Abstract
To compare the safety and efficacy of clipping and coiling in patients with ruptured anterior circulation aneurysms. A systematic search of four databases (PubMed, Web of Science, Cochrane Library, and Embase) was conducted to identify comparative articles on endovascular coiling and surgical clipping in patients with ruptured anterior circulation aneurysms. Meta-analyses were conducted using random-effects models. Nineteen studies, including 1981 patients, were included. The meta-analysis showed that neurosurgical clipping was associated with a lower incidence of retreatment (OR:0.28, 95% CI (0.11, 0.70), P = 0.006) than endovascular coiling, which seemed to be a result of incomplete occlusion (OR:0.22, 95% CI (0.11, 0.45), P < 0.001). Neurosurgical clipping was associated with lower mortality (OR:0.45, 95% CI (0.25, 0.82), P = 0.009) at short-term follow-up than endovascular coiling. However, neurosurgical clipping showed a higher incidence of ischemic infarction (OR:2.28, 95% CI (1.44, 3.63), P < 0.001) and a longer length of stay (LOS) (WMD:6.12, 95% CI (4.19, 8.04), P < 0.001) after surgery than endovascular coiling. Furthermore, the pooled results showed no statistically significant differences between the two groups regarding poor outcome, long-term mortality, rebleeding, vasospasm, and hydrocephalus. Evidence from this systematic review illustrates that neurosurgical clipping may be superior to endovascular coiling for ruptured anterior circulation aneurysms. Large-scale RCTs should be conducted to verify these outcomes and provide results according to patient status.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Subarachnoid hemorrhage (SAH) from a ruptured intracranial aneurysm accounts for approximately 5% of all stroke cases and is associated with exceptionally high mortality and disability [1, 2]. Compared with previous studies, the worldwide crude incidence of SAH has decreased to approximately 7.9 per 100000 people but still shows large regional differences [3, 4].
Therefore, proper management of ruptured aneurysms is particularly important. Aneurysms of the anterior circulation appear more common than those of the posterior circulation [5]. Two definitive treatment modalities were used: microsurgical clipping and endovascular aneurysm coiling. In the last century, microsurgical clipping has become the gold standard for treating intracranial aneurysms. Over the past 20 years, improvements in and popularity of endovascular coiling techniques have led to significant controversy over the ideal treatment strategy for ruptured intracranial aneurysms [6,7,8,9]. Endovascular coiling avoids a large incision and craniotomy, thereby reducing the recovery time. Although surgical clipping is more invasive, permanent metal clips can be placed accurately on the neck of an aneurysm under a microscope to block the blood flow to the aneurysm, which is associated with better durability [8, 10]. The results of several recently published meta-analyses favor endovascular coiling as the primary modality for the treatment of intracranial aneurysms [8, 11,12,13]. However, these studies included a very important heterogeneous subgroup: anterior–posterior circulation. Intracranial aneurysms within posterior circulation pose a significant obstacle to microsurgical operation due to their deep location, complexity of anatomical exposure, limited space for manipulation, and floating complexity of adjacent structures [14]. Posterior circulation aneurysms are more likely to be treated with endovascular treatment. Therefore, it is not appropriate to extrapolate the results to aneurysms located in the anterior circulation. Moreover, in the last 10 years, many new high-quality studies have been conducted around the world. Therefore, we performed a meta-analysis to compare the efficacy and safety of clipping and coiling in patients with ruptured anterior circulation aneurysms based on existing published studies and a strictly limited inclusion population.
Material and methods
This study followed the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [15] and SRMA [16]. Our protocol was registered with PROSPERO (CRD42022375852).
Data sources and searches
Two authors (H Q and J-H L) searched for comparative articles involving endovascular coiling and surgical clipping in patients with anterior circulation aneurysms. Where necessary, a third investigator (N-J W) reviewed the selection). The PubMed, Web of Science, Cochrane Library, and Embase electronic databases were searched for studies published up to December 2022. The search strategy combined search terms for clipping, coiling, and ruptured anterior circulation aneurysms, using multiple versions of medical terms and text words (Supplementary Materials, strategy). We imposed restrictions on text availability (full text only), species (humans only), and language (English only) and double-checked them. Reference lists of the retrieved articles were manually checked to identify other potentially eligible studies.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) patients with ruptured anterior circulation aneurysms; (2) studies including endovascular coiling and surgical clipping; (3) studies comparing the results of clipping and clipping; and (4) studies reporting at least one of the following outcomes: poor outcome (modified Rankin scale (mRS; > 2) or Glasgow Outcome Scale (GOS; 1–3)), mortality, incomplete occlusion, rebleeding, hydrocephalus, ischemic infarction, vasospasm, retreatment and length of stay.
