Abstract
Skull base dural reflections are complex, and along with various ligaments joining sutures of the skull base, are related to most important vessels like internal carotid arteries (ICA), vertebral arteries, jugular veins, cavernous sinus, and cranial nerves which make surgical approaches difficult and need thorough knowledge and anatomy for a safe dissection and satisfactory patient outcomes. Cadaver dissection is much more important for the training of skull base anatomy in comparison to any other subspecialty of neurosurgery; however, such facilities are not available at most of the training institutes, more so in low- and middle-income countries (LMICs). A glue gun (100-Watt glue gun, ApTech Deals, Delhi, India) was used to spread glue over the superior surface of the bone of the skull base over desired area (anterior, middle, or lateral skull base). Once glue was spread over the desired surface uniformly, it was cooled under running tap water and the glue layer was separated from the skull base. Various neurovascular impressions were colored for ease of depiction and teaching. Visual neuroanatomy of the inferior surface of dural reflections of the skull base is important for understanding neurovascular orientations of various structures entering or exiting the skull base. It was readily available, reproducible, and simple for teaching neuroanatomy to the trainees of neurosurgery. Skull base dural reflections made up of glue are an inexpensive, reproducible item that may be used for teaching neuroanatomy. It may be useful for trainees and young neurosurgeons, especially at resource-scarce healthcare facilities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Skull base surgery has advanced tremendously in the last couple of decades after the emergence of advancements in techniques and technologies of endoscope optics, illumination in addition to increasing use of neuronavigation, neurophysiological monitoring, and specially designed instruments and drills [1-3]. However, training in skull base surgery is demanding as it needs strong concepts of neuroanatomy pertaining to neurovascular structures and their relationships to dural reflections of the skull base. Cadaver dissection is much more important for skull base surgery training than any other subspecialty of neurosurgery [4, 5]. However, access to cadaver laboratories is a limiting factor in most of developing countries including India. Recent data suggests approximately 112 public sector healthcare institutions are having training courses in neurosurgery and barring 7–8, none has the facility of cadaver dissection even for basic neurosurgical training [6].
The authors use artificial visual neuroanatomical models of skull base dural reflections for teaching neurosurgery trainees and young neurosurgeons and describe the technique of the same. It may be useful for neuroanatomy teaching at resource-scarce centers, especially those without cadaver dissection facilities, around the world.
Materials and methods
The term “dural reflection” is used for the morphological appearance of the dura of the skull base, seen from below after the removal of bone. It also includes the appearance/impressions of various neurovascular structures crossing through the dura, most importantly internal carotid arteries and cranial nerves.
These models were made for visual neuroanatomy teaching for the neurosurgery trainees in the department, especially for endoscopic skull base neuroanatomy. These models were not made for hands-on training.
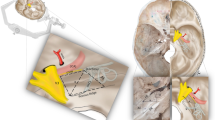
Upper surface skull base bone (Fig. 1), which represents the skull base dura, was used to make these models. Four regions of skull base were selected which are important from neuroanatomical perspectives of skull base surgery teaching.
-
1.
Anterior cranial fossa (ACF) starting from planum sphenoidale, tuberculum sella, anterior and posterior clinoids, sella and upper part of clivus, and paramedian parts of middle cranial fossa beyond the foramen ovale and the foramen spinosum (Figs. 2a and 3a).
-
2.
One-half of anterior, middle, and posterior cranial fossae on the right side. (Figs. 2b and 3b).
-
3.
Planum sphenoidale, tuberculum sella, sella, anterior and posterior clinoids, middle cranial fossa until its lateral extent and upper parts of clivus, and bilateral petroclival regions along with posterior surfaces of petrous bones (Fig. 3c).
-
4.
Tuberculum sella, sella, anterior and posterior clinoids, paramedian middle cranial fossae, and anterior half of posterior fossa extending laterally up to sigmoid sinuses and up to the anterior margin of the foramen magnum including occipital condyles, jugular foramen, hypoglossal foramen, and internal auditory meatus (Fig. 3d).
