Abstract
The objective of this study was to determine risk factors of pejorative evolution course in patients suffering from postoperative cranial infection. The data of patients who developed an intracranial infection after craniocerebral surgery in the neurosurgical intensive care unit of the First Affiliated Hospital of Nanjing Medical University in Nanjing, Jiangsu, China, from February 2018 to August 2019 were retrospectively analyzed. Logistic regression was used to analyze the factors influencing the prognosis of intracranial infection treatment. Sixty-four patients developed an infection after craniocerebral surgery, and 48 of them with negative CSF cultures received experimental anti-infectives. In 16 patients, cerebrospinal fluid culture showed pandrug-resistant pathogens, including 11 Acinetobacter baumannii (11), Klebsiella pneumoniae (3), Escherichia coli (1), and Candida glabrata (1). Nine patients received intraventricular or intrathecal injections of polymyxin B. The mean duration of infection treatment was 22.2 ± 9.9 days, and the clinical cure rate was 85.9% (55/64). Logistic multivariate regression analysis showed that inadequate CSF drainage (OR, 6.839; 95% CI, 1.130–41.383; P = 0.036) and infection with drug-resistant bacteria (OR, 24.241; 95% CI, 2.032–289.150; P = 0.012) were independent risk factors for postoperative intracranial infection. Intracranial infection with positive CSF culture and inadequate CSF drainage are factors contributing to the poor prognosis of intracranial infection. Moreover, early anti-infection treatment and adequate CSF drainage may improve patient outcomes. In particular, intraventricular or intrathecal injection of polymyxin B may be a safe and effective treatment strategy for MDR/XDR gram-negative bacilli infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intracranial infection is a serious complication that can occur after craniocerebral surgery, and the incidence of intracranial infection is approximately 4.6–25%, with bacteria being the most common cause [1]. Reportedly, the mortality rate of nosocomial meningitis (NM) is approximately 14–25%, and the mortality rate of pan drug-resistant bacterial infection can reach up to 55% [2, 3]. At present, there are many reports on the factors related to intracranial infection. However, there are few studies on the factors negatively affecting the prognosis of NM. The treatment of intracranial infections is complex and variable. To further understand these varied responses to intracranial infection treatment, the data of 64 patients with NM who were admitted to our hospital’s NICU from February 2018 to August 2019 were analyzed. A summary of the treatment experience and analysis of factors affecting the prognosis is presented.
Materials and methods
Population study
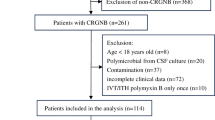
A review of 734 patients undergoing craniocerebral surgery admitted to the NICU of the First Affiliated Hospital of Nanjing Medical University from February 2018 to August 2019 was conducted. The research protocol was approved by our hospital ethical committee (No. 2022-NT-07). Head skin was prepared 2 h before surgery in all patients, and second-generation cephalosporin antibiotics were administered intravenously as prophylaxis 30 min before surgery. Antibiotics were routinely given for 24 h. The incision dressing was changed regularly after the operation, and the drainage tube under the scalp was removed within 48 h. According to the inclusion and exclusion criteria, 670 patients were excluded, and 64 patients who developed NM were included (Fig. 1).
Admission criteria
Inclusion criteria were as follows: (1) patients who underwent craniocerebral surgery and standard treatment after surgery; (2) patients who developed NM, as defined by the criteria for intracranial infection proposed by the Neurosurgery Branch of the Chinese Medical Association [4]; (3) patients who were 18–80 years old; and (4) patients who themselves or their family members signed informed consent forms.
Exclusion criteria were as follows: (1) patients had a prior history of craniotomy or had a history of intracranial infection; (2) patients with immunodeficiency diseases and severe chronic heart, lung, kidney, and other serious diseases; (3) patients with an intracranial infection before surgery; (4) patients who did not receive standardized treatment or voluntarily withdrew from treatment after the surgery.