Studies were excluded if they evaluated the outcomes of endovascular coiling and surgical clipping without reporting our specified outcomes or if they included pediatric cases. Studies focusing on only one of these two methods were excluded. Moreover, we excluded studies that did not provide sufficient information for us to extract or calculate the absolute number of clinical events. We excluded patients with repeat or hybrid procedure, and included only those who underwent clipping or coiling for the first time after admission.
Data collection and quality assessment
The collected data included the name of the first author or study group, country, publication year, covered study period, study design, sample size, sex, mean age, follow-up duration, and reported outcomes. To assess the quality of the extracted data, the Newcastle–Ottawa scale was used for cohort studies, and the Jadad scale was utilized for randomized controlled trials. Two investigators (H Q and J-H L) independently extracted data. Discrepancies were resolved by senior authors (X-M D). Clinical outcomes that appeared in the hospital or within 30 days were defined as short-term outcomes.
Statistical analysis
The data were entered into Stata (version 17.0; StataCorp, TX, USA) and analyzed using a forest plot for visual estimation of the meta-analysis. The results of each study were assigned to binary frequency data, and the odds ratios and 95% confidence intervals were calculated and combined. The heterogeneity of all included studies was evaluated using Cochran’s Q test and the I2 statistic. Considering the prevalent clinical and statistical heterogeneity between the two groups, this meta-analysis only reported the random-effect model. All studies were included in sensitivity analyses to assess the robustness of the pooled results. Potential publication bias was assessed using the Egger’s and Begg’s tests. Statistical significance was set at P-value < 0.05 unless otherwise specified.
Results
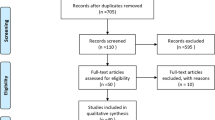
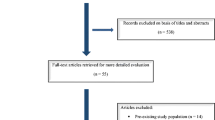
We identified 9541 articles, including 2932 from PubMed, 3665 from Web of Science, 392 from the Cochrane Library, and 2562 from Embase. After removing duplicates, screening titles and abstracts, and conducting a full-text review, 19 articles were selected for the final meta-analysis [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35]. A Flow diagram of the data extraction strategy is shown in Fig. 1. Of the 19 studies, 2 were RCTs [27, 34], 2 were prospective cohort studies [17, 33], and the remaining 15 were retrospective studies [18,19,20,21,22,23,24,25,26, 28,29,30,31,32, 35]. The total study population comprised 1981 patients, of which 1019 were treated with surgical clipping and 962 were treated with endovascular coiling. Table 1 shows the baseline characteristics of the included studies.
Effectiveness outcome
Seventeen studies reported poor outcome. Meta-analysis of the data revealed no significant difference between the 2 groups in the rate of poor outcome at short-term (OR: 0.85, 95% CI (0.62, 1.18), P = 0.343), at 3 months (OR: 1.10, 95% CI (0.67, 1.81), P = 0.711), at 6 months (OR: 1.03, 95% CI (0.67, 1.59), P = 0.859), at 1-year follow-up, or more (OR: 1.20, 95% CI (0.86, 1.68), P = 0.286, Fig. 2). Seven studies with 653 patients reported the postoperative incomplete occlusion rate and the results showed that clipping was associated with a lower incidence of incomplete occlusion than coiling (OR:0.22, 95% CI (0.11, 0.45), P < 0.001, Fig. 3). Furthermore, six studies reported the retreatment rate of 645 participants, and the results showed that clipping was associated with a lower incidence of retreatment than coiling (OR:0.28, 95% CI (0.11, 0.70), P = 0.006, Fig. 4).
