unconscious and had tonic decerebrate posturing following surgery made progressive and positive clinical improvement. At a follow-up of more than 17 years, he was asymptomatic and functioning as a mathematics teacher. Four patients had hemiplegia and three patients had hemiparesis after reversal from anesthesia. One young boy with posterior parietal AVM who developed hemiplegia following surgery made significant neurological recovery. At a follow-up of more than 15 years, he was normally functioning. The other 3 patients who had hemiplegia following surgery made satisfactory but incomplete recovery but are functioning actively in the society. Three patients had a minor degree of hemiparesis following surgery which recovered completely at follow up. Ten patients presented with preoperative hemiparesis. Out of these, 6 patients recovered completely in their limb function at follow up. Four patients continued to have mild residual weakness. All the patients are back to their routine life and are able to perform their functions independantly. Two patients presented with altered sensorium. Both the patients improved in their sensorium following surgery. Apart from other symptoms, one patient had hair loss over the site of AVM. At follow up of 5 years, some hair growth was seen on the patch of hair loss. Five patients developed homonymous hemianopia following surgery which improved incompletely at follow up. Visual function and field testing was inadequately performed both preoperatively and on follow-up examination hence the exact number of patients with visual deficit could not be quantified. One patient with a large parietotemporal AVM (Fig. 7) developed significant parietal lobe signs that improved only marginally at a follow-up of more than 5 years. All other patients are leading normal and essentially symptom free life. All patients improved in their symptoms of headache and giddiness. Out of the 26 patients who presented with convulsions, 5 continued to have convulsions but the intensity and frequency of the seizures decreased.
Radiological outcome: (Figures 1, 2, 3, 4, 5, 6 and 7)
Postoperative digital subtraction angiography revealed a residual AVM nidus in one patient, wherein the operation was deliberately prematurely terminated due to the reason of excessive blood loss. In another patient post-operative CT angiogram revealed a residual nidus. This AVM was clinically observed as the patient was unwilling for additional surgery and essentially had no symptoms. A repeat CT angiogram at 5 years after surgery showed spontaneous thrombosis of the residual nidus. In one patient a small residual nidus was observed on post-operative imaging done after 48 h of surgery. The patient was re-operated and the residual nidus was excised. In none of the patients in the entire series there were clinical events that suggested normal perfusion pressure break-through bleeding or delayed hemorrhage in the operative area or in the adjoining brain [22]. Complete angiographic obliteration of all other AVMs was achieved.
Discussion
The ‘philosophical’ understanding of the subject and the art of surgery on AVMs have to be learnt and perfected over several years. The important key for success of surgery in a case with AVM is to select the correct patient. Despite the mandate of superiority of surgical resection of AVMs over all other therapeutic modalities, only relatively few case series on the subject in general and large AVMs in particular could be garnered from the literature [5, 9, 11, 18,19,20]. A number of clinical trials have been conducted that analyze the validity of surgery and other forms of treatment for AVMs [6, 23, 24]. The role of embolization and gamma knife in the treatment of AVMs is still under intense evaluation. ARUBA trial initiated significant discussion about the validity of any kind of treatment for AVMs [24]. The prevalence of intracranial AVM is 0.02% of the adult population with a detection rate of 1 per 100,000 adults per year [2]. AVMs constitute the most common cause of spontaneous intracerebral hemorrhage. The overall risk of hemorrhage for AVM’s is estimated to be 2 to 4% per year.[2,25 ]The likelihood of future rupture or second hemorrhage is higher in patients with ‘large’ size AVMs [25].
The danger of uncontrollable bleeding, potential of damaging critical neural structures during dissection and possibility of devastating complications related to compromise of critical blood vessel or blood flow during dissection weighs heavily on the minds of even those who have significant experience and/or are highly skilled.