Diagnostic criteria for intracranial infection were as follows: (1) clinical manifestations—systemic inflammatory reaction, altered consciousness, symptoms of cranial hypertension, meningeal stimulation, and concomitant symptoms; (2) imaging manifestations—diffuse cerebral edema, dural thickening and enhancement, ventricular system dilation, and annular enhancement; (3) blood-related examination—blood leukocyte count >10.0×109 cells/L or neutrophil ratio >80%; (4) CSF examination—(a) intracranial pressure: lumbar puncture open pressure >200 mmH2O; (b) CSF traits: cloudy, yellow, or purulent in the acute phase; (c) CSF cell count: CSF leukocyte count >100~1000×106 cells/L with neutrophil ratio >70%; (d) CSF biochemistry: glucose <2.6 mmol/L or CSF glucose/serum glucose <0.66; (5) smears and cultures of CSF—positive bacteriology examination of CSF, surgical incision secretion, and surgical specimens. The clinical diagnosis was consistent with (1)–(4), and the etiological diagnosis was consistent with (1)–(5) [5].
Study protocol
Sixty-four patients with NM as defined in the inclusion criteria underwent lumbar cistern drainage or ventricular drainage. The patients with negative CSF cultures were given broad spectrum empirical anti-infective treatment, and those with positive CSF cultures were given targeted anti-infective treatment based on the patient’s drug sensitivity test. Intraventricular or intrathecal medication was given to patients with drug-resistant bacterial infection. CSF was regularly collected from the lumbar cistern or ventricle for routine, biochemical, and culture examinations. We retrospectively evaluated age, sex, route of admission, number of operations, daily CSF drainage, primary lesions, intracranial infection sites, CSF sugar content, CSF white blood cell count, CSF culture results, artificial material placement, and route of antibiotic administration. The observation endpoint of the study was 1 month of treatment for intracranial infection and three months of postoperative follow-up. Cure was defined as 3 consecutive normal values of the following parameters within l~2 weeks: (1) normal body temperature; (2) disappearance of clinical signs of infection; (3) normal CSF sugar content, CSF sugar content/serum glucose content ≥ 0.66; (4) normal white blood cells in CSF; (5) negative CSF bacterial culture; (6) normal white blood cells and neutrophils. According to the prognosis, the patients were divided into a clinically cured group and a deteriorated group.
Statistical analysis
The data were statistically analyzed using IBM SPSS Statistics, version 19 software (SPSS Inc., Chicago, IL, USA). Categorical variables are described by count. Continuous variables are described using x2 ± s or median (range). Univariate binary logistic regression was initially used to identify potential predictor variables associated with clinical efficacy. Variables with P values less than 0.10 were included to assess independent risk factors by multivariate binary logistic regressions. Statistical significance was set at a two-sided P value less than 0.05.
Results
Population study
Among the 64 patients with NM, 49 were males and 15 were females, and they were aged 19 to 80 years old. According to the type of primary disease, there were 35 cases of cerebral hemorrhage, 10 cases of brain trauma, 7 cases of brain tumor, and 12 cases of other diseases. The lesions were above the tentorium in 55 patients and below the tentorium in 9 patients. The surgical approaches included 33 hematoma evacuations, 15 external ventricular drainage procedures, 5 craniocerebral tumorectomies, 7 aneurysm clipping techniques, and 4 ventriculoperitoneal shunt procedures. There were no significant differences in primary diseases or surgical approaches between the cured group and the deteriorated group (Table 1).
NM occurred on 8.4 ± 3.6 (mean and standard deviation) days after surgery. The white blood cell counts in the CSF of all patients increased to varying degrees. There were 20 patients with a white blood cell count ≥ 10,000/μL and 44 with a count < 10,000/μL, while there were 54 patients with a CSF glucose content ≥ 1.1 mmol/L and 10 patients with a CSF glucose content < 1.1 mmol/L. Combined with brain computed tomography or magnetic resonance, 19 patients were diagnosed with ventriculitis, and 45 patients were diagnosed with meningitis. Artificial materials such as intracranial meninges, skull repair titanium plates, hemostatic materials, and ventriculoperitoneal shunts were placed in 50 patients, while no artificial materials were placed in 14 patients. Of all the patients who underwent external ventricular or lumbar drainage, drainage was adequate (≥ 50 mL/day) in 50 patients and inadequate (< 50 mL/day) in 14 patients.