Safety outcome
Seven articles, including 673 patients, reported short-term mortality. Meta-analysis of the data revealed that clipping was associated with a lower incidence of short-term mortality than coiling (OR:0.45, 95% CI (0.25, 0.82); P = 0.009, Fig. 5). However, this advantage was no longer sustained at the 3-month follow-up or more (OR:0.78, 95% CI (0.54, 1.13), P = 0.192, Fig. 5), according to the meta-analysis of 12 studies. Ten studies with 1144 patients reported postoperative rebleeding. Meta-analysis showed that there was no significant difference between the two groups in the rate of rebleeding (OR:0.75, 95% CI (0.42, 1.36), P = 0.345, Fig. 6). Nine articles, including 832 patients, reported on postoperative vasospasm. Meta-analysis of the data revealed no significant difference between the two groups in the rate of vasospasm (OR:1.16, 95%CI (0.67, 2.01), P = 0.600, Fig. 7). Six studies with 585 patients reported postoperative ischemic infarction and the results showed that clipping was associated with a higher incidence of ischemic infarction compared to coiling (OR:2.28, 95% CI (1.44, 3.63), P < 0.001, Fig. 8).
Six studies involving 694 patients reported postoperative hydrocephalus. Meta-analysis of the data revealed no significant difference between the two groups in the rate of hydrocephalus (OR:0.86, 95%CI (0.40, 1.85), P = 0.695, Fig. 9).
Other outcomes of interest
The data on LOS were available from four studies that included 475 patients. The meta-analysis showed that clipping was associated with a longer length of stay than coiling (WMD:6.12, 95% CI (4.19, 8.04), P < 0.001, Fig. 10).
Publication Bias
Although there were less than 10 studies for some outcomes, we assessed publication bias for each outcome, and no significant publication bias was observed. A summary of these results is presented in Table 2.
Discussion
Appropriately managing ruptured anterior circulation aneurysms remains a major challenge for modern neurosurgeons. In previous studies, there was no certain evidence that the clinical safety and efficacy of one procedure is superior to another [8, 22, 30]. The decision to use clipping or coiling techniques for treatment is influenced by the patient’s preference or the experience of the institution and the surgeon [26]. As a result, the optimal management of ruptured anterior circulation aneurysms is not easily determined. This meta-analysis evaluated the clinical benefits of endovascular coiling and surgical clipping in patients with ruptured anterior circulation aneurysms.
Compared to previous meta-analysis [36] involving patients with ruptured anterior circulation aneurysms, the present study reported inconsistent conclusions regarding the efficacy of clipping versus coiling. A limitation of the previous meta-analysis was that the pooled results were based on only eight studies, and numerous studies were not updated [36]. In our study, the pooled results of all identified studies on neurosurgical clipping versus endovascular coiling in patients with SAH from a ruptured anterior circulation aneurysm showed a reduction in retreatment and incomplete occlusion after treatment by clipping compared with coiling. Li et al. [37] reported that clipping could increase the incidence of complete occlusion (OR, 2.43; 95% CI, 1.88–3.13) compared to coiling. Zhu et al. [12] found that clipping could increase the incidence of complete aneurysmal occlusion by 33% compared to coiling. Therefore, follow-up angiography is necessary to detect incomplete occlusions after the embolization of ruptured anterior circulation aneurysms. Due to the low recurrence rate of completely occlusive aneurysms, late follow-up angiography may not be necessary for patients with complete occlusion [38, 39]. Moreover, several publications [40, 41], including ruptured anterior and posterior circulation aneurysms, have shown no significant differences in the incidence of poor outcome between neurosurgical clipping and endovascular coiling. A large and well-structured meta-analysis demonstrated that surgical clipping was associated with a higher rate of poor outcome than endovascular coiling [12]. However, subgroup analyses of this large and well-structured meta-analysis showed that clipping was not associated with a higher rate of poor outcome compared to coiling for the treatment of anterior cerebral artery, anterior communicating artery (ACA-AComA), or middle cerebral artery (MCA) aneurysms. Nevertheless, these studies included fewer RCTs in the meta-analyses, and more trials with long-term follow-up are required for further evaluation of both techniques.