Clinical presentation
More than patient related factors, personal experience in surgically dealing with AVMs will define the indication for surgery. Clinical evidence of previous bleeding episodes, severe ‘steal’ phenomenon and symptoms not responding to pharmacological treatment like mental deterioration like memory disturbance and altered behavior, unresponsive headache, disabling giddiness or intractable seizures can indicate the need for surgery. (Table 1). Twenty six patients presented with the primary symptom of focal or generalised convulsions. Seventy nine patients presented with the chief symptom of moderate to severe headache. Our observation was that AVM’s in patients presenting with ‘severe’ headaches harbored higher flow AVMs. The relationship of presentation with seizures and degree of blood flow in the AVM could not be exactly correlated. Other modes of presentation in large AVMs include memory disturbance, behavioral abnormality, worsening in performance and focal neurological deficits [26]. In the series, 19 patients presented with sudden onset symptoms and bleeding. Ten patients presented with preoperative hemiparesis. Out of these 6 patients recovered completely in their limb function at follow up. Four patients continued to have ‘mild’ residual weakness. All the patients are back to their routine life and are able to perform their functions independantly. In 21 patients, there was evidence of hemorrhage around the nidus. It is obvious that AVMs that have spontaneously bled will be inherently ‘high-flow’ in nature. In 16 cases, the ages of the patients was less than 20 years. Although the difference in conduct of surgery related to age is difficult to speculate on the basis of the experience, it appears that surgery on younger patients was relatively simpler when compared to that in the ‘elderly’ patients. It was observed that presence of preoperative focal neurological deficit was an indicator of high-flow nature of AVM and consequent related surgical difficulties. Lawton observed better clinical outcome in younger patients and those with intact preoperative clinical neurological status [19].
Investigations
Both DSA and MRI are Gold-standard investigations and essential in the preoperative work-up of large AVMs. DSA identifies the general cerebral vascular alterations in the presence of AVM, clarifies the nature of feeding and draining vascular channels and demonstrates the characteristics and size of the nidus. Some authors have stressed the importance of direct observation of conduct of DSA by the treating surgeon to assess the rate of blood flow in the AVM [27, 28]. Such observation helped to understand better the degree of blood flow in the AVM, visualize the direction of blood flow in the AVM nidus and directed the focus and sequence of surgical targets.
In 19 patients 3-D models of AVM were made. Technical details of the manufacture of the model have been discussed by us earlier [21]. Such 3-D models and its correlation with the images of DSA were identified to be critical in specifying the physical characteristic of the AVM and in planning and executing the surgical procedure.
Classification
The validated and universally recognized Spetzler-Martin classification formed the basis of assessment of the AVMs. (Table 3) In addition, to simplify the characteristics of AVM the authors designed a personalised classification scheme. According to the anticipated difficulties during surgery, the AVMs were graded into simple (Grade 1), difficult but possible (Grade 2), very difficult but possible (Grade 3) and impossible (Grade 4). This classification was essentially individual surgeon based and the senior authors personal grading of AVM and depended on various characteristics of the AVM that included the parameters that form the basis of Spetzler-Martin classification [29]. Also the gradation of the AVM will change with increasing experience. For this reason, the senior author preferred to label it as a dynamic classification. All the cases in the presented series were graded as ‘very difficult but possible’ or Grade 3. As the classification is based on subjective considerations and will depend of individual surgeon’s experience, the utility of such personalized grading system will have to be assessed by other surgeons.
Several authors have identified size of the AVM to be a major factor that determined the potential risks during surgery [30,31,32]. Spetzler observed that smaller AVMs presented more frequently with hemorrhage whilst large AVMs presented with functional and neurological symptoms [30]. However, others have identified ‘equally’ high incidence of presentation with hemorrhage in large and small AVMs [31]. In the presented series 19 patients presented with acute symptoms (other than convulsions) and there was an evidence of hemorrhage in the confines of the AVM nidus in 21 cases. In Spetzler-Martin classification, size has been included as one of the three most determining parameters that characterised AVMs [30]. Spetzler and Martin mentioned that blood flow quantity is related to AVM size. In 1977 Luessenhop proposed a classification of AVM on the basis of their size as measured on angiographic imaging [16, 17]. Grade 1 AVM were of size less than 2 cm, Grade 2 were of size between 2 and 4 cm, Grade 3 were of size 4–6 cm and Grade 4 were of size more than 6 cm. AVM nidus size larger than 4 cm in their maximum dimensions has been defined as ‘large’ AVM. In the presented series, in 29 patients the size of the AVM nidus was more than 6 cm and in 5 patients it was more than 8 cm in either (3 patients) or both lateral and anteroposterior view (2 patients) of angiogram.