Treatment and outcome
Forty-eight patients with negative CSF cultures were given anti-infectives experimentally. Vancomycin or linezolid was combined with cephalosporin or meropenem for anti-infective therapy. The CSF cultures of 16 patients were positive for pandrug-resistant pathogens, including 11 Acinetobacter baumannii (11), Klebsiella pneumoniae (3), Escherichia coli (1), and Candida glabrata (1). A combination of two or three drugs, including meropenem, tigecycline, or polymyxin B, was selected for intravenous anti-infective therapy. After 72 h of intravenous treatment, polymyxin B was given to 9 patients via drainage tube or intrathecal injection at a dose of 50,000 units each time. After injection, the tube was clamped for 1 h once a day, and the course of treatment was 5 to 7 days. Three patients developed cerebral empyema and were treated with endoscopic lavage and intermittent ventricular flushing. The mean duration of infection treatment in all patients was 22.2 ± 9.9 (mean and standard deviation) days. After active treatment, clinical cure was achieved in 50 patients, and deterioration occurred in 14 patients. The percentage of patients with overall deterioration was 22%. Among the 16 patients with drug-resistant bacteria in cerebrospinal fluid culture, 9 patients were treated with topical polymyxin B, and the clinical cure rate was 55.5% (5/9). The other 7 patients were not treated with polymyxin B, and the clinical cure rate was 28.6% (2/7). After 3 months of follow-up, the Glasgow Outcome Score was 5 in 7 patients, 4 in 14 patients, 3 in 16 patients, 2 in 8 patients, and 1 in 19 patients. Thirty-one patients had secondary complications, including pulmonary infection in 15 patients, hydrocephalus in 10 patients, and epilepsy in 6 patients.
Prognostic single-factor analysis
According to the prognosis, patients were divided into the cured group (50 patients) or the deteriorated group (14 patients). Single-factor analysis was used to compare age, sex, route of admission, number of operations, CSF drainage, primary lesions, intracranial infection sites, CSF glucose content, CSF white blood cell count, CSF culture results, artificial material placement, and route of antibiotic administration between the two groups. The results showed that the number of operations, CSF glucose content, intracranial infection sites, CSF drainage, CSF culture, and route of antibiotic administration were important factors affecting prognosis (P < 0.1, Table 2).
Logistic multivariate regression analysis
Binary logistic multivariate regression analysis was performed on statistically significant variables. The results showed that inadequate drainage of CSF and positive CSF cultures were independent risk factors for deterioration of NM (P < 0.05, Table 2).
Discussion
The incidence of NM varied among operations. According to research, the incidence of postoperative meningitis ranges from 1.5 to 8.6%, and the incidence of EVD-related infections ranges from 8 to 22% [6]. The various clinical manifestations of NM and the low positive rate of cerebrospinal fluid pathogen culture lead to difficulties in early diagnosis. In addition, the blood–brain barrier, drug-resistant bacteria, and individual immunity affect the efficacy of NM treatment [7]. Even if patients survive, only a few will fully recover after treatment. In our study, only 7 out of 64 patients returned to normal at 3 months of follow-up .The vast majority of patients were accompanied by varying degrees of disability or death. Therefore, it is necessary to summarize the following treatment experience.
Early diagnosis and early anti-infective treatment
The content of pathogenic bacteria in the CSF was low, and the infection symptoms were mild in the early stage of NM. As the number of bacteria increased, the symptoms of infection gradually worsened. For all patients with NM, external lumbar cisterna drainage or ventricular drainage should be performed early to drain out infected CSF, promote CSF circulation, reduce the bacterial concentration of infected CSF, and facilitate anti-infection [8]. Among the 64 patients in this group, 48 had a negative cerebrospinal fluid culture, with a cure rate of 91%. However, the CSF cultures were positive in 16 patients with drug-resistant bacterial infections, and the cure rate was only 38%. Multivariate regression analysis suggested that NM with positive CSF culture was an independent risk factor for deterioration. The deterioration rate of the CSF culture-positive patients was 24.4 times that of the CSF culture-negative patients. The patients with positive CSF culture had high CSF bacterial density and severe symptoms of infection. Moreover, cerebrospinal arachnoid adhesion leads to CSF circulation disorder and poor anti-infective effects. Therefore, early initiation of anti-infective treatment is critical to the prognosis of patients. Clinically, the culture period of CSF is approximately 3 to 5 days, and the positive rate of traditional CSF culture is lower than 40% [9]. Macrogene sequencing improves the detection of bacteria in CSF, but the disadvantage is that drug sensitivity results cannot be obtained [10,11,12]. The frequency of positive cultures in this study was only 25%. It is not realistic to detect pathogens during the early stages of infection. Clinical diagnosis should be based on the combination of clinical symptoms, imaging, blood, and CSF examinations, and empirical anti-infective treatment should be performed as soon as possible after the specimens are taken. The choice of empirical medication is based on (1) pathogen epidemiology and bacterial resistance in this ward; (2) recent use of antibiotics; (3) the patient’s systemic organ function; and (4) the pharmacokinetics and pharmacodynamic characteristics of the drug [13].