The meta-analysis of ischemic infarction after treatment showed a significantly higher risk in the clipping group, but the vasospasm endpoint showed no statistical difference. Even though surgical clipping causes greater damage to brain tissue and blood vessels, our results showed no difference in vasospasm between clipping and coiling. A recent meta-analysis [42] investigated the role of antiplatelet therapy (AT) in coiling and clipping on vasospasm and found that surgically treated aSAH was associated with lower rates of symptomatic and angiographic vasospasm in the AT group. Although endovascular coiling has been associated with a higher risk of ischemic infarction in previous studies [43, 44], different results were observed in our study. Early studies suggested that ischemic infarction is caused by emboli that escape from aneurysms [45]. However, a recent high-quality meta-analysis [8] showed that the risk of ischemic infarction was lower in patients in the endovascular coiling group, which is consistent with our results. This may be due to the fact that clipping is more invasive to brain tissue. The continuous improvements in endovascular technology greatly reduce the probability of emboli escape [46]. Analysis of short-term mortality after operation showed a significantly higher risk in the coiling group; however, long-term mortality showed no statistical difference. These results are inconsistent with those of a previous meta-analysis conducted in 2017 [36], which revealed no significant difference in the risk of short-term mortality between coiling and clipping. The main difference may lie in including one study involving 94 patients with ruptured poor-grade anterior circulation aneurysms, which found a significant difference. Moreover, most studies provided only all-cause mortality data and failed to provide case fatality data, thus reducing the reliability of the results. Previous studies have shown that patients in the endovascular coiling group have a higher risk of rebleeding [8, 37, 41, 47]. However, most studies included heterogeneous subgroups (such as rupture-unruptured and anterior–posterior circulation) and were affected by ISAT. Thus, generalizing the findings of those studies to all intracranial aneurysms is inadequate. Additionally, we compared the LOS results and revealed a significantly shorter LOS in the endovascular coiling group than in the surgical clipping group because endovascular coiling is a minimally invasive treatment without craniotomy.
Owing to constant technological advancements and the introduction of new therapeutic approaches, there is a continuing impetus for less risky treatments. Nevertheless, the evolution of clinical practice tends to occur gradually rather than suddenly. Neurosurgical clipping for ruptured anterior circulation aneurysms is currently not a substitute. This study included all contemporary comparative studies and provided important information for neurosurgeons regarding treating ruptured anterior circulation aneurysms.
Limitations
There are several potential limitations to this study. Firstly, three kinds of study designs were included in the present study, of which only two randomized controlled trials and two prospective studies, mostly retrospective. Secondly, 21% of the included studies did not provide sufficient information regarding the baseline characteristics of patients. The uncertainty between the two treatment groups could have introduced a confounding bias. Moreover, we intended to conduct more subgroup analyses of patients with ruptured anterior circulation aneurysms. However, some studies lack specific information on the outcomes of this subgroup of participants.
Conclusions
According to our study, surgical clipping resulted in lower incomplete occlusion and retreatment rates and was associated with a lower incidence of short-term mortality. However, endovascular coiling was associated with a shorter LOS and a lower rate of ischemic infarction. Evidence from this systematic review illustrates that neurosurgical clipping may be superior to endovascular coiling for ruptured anterior circulation aneurysms. Large randomized controlled trials are needed to validate these outcomes and provide results based on patient status.
Data availability
No datasets were generated or analysed during the current study.