Surgical strategy and nuances
More than anything else, presence of an AVM and the feasibility and possibility of its safe surgical resection is the primary indication for surgery. Presence of large arterial feeders, large draining veins, intranidal aneurysmal dilatations of blood vessels, high flow observed during angiography and presentation of the patient with bleed or with severe headache are suggestions that the AVM will be high flow, bleeding can be excessive and that the dissection can be difficult [1]. Surgery on large AVMs has to be necessarily done by circumferential dissection around the dome of the nidus. It has been extensively discussed in the literature that voluntary piecemeal AVM resection is not an option.
AVMs located in the posterior cranial fossa are in general relatively easier and safer to operate than supratentorial AVMs. In 7 cases, the AVM nidus was located in the posterior cranial fossa. The posterior parieto-occipital AVMs are relatively more common. In the present series 23 patients had posterior parieto-occipital AVM. It was observed that medially located frontal AVMs (10 cases), anterior temporal lobe (6 cases) and posterior parieto-occipital AVMs (23 cases) formed a relatively favorable subset for surgery. Apart from obvious dangers of operating in the region of motor strip, left parietal AVMs formed a complex surgical issue. Cerebral circulation was divided into 6 territories namely right and left anterior cerebral artery, right and left middle cerebral artery, posterior circulation and external carotid artery. More the number of territories that feed AVM’s, higher is the degree of flow. Several authors have identified the rate of blood flow within the AVM nidus to be the more critical factor that determines difficulties during the conduct of the surgical procedure [29,30,31]. In general, it has been identified that larger the size of the AVM, higher is the degree of blood flow and more difficult is the conduct of the surgical procedure.
Our literature review observed that in AVMs the incidence of intracranial aneurysms varies and is approximately 2.7–58% [33]. Presence of ‘flow-related’ aneurysms was seen in 8 cases. Intranidal aneurysms were observed in 21 cases. Aneurysmal dilatation of draining venous channel was observed in 18 cases. Presence of nidal and para-nidal aneurysms was observed to be an indicator of high-flow nature of AVM and consequent difficulties during surgery. AVMs fed by large arteries and drained by large and multiple veins are generally high-flow in nature. The sizes and numbers of draining veins form an indicator of volume, intensity and degree of blood flow from the nidus. Thirty AVMs were fed by both anterior and posterior circulation vessels. In 2 cases, AVM was additionally fed by branches of external carotid artery. It was generally observed that AVMs fed by arteries from more than one teritories and those fed by branches of external carotid artery were of high-flow nature. In 48 cases there were feeding arterial vessels arising from the choroidal blood vessels. The blood flow from the choroidal vessels was ‘always’ high flow. As this bleeding occurred in the later part of the dissection, it formed the more tedious part of the surgical procedure. In 29 cases in the presented series both superficial and deep venous drainage was present. In general, AVMs draining into both superficial and deep venous channels had higher grade blood flow.
In the series, AVM in one patient was resected partially due to intraoperative issues with controlling bleeding. This patient fared well after surgery and was asymptomatic when seen after 8 years of surgery. Repeat investigation was not done in this case. Despite this positive experience it is clear to the authors from their experience and that reported in the literature that staged surgery is not a good option for large AVMs. Although embolization of AVMs is generally a staged procedure, several authors have alluded to the fact that partial and staged surgical resections are not safe.
Complications
Complications following surgery can be unforgiving in these ‘benign’ lesions as the patient will have to live with them for rest of their otherwise normal lifespan. Intraoperative bleeding disasters have been reported and probably under-reported, particularly during surgery on large AVMs. and can have influence over the psyche of the surgeon during the surgical procedure.
Five patients died in the immediate postoperative period. Additionally, one patient developed altered consciousness and four developed major unilateral hemispheric motor deficits. Spetzler discussed the issue of edema, hyperaemia and hemorrhage in the AVM adjoining parenchyma following the resection of AVM in the ‘immediate’ postoperative phase [22]. None of the patients in the presented series presented with such hemorrhage or symptoms related to abnormal brain effects like edema and hyperaemia. It appears that complete resection of AVM is necessary to avoid postoperative hemorrhagic complications. In this context, it appears that the phenomenon of normal perfusion pressure break-through as proposed by Spetzler [22] following resection of AVM needs to be re-evaluated.