In recent years, according to the monitoring of bacterial resistance in China, NM after neurosurgery is still dominated by gram-positive bacteria. Gram-positive bacteria maintain high sensitivity to vancomycin, teicoplanin, and linezolid [14]. Third- or fourth-generation cephalosporins or carbapenems are preferred for treating gram-negative bacilli. In this study, 16 CSF cultures were positive, of which 15 were gram-negative bacilli and 1 was fungal. Gram-positive cocci are mostly nonresistant bacteria and are easily killed by drugs, which may be the reason for the low rate of cultures testing positive for cocci. A combination of drugs to treat both positive and negative bacilli is recommended for patients whose pathogen cannot be determined [15].
It is difficult to achieve an effective antibiotic concentration in the CSF due to the blood–brain barrier, so it is recommended to use the maximum drug dose that is allowed in the instruction manual and possibly a long course of treatment [6]. When empirical treatment given for at least 72 h is not effective, it is necessary to consider the occurrence of new pathogens or the effects of pharmacology and pharmacodynamics of antibiotics and to adjust the anti-infection program accordingly.
Clearance of the infected area and continuous adequate drainage of CSF
Numerous guidelines and expert consensus suggest that surgical placement of ventricular shunts, skull repair materials, artificial meninges, electrode plates, and other artificial materials are risk factors for NM, as artificial materials can carry external pathogens into the brain [16, 17]. Ependymal inflammatory infiltration, intraventricular adhesion, and diaphragm formation easily cause hydrocephalus and intraventricular infectious separation, resulting in deterioration of examination outcome. The removal of infected foreign bodies and adequate CSF purification drainage are the basic principles for the treatment of NM.
CSF drainage is a safe and potentially beneficial therapy in carefully selected patients with acute bacterial meningitis, particularly in the context of intracranial hypertension. Both LD and EVD are suitable for drainage of intracranial infections, but severe cranial hypertension should be excluded before LD placement. In addition, when topical antibiotics are required, the effective CSF drug concentration is more easily achieved by EVD than by LD [18]. EVD and LD play important roles in draining pathogens in the subarachnoid cerebrospinal fluid exudates and inflammatory factors, reduce the concentration of bacteria in the cerebrospinal fluid, accelerate the circulation of the cerebrospinal fluid, prevent the ependymal and subarachnoid adhesion, and reduce the occurrence of hydrocephalus [19]. In addition, when accompanied by severe intracranial infection, drainage can be placed through the ventricle or intrathecal administration of drugs to treat the infection [20]. However, cerebral hemorrhage, turbid CSF, and improper drainage tube positioning may cause drainage tube obstruction. Inadequate drainage of infectious CSF may lead to more severe intracranial infection and a vicious cycle.
All the patients in this study underwent CSF drainage, and nine of the 14 patients with inadequate drainage had a worse prognosis. Multivariate statistical analysis suggested that patients with inadequate CSF drainage (less than 50 ml/day) were 6.8 times more likely to experience deterioration of the prognosis than those with adequate CSF drainage. In recent years, many studies have suggested that continuous extracranial drainage plays an important role in the treatment of intracranial infection. There is no definitive research on optimal drainage of CSF [19]. The optimal drainage varies from patient to patient due to the degree of intracranial infection and intracranial pressure. Insufficient drainage may cause cranial hypertension or low anti-infection efficiency. Excessive drainage may lead to intracranial hemorrhage or even cerebral hernia [21]. In our study, patients with a daily drainage volume more than 50ml had a better prognosis than those with less than 50ml, and the optimal drainage volume still needs to be confirmed by a large number of studies.