Change history
12 April 2024
A Correction to this paper has been published: https://doi.org/10.1007/s10143-024-02390-4
References
Maher M, Schweizer TA, Macdonald RL (2020) Treatment of Spontaneous Subarachnoid Hemorrhage: Guidelines and Gaps. Stroke 51(4):1326–1332. https://doi.org/10.1161/strokeaha.119.025997
van Gijn J, Kerr RS, Rinkel GJ (2007) Subarachnoid haemorrhage. Lancet 369(9558):306–318. https://doi.org/10.1016/s0140-6736(07)60153-6
Rinkel GJ, Algra A (2011) Long-term outcomes of patients with aneurysmal subarachnoid haemorrhage. Lancet Neurol 10(4):349–356. https://doi.org/10.1016/s1474-4422(11)70017-5
Etminan N, Chang HS, Hackenberg K et al (2019) Worldwide Incidence of Aneurysmal Subarachnoid Hemorrhage According to Region, Time Period, Blood Pressure, and Smoking Prevalence in the Population: A Systematic Review and Meta-analysis. JAMA Neurol 76(5):588–597. https://doi.org/10.1001/jamaneurol.2019.0006
Tykocki T, Kostkiewicz B (2014) Aneurysms of the anterior and posterior cerebral circulation: comparison of the morphometric features. Acta Neurochir (Wien) 156(9):1647–1654. https://doi.org/10.1007/s00701-014-2173-y
Anxionnat R, Tonnelet R, Derelle AL et al (2015) Endovascular treatment of ruptured intracranial aneurysms: Indications, techniques and results. Diagn Interv Imaging 96(7–8):667–675. https://doi.org/10.1016/j.diii.2015.06.002
Lin N, Cahill KS, Frerichs KU et al (2018) Treatment of ruptured and unruptured cerebral aneurysms in the USA: a paradigm shift. J Neurointerv Surg 10(Suppl 1):i69–i76. https://doi.org/10.1136/jnis.2011.004978.rep
Lindgren A, Vergouwen MD, van der Schaaf I et al (2018) Endovascular coiling versus neurosurgical clipping for people with aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev 8(8):Cd003085. https://doi.org/10.1002/14651858.CD003085.pub3
Lee KS, Zhang JJY, Nguyen V et al (2022) The evolution of intracranial aneurysm treatment techniques and future directions. Neurosurg Rev 45(1):1–25. https://doi.org/10.1007/s10143-021-01543-z
Molyneux AJ, Birks J, Clarke A et al (2015) The durability of endovascular coiling versus neurosurgical clipping of ruptured cerebral aneurysms: 18 year follow-up of the UK cohort of the International Subarachnoid Aneurysm Trial (ISAT). Lancet 385(9969):691–697. https://doi.org/10.1016/s0140-6736(14)60975-2
Luo M, Yang S, Ding G et al (2019) Endovascular coiling versus surgical clipping for aneurysmal subarachnoid hemorrhage: A meta-analysis of randomized controlled trials. J Res Med Sci 24:88. https://doi.org/10.4103/jrms.JRMS_414_18
Zhu W, Ling X, Petersen JD et al (2022) Clipping versus coiling for aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis of prospective studies. Neurosurg Rev 45(2):1291–1302. https://doi.org/10.1007/s10143-021-01704-0
de Liyis BG, Surya SC, Tini K (2023) Effectivity and safety of endovascular coiling versus microsurgical clipping for aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis. Clin Neurol Neurosurg 236:108058. https://doi.org/10.1016/j.clineuro.2023.108058
You W, Meng J, Yang X et al (2022) Microsurgical Management of Posterior Circulation Aneurysms: A Retrospective Study on Epidemiology, Outcomes, and Surgical Approaches. Brain Sci 12(8):1066. https://doi.org/10.3390/brainsci12081066
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700. https://doi.org/10.1136/bmj.b2700
Lee KS, Zhang JJY, Nga VDW et al (2022) Tenets for the Proper Conduct and Use of Meta-Analyses: A Practical Guide for Neurosurgeons. World Neurosurg 161:291-302.e1. https://doi.org/10.1016/j.wneu.2021.09.034
Ahmed AZ, Zohdi AM, Zaghloul MS et al (2013) Endovascular coiling versus surgical clipping in the treatment of ruptured anterior communicating artery aneurysm in Cairo University Hospitals. Egypt J Radiol Nucl Med 44(3):523–530. https://doi.org/10.1016/j.ejrnm.2013.06.007
Ba YF, Zhang CY, Huang JB et al (2021) Microsurgical clipping vs. arterial embolization in the treatment of ruptured anterior circulation aneurysms. Am J Transl Res 13(7):8040–8