Role of pre-operative embolization
The subtleties of the techniques and indication regarding preoperative embolization has been elaborately discussed in the literature. [34, 35] Preoperative embolization of the feeding vessels particularly of those originating in the depths and AVM nidus has been recommended by most surgeons. Other indications for embolization include treatment of feeding vessel or intranidal aneurysms or handling the mechanically ‘weak’ parts of the AVM that are prone for rupture. However, the senior author did not prefer preoperative embolization. Although reasons for not favouring preoperative embolization are several, the critical issue was that the authors did not find preoperative embolization to remarkably alter the conduct of surgery, and subjected the patient to additional procedures that itself carried potential risks and possibly altered unfavorably the dynamics and circulation of the AVM.
Conclusions
Our clinical results following surgery on large AVMs suggest that surgery on these lesions is a reasonable option. However, surgery can be a formidable task and the surgeon needs to be appropriately geared up to accept this challenge.
Images of a 14 year old boy. (A) Reconstructed sagittal angiographic image showing a large right frontal compact arteriovenous malformation. (B) Antero-posterior angiographic image showing the AVM being fed by the anterior and middle cerebral arteries. (C) Lateral run of angiogram showing the nidus. (D) Coronal image of CT scan showing the nidus and the multiple venous aneurysms. (E) Post-operative sagittal CT image showing excision of the AVM. (F) Post-operative coronal CT image. (G) Image showing the resected AVM specimen
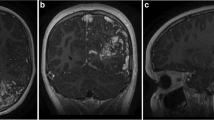
Image of a 25 year old female patient. (A) T2-weighted MRI shows a large vascular malformation in the left frontal brain. (B) CT angiography shows the AVM with feeders from anterior and middle cerebral arteries. (C) Coronal view showing the AVM and the feeding arteries. (D) 3-D model showing the AVM. (E) Pre-operative image of the patient showing the severity of headaches. (F) Image of the cortex on opening of the dura. (G) Indocyanine green dye injection performed after opening of dura. (H) Postoperative CT angiography showing resection of the AVM. (I) Another view of angiogram showing resection of the AVM. (J) Clinical photograph of the patient in the postoperative phase
Images of a 25 year old male patient. (A) Sagittal cut of CT angiogram showing the AVM in the region of the velum interpositum. (B) Coronal cut of CT angiogram showing the AVM. (C) DSA showing AVM with feeders from the posterior cerebral artery. E. DSA showing the AVM with feeders from the anterior cerebral artery. F. Postoperative sagittal cut of CT angiogram showing resection of the AVM. G. Postoperative axial cut of CT angiogram showing resection of the AVM. H. Postoperative specimen. I. Postoperative carotid run of DSA showing resection of the AVM. F. Postoperative vertebral run of DSA showing resection of the AVM.
Images of a 21 year old male patient. (A) Large AVM nidus fed mainly by anterior cerebral artery and draining into the superior sagittal sinus. (B) 3D reconstructed sagittal image of CT angiogram showing the AVM. (C) 3D reconstructed coronal image of CT angiogram. (D) Axial CT image showing the left frontal AVM. (E) Resected AVM specimen. (F) Postoperative angiogram shows resection of the AVM. (G) Postoperative status of the patient
Images of a 30 year old male patient. A.Sagittal image of CT scan showing the large AVM. B. Axial cut of CT angiogram showing the AVM fed by large branches of ACA. C. 3D reconstructed coronal image of the large AVM. D. Axial image showing the AVM fed by the ACA and the MCA. E. Intra-operative image on opening of dura. F. Post-operative resected specimen
Image of a 39 year old female patient. (A) CT angiogram showing large medial posterior parietal AVM. The AVM is fed by branches of ACA and PCA. The AVM drains into the superior sagittal sinus and into the vein of Galen. The only symptom of the patient was severe giddiness. (B) Axial CT angiography images showing the multiple large intranidal aneurysms. (C) Sagittal image showing the AVM. (D) Picture of brain on opening of the dura. (E) Resected specimen. (F) Post-operative DSA showing excision of the AVM.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- AVM:
-
Arteriovenous malformation
- ACA:
-
Anterior cerebral artery
- MCA:
-
Middle cerebral artery
- PCA:
-
Posterior cerebral artery
- SCA:
-
Superior cerebellar artery
- AICA:
-
Anterior Inferior cerebellar artery
- PICA:
-
Posterior Inferior cerebellar artery
References
Goel A (2005) Arteriovenous malformations: current status of surgery. Neurol India 53:11–35
Luther E, Govindarajan V, McCarthy DJ et al (2022) Brain arteriovenous malformations: Status of Open surgery after a Randomized Trial of Unruptured Brain Arteriovenous malformations. Neurosurg Clin N Am 33(4):443–448
Park MT, Essibayi MA, Srinivasan VM, Catapano JS, Graffeo CS, Lawton MT (2022) Surgical management outcomes of intracranial arteriovenous malformations after preoperative embolization: a systematic review and meta-analysis. Neurosurg Rev 45(6):3499–3510
Kim MJ, Jung HH, Kim YB et al (2023) Comparison of Single-Session, Neoadjuvant, and Adjuvant Embolization Gamma Knife Radiosurgery for Arteriovenous Malformation. Neurosurgery 92(5):986–997
Jiang H, Tang X, Weng R et al (2022) Long-term outcome of a tailored embolization strategy with Gamma Knife radiosurgery for high-grade brain arteriovenous malformations: a single-center experience. J Neurosurg 30:1–8
Dumot C, Picart T, Eker O, Guyotat J, Berhouma M, Pelissou-Guyotat I (2022) Outcomes of Unruptured Low-Grade Brain Arteriovenous malformations using TOBAS (treatment of Brain Arteriovenous malformations Study) Criteria. World Neurosurg 167:e1050–e1061
Hasegawa T, Kato T, Naito T et al (2022) Effect of embolization before stereotactic radiosurgery for brain arteriovenous malformations: a case-control study with propensity score matching. J Neurosurg 138(4):955–961
Niwa R, Ichi S, Nomura R, Sato K (2022) Hypofractionated stereotactic radiotherapy with CyberKnife for large arteriovenous malformations and arteriovenous malformations located in eloquent areas. Neurol Med Chir (Tokyo) 62(10):445–450
Cannizzaro D, Scibilia A, Frio F et al (2022) IV and V grade arteriovenous malformations: a multicenter surgical experience. Use of multiple grading system to predict surgical risk. J Clin Neurosci 104:96–102
De Leacy R, Ansari SA, Schirmer CM et al (2022) SNIS Standards and Guidelines Committee, SNIS Board of Directors. Endovascular treatment in the multimodality management of brain arteriovenous malformations: report of the Society of NeuroInterventional Surgery Standards and Guidelines Committee. J Neurointerv Surg 14(11):1118–1124
Abla AA, Rutledge WC, Seymour ZA et al (2015) A treatment paradigm for high-grade brain arteriovenous malformations: volume-staged radiosurgical downgrading followed by microsurgical resection. J Neurosurg 122(2):419–432
Shah A, Patni N, Ramdasi R, Goel A (2017) Progression in the size of an arterio-venous malformation. Asian J Neurosurg 12(2):207–210
Abdulrauf SI (2015) Awake craniotomies for aneurysms, arteriovenous malformations, skull base tumors, high flow bypass and brainstem lesions. J Craniovertebr Junction Spine 6(1):8–9
Gabarrós A, Young WL, McDermott MW, Lawton MT (2011) Language and motor mapping during resection of brain arteriovenous malformations: indications, feasibility, and utility. Neurosurgery 68(3):744–752
Goel A (1997) Bilateral parafalcine approach for arteriovenous malformations located in the interhemispheric fissure. In: Kobayashi S, Goel A, Hongo K (eds) edsNeurosurgery of Complex tumours and vascular lesions. Churchill Livingstone, New York, p 143
Luessenhop AJ (1984) Natural history of cerebral arteriovenous malformations. Williams & Wilkins, Baltimore
Luessenhop AJ, Rosa L (1984) Cerebral arteriovenous malformations. Indications for and results of surgery, and the role of intravascular techniques. J Neurosurg 60(1):14–22
Li N, Yan D, Li Z et al (2022) Long-term outcomes of Spetzler-Martin grade IV and V arteriovenous malformations: a single-center experience. Neurosurg Focus 53(1):E12