However, at the same time, catheter-related ventriculitis and meningitis are serious complications of CSF drainage [22], for example, the time of ventricle or lumbocisterna drainage tube placement > 5 days, frequent retention of cerebrospinal fluid samples during the indwelling of drainage tube, cerebrospinal fluid leakage at the opening of drainage tube, puncture canal bleeding, and simultaneous EVD on both sides and other factors related to drainage tube [6]. Studies have reported a decrease in the rate of infection of LD to 0.8% with a strict protocol, including no monitoring of cerebrospinal fluid sampling, drainage no longer than 5 days, strict aseptic procedures when disconnected or damaged drainage tubes need to be reconnected, and removal of drainage tubes after a second disconnection or fracture [23]. Other studies reported the risk of infection can be reduced by making timely dressing changes for the incision, using broad-spectrum antibiotics, shortening the drainage time, and reducing the number of drainage tube operations such as sampling and drug injection [24, 25]. It was confirmed that longer duration of external ventricular CSF drain placement increased the risk of infection. However, we agree with the IDSA’s strong recommendation that regular replacement of the external drainage tube (such as every 5 days) cannot reduce the infection rate. For those who need long-term external ventricular drainage, replacement should be considered unless a change is necessary because of CSF infection or catheter malfunction, and regular replacement of the drainage tube is not recommended [6].
Treatment of multidrug-resistant infections
In recent years, the number of resistant bacteria in intracranial infection patients has gradually increased. In the intensive care unit, patients with severe conditions need to undergo many invasive medical procedures, which may lead to the increased prevalence of drug-resistant bacteria. In this study, CSF cultures of 16 patients with NM were positive, and all were positive for multidrug-resistant bacteria. The high rate of drug resistance may be related to the rapid mutation of drug-resistant bacteria caused by the application of antibiotics in recent years. At the same time, the application of broad-spectrum antimicrobial agents as empirical anti-infective treatment results in reduced culture rates of susceptible bacteria and detection of resistant bacteria. Vancomycin-resistant cocci were not cultured in the CSF in this study. However, carbapenem-resistant Enterobacter, Acinetobacter baumannii, and multidrug/pandrug-resistant Pseudomonas aeruginosa were less responsive to anti-infection treatment. Therefore, infection progresses rapidly, especially in patients with carbapenem-resistant Klebsiella pneumoniae, causing systemic septic shock or even death. In this study, all five patients with NM due to carbapenem-resistant Klebsiella pneumoniae died after treatment.
At present, the vast majority of multidrug-resistant gram-negative bacilli are sensitive to polymyxin B [5, 26, 27]. Although polymyxin B does not easily penetrate the blood–brain barrier, intraventricular or intrathecal administration is feasible. The advantage of local administration is the high concentration of drugs in the cerebrospinal fluid, which reduces the need for high-dose intravenous injections of drugs [28, 29]. Nine patients with multidrug-resistant infections were given intraventricular or intrathecal polymyxin, and five patients were cured in our study. The univariate analysis suggested that intravenous combined with local administration had a better clinical effect than intravenous administration alone.
The local administration dose should be appropriately increased during external drainage of CSF and ventricular irrigation. The effective therapeutic dose and interval should ensure that the minimum drug concentration in CSF is 10–20 times the minimum inhibitory concentration (MIC) of the pathogen. Therefore, the dose is mainly determined according to the MIC value of the drug and the volume of CSF when the CSF drug concentration cannot be measured. However, local administration can increase the risks of reinfection and intracranial hypertension, and excessive drug concentrations will cause chemical encephalitis and nerve root irritation. Excessive drug administration will also lead to convulsions, coma, and other adverse reactions [30].
The consequences of NM are serious. It is characterized by a high disability rate and a high death rate. In our study, 19 patients died after 3 months of follow-up. Among them, 14 died of intracranial infection accompanied by shock, 4 died of secondary intractable hydrocephalus and cerebral edema, and 1 died of respiratory failure caused by pulmonary infection. The high mortality rate (19/64) was considered to be related to the patients' origin from NICU. Patients in NICU have low GCS score, critical illness, low resistance, and high risk of drug-resistant bacterial infection. Studies have reported that GCS < 9 score also is a risk factor for intracranial infection [31]. Increased intracranial pressure caused by meningoencephalitis can be treated with sedation, hyperventilation, corticosteroids, osmotherapy, hypothermia, cerebrospinal fluid drainage, and other measures. Cranial decompression is an extreme measure. Studies have reported that the treatment of decompressive craniotomy can be chosen when the treatment of craniostatic pressure is not effective [32]. At the same time, complications such as hydrocephalus, epilepsy, and disturbance of consciousness often occur in the later stage and should be actively prevented in clinical practice [33].