Bäcker HC, Shoap S, Vajda J et al (2020) Anterior communicating artery aneurysm rupture and functional outcome in short-term: clipping versus coiling. J Integr Neurosci 19(2):349–54. https://doi.org/10.31083/j.jin.2020.02.125
De Los Reyes K, Patel A, Bederson JB et al (2013) Management of subarachnoid hemorrhage with intracerebral hematoma: clipping and clot evacuation versus coil embolization followed by clot evacuation. J Neurointerv Surg 5(2):99–103. https://doi.org/10.1136/neurintsurg-2011-010204
Ghorbani M, Griessenauer CJ, Wipplinger C et al (2020) Surgical clipping compared to endovascular coiling of ruptured coil able middle cerebral aneurysms: A single-center experience. Interdiscip Neurosurg: Adv Tech Case Manag 21:100. https://doi.org/10.1016/j.inat.2020.100708
Groden C, Kremer C, Regelsberger J et al (2001) Comparison of operative and endovascular treatment of anterior circulation aneurysms in patients in poor grades. Neuroradiology 43(9):778–783. https://doi.org/10.1007/s002340100573
Harris L, Hill CS, Elliot M et al (2021) Comparison between outcomes of endovascular and surgical treatments of ruptured anterior communicating artery aneurysms. Br J Neurosurg 35(3):313–318. https://doi.org/10.1080/02688697.2020.1812517
Heit JJ, Ball RL, Telischak NA et al (2017) Patient outcomes and cerebral infarction after ruptured anterior communicating artery aneurysm treatment. Am J Neuroradiol 38(11):2119–2125. https://doi.org/10.3174/ajnr.A5355
Lee SH, Park JS (2022) Outcome of ruptured anterior communicating artery aneurysm treatment compared between surgical clipping and endovascular coiling: A single-center analysis. Medicine (Baltimore) 101(38):e30754. https://doi.org/10.1097/md.0000000000030754
Liao CC, Huang YH, Fang PH et al (2013) Surgical and endovascular treatment for ruptured anterior circulation cerebral aneurysms: a comparison of outcomes–a single centre study from Taiwan. Int J Surg 11(9):998–1001. https://doi.org/10.1016/j.ijsu.2013.05.038
Moon K, Levitt MR, Almefty RO et al (2015) Treatment of Ruptured Anterior Communicating Artery Aneurysms: equipoise in the Endovascular Era? Neurosurgery 77(4):566–71. https://doi.org/10.1227/NEU.0000000000000878. (discussion 71)
Niskanen M, Koivisto T, Ronkainen A et al (2004) Resource use after subarachnoid hemorrhage: comparison between endovascular and surgical treatment. Neurosurgery 54(5):1081–6. https://doi.org/10.1227/01.neu.0000119350.80122.43. (discussion 6‐88)
Park KY, Kim BM, Lim YC et al (2015) The role of endovascular treatment for ruptured distal anterior cerebral artery aneurysms: comparison with microsurgical clipping. J Neuroimaging 25(1):81–86. https://doi.org/10.1111/jon.12073
Shen J, Huang K, Shen J et al (2019) Clinical Efficacy Between Microsurgical Clipping and Endovascular Coiling in the Treatment of Ruptured Poor-Grade Anterior Circulation Aneurysms. World Neurosurg 127:e321–e329. https://doi.org/10.1016/j.wneu.2019.02.248
Suzuki S, Kurata A, Yamada M et al (2011) Outcomes analysis of ruptured distal anterior cerebral artery aneurysms treated by endosaccular embolization and surgical clipping. Interv Neuroradiol 17(1):49–57. https://doi.org/10.1177/159101991101700108
Taweesomboonyat C, Tunthanathip T, Kaewborisutsakul A et al (2019) Outcome of Ruptured Posterior Communicating Artery Aneurysm Treatment Comparing Between Clipping and Coiling Techniques. World Neurosurg 125:e183–e188. https://doi.org/10.1016/j.wneu.2019.01.037
Vanninen R, Koivisto T, Saari T et al (1999) Ruptured intracranial aneurysms: Acute endovascular treatment with electrolytically detachable coils - A prospective randomized study. Radiology 211(2):325–336. https://doi.org/10.1148/radiology.211.2.r99ap06325
Wadd IH, Haroon A, Habibullah AS et al (2015) Aneurysmal subarachnoid hemorrhage: outcome of aneurysm clipping versus coiling in anterior circulation aneurysm. J Coll Phys Surg Pak 25(11):798–801
Zhao B, Xing H, Fan L et al (2019) Endovascular Coiling versus Surgical Clipping of Very Small Ruptured Anterior Communicating Artery Aneurysms. World Neurosurg 126:e1246–e1250. https://doi.org/10.1016/j.wneu.2019.03.074