Winkler EA, Lu A, Morshed RA, Yue JK et al (2020) Bringing high-grade arteriovenous malformations under control: clinical outcomes following multimodality treatment in children. J Neurosurg Pediatr 26(1):82–91
Eliava S, Gorozhanin V, Shekhtman O, Pilipenko Y, Kuchina O (2021) Surgical Treatment of Unruptured Brain AVMs: short- and long-term results. Acta Neurochir Suppl 132:87–90
Shah A, Jankharia B, Goel A (2017) Three-dimensional model printing for surgery on arteriovenousmalformations. Neurol India 65(6):1350–1354
Spetzler RF, Hargraves RW, McCormick PW et al (1992) Relationship of perfusion pressure and size to risk of hemorrhage from arteriovenous malformations. J Neurosurg 76:918–923
Magro E, Gentric JC, Batista AL et al (2018) The treatment of Brain AVMs Study (TOBAS): an all-inclusive framework to integrate clinical care and research. J Neurosurg 128(6):1823–1829
Mohr JP, Parides MK, Stapf C et al (2014) Medical management with or without interventional therapy for unrupturedbrainarteriovenous malformations (ARUBA): a multicentre, non-blinded, randomisedtrial. Lancet 15(9917):383
Brown RD Jr, Wiebers DO, Forbes G et al (1988) The natural history of unruptured intracranial arteriovenous malformations. J Neurosurg 68:352–357
Lawton MT, Rutledge WC, Kim H et al (2015) Brain arteriovenous malformations. Nat Rev Dis Primers 1:15008
Shimada K, Miyake K, Yamaguchi I et al (2023) Efficacy of utilizing both 3-Dimensional Multimodal Fusion Image and Intra-arterial Indocyanine Green Videoangiography in cerebral arteriovenous malformation surgery. World Neurosurg 169:e260–e269
Tao S, Zhang T, Zhou K et al (2022) Intraoperative monitoring cerebral blood Flow during the treatment of Brain Arteriovenous malformations in Hybrid operating room by laser speckle contrast imaging. Front Surg 9:855397
Spetzler RF, Martin NA (1986) A proposed grading system for arteriovenous malformations. J Neurosurg 65:476–483
Lawton MT, Kim H, McCulloch CE, Mikhak B, Young WL (2010) A supplementary grading scale for selecting patients with brain arteriovenous malformations for surgery. Neurosurgery 66:702–713 discussion 713
Tayebi Meybodi A, Lawton MT (2018) Modern classification and outcome predictors of surgery in patients with brain arteriovenous malformations. J Neurosurg Sci 62(4):454–466
Drake CG (1979) Cerebral arteriovenous malformations: considerations for and experience with surgical treatment in 166 cases. Clin Neurosurg 26:145–208
Rammos SK, Gardenghi B, Bortolotti C, Cloft HJ, Lanzino G (2016) Aneurysms Associated with Brain Arteriovenous malformations. AJNR Am J Neuroradiol 37(11):1966–1971
Perret G, Nishioka H (1966) Report on the cooperative study of intracranial aneurysms and subarachnoid hemorrhage. Section VI. Arteriovenous malformations. An analysis of 545 cases of cranio-cerebral arteriovenous malformations and fistulae reported to the cooperative study. J Neurosurg 25:467–490
Chang SD, Marcellus ML, Marks MP et al (2003) Multimodality treatment of giant intracranial arteriovenous malformations. Neurosurgery 53:1–11, discussion 11–13
Funding
No funding was obtained.
Author information
Authors and Affiliations
Contributions
A.G.: Conceptualization, Methodology, Validation, Formal analysis, Writing – Original draft, Writing – Review and Editing, Supervision, Project Administration. R.V.: Methodology, Validation, Formal analysis, Writing – Review and Editing. A.S: Conceptualization, Methodology, Validation, Formal analysis, Writing – Original draft, Writing – Review and Editing, Supervision. A.P: Methodology, Validation, Formal analysis, Writing – Review and Editing. K.A: Methodology, Validation, Writing – Review and Editing. A.S: Methodology, Validation, Writing – Review and Editing.
Corresponding author
Ethics declarations
Ethical approval
This is a retrospective review hence ethics committee permission was not deemed necessary.
Informed consent
Not applicable.
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Goel, A., Vutha, R., Shah, A. et al. Clinical analysis of surgical outcome of 89 patients having large cerebral arteriovenous malformations. Neurosurg Rev 47, 224 (2024). https://doi.org/10.1007/s10143-024-02447-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-024-02447-4