Our study also has several limitations, including its small sample size and retrospective and single-center design. Due to the short follow-up time of this retrospective study, patients who experienced recurrence and long-term complications were not included in the study. A multicenter randomized controlled trial is required to verify the findings of the present study in the future.
Conclusion
In conclusion, intracranial infection with positive CSF culture and inadequate drainage of CSF are all factors correlated with a deterioration of the prognosis for patients with NM. In contrast, early reasonable anti-infection treatment and adequate cerebrospinal fluid drainage are effective for the treatment of infection. In particular, intraventricular or intrathecal injection of polymyxin B may be a safe and effective treatment strategy for MDR/XDR gram-negative bacilli infection.
Data availability
The data that support the findings of this study are available from the corresponding author, Dr. Jing Ji, upon request.
References
Ortiz OH, García HI, Ramírez FM, Flórez JS, Valencia BA, Mantilla SE, Ochoa MJ, Ochoa JE, Jaimes F (2018) Development of a prediction rule for diagnosing postoperative meningitis: a cross-sectional study. J Neurosurg 128(1):262–271. https://doi.org/10.3171/2016.10.JNS16379
De Bonis P, Lofrese G, Scoppettuolo G, Spanu T, Cultrera R, Labonia M, Cavallo MA, Mangiola A, Anile C, Pompucci A (2016) Intraventricular versus intravenous colistin for the treatment of extensively drug resistant Acinetobacter baumannii meningitis. Eur J Neurol 23(1):68–75. https://doi.org/10.1111/ene.12789
Shahan B, Choi EY, Nieves G (2021) Cerebrospinal fluid analysis. Am Fam Physician 103(7):422–428 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/33788511
Society, C. N., & (2017), CNCMCG Consensus of experts in the diagnosis and treatment of infection in Chinese neurosurgical critical patients (2017). Natl Med J China(21).
Chen L, Li X, Li D, Dong X, Chen H (2022) Efficacy and safety of intraventricular polymyxin B plus continuous ventricular drainage for the treatment of intracranial infection caused by drug-resistant Acinetobacter baumannii. Ann Palliat Med 11(2):490–497. https://doi.org/10.21037/apm-21-3149
Tunkel, A. R., Hasbun, R., Bhimraj, A., Byers, K., Kaplan, S. L., Scheld, W. M., . . . Zunt, J. R. (2017). 2017 Infectious Diseases Society of America's Clinical Practice Guidelines for Healthcare-Associated Ventriculitis and Meningitis. Clin Infect Dis, 64(6), e34-e65. https://doi.org/10.1093/cid/ciw861
Nau R, Seele J, Djukic M, Eiffert H (2018) Pharmacokinetics and pharmacodynamics of antibiotics in central nervous system infections. Curr Opin Infect Dis 31(1):57–68. https://doi.org/10.1097/QCO.0000000000000418
Kural C, Kirmizigoz S, Ezgu MC, Bedir O, Kutlay M, Izci Y (2019) Intracranial infections: lessons learned from 52 surgically treated cases. Neurosurg Focus 47(2):E10. https://doi.org/10.3171/2019.5.FOCUS19238
Zhang, Y., Cui, P., Zhang, H. C., Wu, H. L., Ye, M. Z., Zhu, Y. M., . . . Zhang, W. H. (2020). Clinical application and evaluation of metagenomic next-generation sequencing in suspected adult central nervous system infection. J Transl Med, 18(1), 199. https://doi.org/10.1186/s12967-020-02360-6
Dabrowski P, Jurkiewicz J, Czernicki Z, Koszewski W, Jasielski P (2017) Polymerase chain reaction based detection of bacterial 16S rRNA gene in the cerebrospinal fluid in the diagnosis of bacterial central nervous system infection in the course of external cerebrospinal fluid drainage. Comparison with standard diagnostics currently used in clinical practice. Neurol Neurochir Pol 51(5):388–394. https://doi.org/10.1016/j.pjnns.2017.06.013