Fotakopoulos G, Tsianaka E, Fountas K et al (2017) Clipping Versus Coiling in Anterior Circulation Ruptured Intracranial Aneurysms: A Meta-Analysis. World Neurosurg 104:482–488. https://doi.org/10.1016/j.wneu.2017.05.040
Li H, Pan R, Wang H et al (2013) Clipping versus coiling for ruptured intracranial aneurysms: a systematic review and meta-analysis. Stroke 44(1):29–37. https://doi.org/10.1161/strokeaha.112.663559
David CA, Vishteh AG, Spetzler RF et al (1999) Late angiographic follow-up review of surgically treated aneurysms. J Neurosurg 91(3):396–401. https://doi.org/10.3171/jns.1999.91.3.0396
Brown MA, Parish J, Guandique CF et al (2017) A long-term study of durability and risk factors for aneurysm recurrence after microsurgical clip ligation. J Neurosurg 126(3):819–824. https://doi.org/10.3171/2016.2.Jns152059
Shao B, Wang J, Chen Y et al (2019) Clipping versus Coiling for Ruptured Intracranial Aneurysms: A Meta-Analysis of Randomized Controlled Trials. World Neurosurg 127:e353–e365. https://doi.org/10.1016/j.wneu.2019.03.123
Jiang Z, Chen Y, Zeng C et al (2020) Neurosurgical Clipping versus Endovascular Coiling for Patients with Intracranial Aneurysms: A Systematic Review and Meta-Analysis. World Neurosurg 138:e191–e222. https://doi.org/10.1016/j.wneu.2020.02.091
Lee KS, Lee C, Dhillon PS et al (2023) Antiplatelet therapy in aneurysmal subarachnoid hemorrhage: an updated meta-analysis. Neurosurg Rev 46(1):221. https://doi.org/10.1007/s10143-023-02120-2
Rordorf G, Bellon RJ, Budzik RE Jr et al (2001) Silent thromboembolic events associated with the treatment of unruptured cerebral aneurysms by use of Guglielmi detachable coils: prospective study applying diffusion-weighted imaging. AJNR Am J Neuroradiol 22(1):5–10
Kang XK, Guo SF, Lei Y et al (2020) Endovascular coiling versus surgical clipping for the treatment of unruptured cerebral aneurysms: Direct comparison of procedure-related complications. Medicine (Baltimore) 99(13):e19654. https://doi.org/10.1097/md.0000000000019654
Nanda A, Vannemreddy PS (2006) Cerebral ischemia as a presenting feature of intracranial aneurysms: a negative prognostic indicator in the management of aneurysms. Neurosurgery 58(5):831–7. https://doi.org/10.1227/01.Neu.0000209643.66807.80. (discussion -7)
Starke RM, Turk A, Ding D et al (2016) Technology developments in endovascular treatment of intracranial aneurysms. J Neurointerv Surg 8(2):135–144. https://doi.org/10.1136/neurintsurg-2014-011475
Liu A, Huang J (2015) Treatment of Intracranial Aneurysms: Clipping Versus Coiling. Curr Cardiol Rep 17(9):628. https://doi.org/10.1007/s11886-015-0628-2
Funding
This study was supported by the Scientific Research Initiation Fund for Talent Introduction of Shanxi Bethune Hospital, Shanxi Province, China (2021RC006). The funders had no role in the study design, data collection, and analysis, decision to publish, and manuscript preparation.
Author information
Authors and Affiliations
Contributions
X.D. and L.P. conceived and planned the study. H.Q., J.L., and N.W. did the literature retrieve, extracted data, and evaluated study quality. L.P. and H.Q. analyzed and interpreted data. L.P. wrote the initial draft of the manuscript.X.D., X.W., and L.H. critically reviewed and revised the draft. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The authors regret that one sentence in the abstract section of the published original article is incorrect.
The incorrect sentence is: Nineteen studies, including 1983 patients, were included.
The correct sentence is: Nineteen studies, including 1981 patients, were included.
The original article has been corrected.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peng, L., Qin, H., Liu, J. et al. Neurosurgical clipping versus endovascular coiling for patients with ruptured anterior circulation aneurysms: A systematic review and meta-analysis. Neurosurg Rev 47, 68 (2024). https://doi.org/10.1007/s10143-024-02304-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-024-02304-4