Perdigão Neto LV, Medeiros M, Ferreira SC, Nishiya AS, de Assis DB, Boszczowski Í, Costa SF, Levin AS (2021) Polymerase chain reaction targeting 16S ribosomal RNA for the diagnosis of bacterial meningitis after neurosurgery. Clinics (Sao Paulo) 76:e2284. https://doi.org/10.6061/clinics/2021/e2284
Ruan L, Wu D, Li X, Huang Q, Lin L, Lin J, Chen L, Xu P, Jin J, Yang N, Li X (2017) Analysis of microbial community composition and diversity in postoperative intracranial infection using high-throughput sequencing. Mol Med Rep 16(4):3938–3946. https://doi.org/10.3892/mmr.2017.7082
Tattevin P, Solomon T, Brouwer MC (2019) Understanding central nervous system efficacy of antimicrobials. Intensive Care Med 45(1):93–96. https://doi.org/10.1007/s00134-018-5270-1
Li J, Zhao QH, Huang KC, Li ZQ, Zhang LY, Qin DY, Pan F, Huang WX (2017) Linezolid vs. vancomycin in treatment of methicillin-resistant staphylococcus aureus infections: a meta-analysis. Eur Rev Med Pharmacol Sci 21(17):3974–3979 https://www.ncbi.nlm.nih.gov/pubmed/28975963
Hussein K, Bitterman R, Shofty B, Paul M, Neuberger A (2017) Management of post-neurosurgical meningitis: narrative review. Clin Microbiol Infect 23(9):621–628. https://doi.org/10.1016/j.cmi.2017.05.013
Dang S, J.-X. M., Zhou G, Wang F, Guo (2015). Etiological surveillance of intracranial infections in patients undergoing craniotomy and analysis of risk factors. Chin J Nosocomiology.
Kourbeti IS, Vakis AF, Ziakas P, Karabetsos D, Potolidis E, Christou S, Samonis G (2015) Infections in patients undergoing craniotomy: risk factors associated with post-craniotomy meningitis. J Neurosurg 122(5):1113–1119. https://doi.org/10.3171/2014.8.JNS132557
Chen H, Guo X, Xie D, Dong X, Niu J, Chen G (2020) A clinical study on the use of intraventricular polymyxin B supplemented by continuous external ventricular drainage in the treatment of drug-resistant gram-negative bacilli intracranial infection. Infect Drug Resist 13:2963–2970. https://doi.org/10.2147/IDR.S261510
Abulhasan YB, Al-Jehani H, Valiquette MA, McManus A, Dolan-Cake M, Ayoub O, Angle M, Teitelbaum J (2013) Lumbar drainage for the treatment of severe bacterial meningitis. Neurocrit Care 19(2):199–205. https://doi.org/10.1007/s12028-013-9853-y
Remes F, Tomas R, Jindrak V, Vanis V, Setlik M (2013) Intraventricular and lumbar intrathecal administration of antibiotics in postneurosurgical patients with meningitis and/or ventriculitis in a serious clinical state. J Neurosurg 119(6):1596–1602. https://doi.org/10.3171/2013.6.JNS122126
Wang K, Liu Z, Chen X, Lou M, Yin J (2013) Clinical characteristics and outcomes of patients with cerebral herniation during continuous lumbar drainage. Turk Neurosurg 23(5):653–657. https://doi.org/10.5137/1019-5149.JTN.7954-13.0
Ramanan M, Lipman J, Shorr A, Shankar A (2015) A meta-analysis of ventriculostomy-associated cerebrospinal fluid infections. BMC Infect Dis 15:3. https://doi.org/10.1186/s12879-014-0712-z
Governale LS, Fein N, Logsdon J, Black PM (2008) Techniques and complications of external lumbar drainage for normal pressure hydrocephalus. Neurosurgery 63(4 Suppl 2):379–384. https://doi.org/10.1227/01.NEU.0000327023.18220.88
Bari ME, Haider G, Malik K, Waqas M, Mahmood SF, Siddiqui M (2017) Outcomes of post-neurosurgical ventriculostomy-associated infections. Surg Neurol Int 8:124. https://doi.org/10.4103/sni.sni_440_16
Cui Z, Wang B, Zhong Z, Sun Y, Sun Q, Yang G, Bian L (2015) Impact of antibiotic- and silver-impregnated external ventricular drains on the risk of infections: a systematic review and meta-analysis. Am J Infect Control 43(7):e23–e32. https://doi.org/10.1016/j.ajic.2015.03.015
Li, J., Fu, Y., Zhang, J., Wang, Y., Zhao, Y., Fan, X., . . . Li, C. (2019). Efficacy of tigecycline monotherapy versus combination therapy with other antimicrobials against carbapenem-resistant Acinetobacter baumannii sequence type 2 in Heilongjiang Province. Ann Palliat Med, 8(5), 651-659. https://doi.org/10.21037/apm.2019.11.06
Li Z, An Y, Li L, Yi H (2022) Intrathecal injection of tigecycline and polymyxin B in the treatment of extensively drug-resistant intracranial Acinetobacter baumannii infection: a case report and review of the literature. Infect Drug Resist 15:1411–1423. https://doi.org/10.2147/IDR.S354460
Li X, Sun S, Ling X, Chen K, Wang Q, Zhao Z (2017) Plasma and cerebrospinal fluid population pharmacokinetics of vancomycin in postoperative neurosurgical patients after combined intravenous and intraventricular administration. Eur J Clin Pharmacol 73(12):1599–1607. https://doi.org/10.1007/s00228-017-2313-4
Ye J, Tan LH, Shen ZP, Yu YS, Lai DM, Fan J, Shu Q (2020) Polymyxin for the treatment of intracranial infections of extensively drug-resistant bacteria in children after neurosurgical operation. World J Pediatr 16(5):528–532. https://doi.org/10.1007/s12519-020-00350-8
McClellan N, Swanson JM, Magnotti LJ, Griffith TW, Wood GC, Croce MA, Boucher BA, Mueller EW, Fabian TC (2015) Adjunctive intraventricular antibiotic therapy for bacterial central nervous system infections in critically ill patients with traumatic brain injury. Ann Pharmacother 49(5):515–522. https://doi.org/10.1177/1060028015570466
Göçmez C, Çelik F, Tekin R, Kamaşak K, Turan Y, Palancı Y, Bozkurt F, Bozkurt M (2014) Evaluation of risk factors affecting hospital-acquired infections in the neurosurgery intensive care unit. Int J Neurosci 124(7):503–508. https://doi.org/10.3109/00207454.2013.863773
Choucha A, Boissonneau S, Beucler N, Graillon T, Ranque S, Bruder N, Fuentes S, Velly L, Dufour H (2023) Meningoencephalitis with refractory intracranial hypertension: consider decompressive craniectomy. J Neurosurg Sci 67(2):248–256. https://doi.org/10.23736/S0390-5616.21.05397-2
Hover AR, Sistrunk WW, Cavagnol RM, Scarrow A, Finley PJ, Kroencke AD, Walker JL (2014) Effectiveness and cost of failure mode and effects analysis methodology to reduce neurosurgical site infections. Am J Med Qual 29(6):517–521. https://doi.org/10.1177/1062860613505680
Acknowledgements
The authors wish to thank all the patients and their families who participated in the study.
Funding
This study was funded by the National Natural Science Foundation of China (Grant No. 81972153 and 82120108018), Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD, Grant No. JX10231803), Gusu School, Nanjing Medical University (GSKY202201010), Jiangsu Province’s Key Discipline of Medicine (Grant No. XK201117), and Jiangsu Province Innovative Team (Grant No. NR17).
Author information
Authors and Affiliations
Contributions
Jing Ji: conceptualization, methodology, software. Zhen Yue: data curation, writing—original draft preparation. Liqing Bi: visualization, investigation. Xiaohui Zhi: supervision, software. Lin Zhao: validation, writing—reviewing and editing.
Corresponding author
Ethics declarations
Ethics approval
The research protocol was approved by our hospital ethical committee (No. 2022-NT-07).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
The authors affirm that human research participants provided informed consent for publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yue, Z., Zhi, X., Bi, L. et al. Treatment and prognostic risk factors for intracranial infection after craniocerebral surgery. Neurosurg Rev 46, 199 (2023). https://doi.org/10.1007/s10143-023-02106-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-023-02106-0