Shows skull base dural fold models of ACF (a), right half of skull base (b), sellar region (c), and sellar and anterior half of posterior cranial fossa (d). Neuromuscular structures have been shown using electrical colored wires (for ACF and right half of skull base) or red (ICA), white (optic and V1, V2, and V3 divisions of trigeminal nerve), and blue (sigmoid, superior, and inferior petrosal sinuses) acrylic colors (for sellar and posterior fossa dural reflection models)
A glue gun (100-Watt glue gun, ApTech Deals, Delhi, India) was used with “transparent” glue (11 mm diameter) sticks to make these dural reflection models. Molten glue was uniformly spread over selected regions, one region at a time. Special care was taken to avoid air pockets under the layer of the glue by spreading it in continuity and putting some glue into the skull base foramen, which are important from anatomical point of view like optic foramen, foramen ovale, rotundum, spinosum, lacerum, and superior orbital fissure, which allow the important neurovascular structure to pass through or enter into the cranial cavity. Once the glue was spread over the desired area, it was cooled under running water for 2–3 min. Once cooled, a gradual separation of the glue layer was done without breaking the layer which usually becomes quite strong and separates easily. Colored electrical wires were placed in the foramen before the application of molten glue to depict neurovascular structures like optic nerves by yellow electrical wires, internal carotid arteries (ICA) by red electrical wires, and V1, V2, and V3 branches of trigeminal nerves by yellow wires. Various acrylic colors like red, blue, and yellow were also used for marking relevant neurovascular structures for depicting arteries, veins/venous sinuses, or nerves respectively. Marking of the neurovascular structures was done by a senior faculty member.
The technique of making dural reflection model of the central skull base is shown in the video (Video 1).
Results
The inferior surface (surface in contact with the bone) of the separated glue layer is similar to the morphological appearance of the inferior aspect of the skull base dura along with all the impressions of the skull base including foramina without the bony elements of the skull base. Once settled after cooling, these colored wires were indicative of cranial nerves and arteries.
These models were useful as neuroanatomy visual teaching material especially for endoscopic skull base surgical approaches including extended endoscopic skull base approaches. It helped in providing a 3-dimensional orientation of the neurovascular structures in relation to the skull base dura. These models were not used for hands-on training.
The approximate cost of the glue gun is 4–4.5 USD and glue sticks used for 3 or 4 areas cost less than 25 cents (1/4 USD).
Discussion
Skull base anatomy is considered one of the most difficult to understand in neurosurgical training, especially due to the complex relationships of dural folds, skull base bone sutures, various ligaments, and their complex relationships with the neurovascular structures [1-3]. The inability to see the bare inferior aspect of the skull base dura due to nasal sinuses, pterygoid plates with its muscles, mandible, and craniovertebral junction with its muscular attachments with the skull base makes it much more difficult to understand especially in the early part of the neurosurgical career. Convexity dura and superior views of skull base dura are easily seen in cadaver specimens and in surgeries too. Most of the transnasal cadaver dissection photos are available which only show sellar and some part of the paramedian dura in extended endoscopic skull base approach articles (hard copy or digital) [4, 5]. Even when they are available, it shows anatomical structures deep to the dura and the appearance of basal dura from the inferior aspect after the removal of the bone of the skull base is not available. With the widening applications of endoscopic endonasal/transoral skull base approaches in the last decade, these models may be useful for teaching neuroanatomy.
The authors feel that understanding orientations of neurovascular structures in the skull base region is important for training and glue models of skull base dura visible from the inferior aspect will be immensely helpful in neuroanatomy teaching. It may be useful even for those who have access to cadaver dissection, mainly because even cadaver dissection will not be able to show inferior aspect views of skull base dura except the sellar and to some extent the parasellar area [4].
The models, made by the technique mentioned, represent the inferior surface of the skull base dura without skull base bony elements. One may mark various neurovascular structures over it on the inferior and superior surfaces for easy understanding for trainees. One can pass colored electrical wires to depict arteries, veins/venous sinuses, and cranial nerves before spreading molten glue, in which case marking will not be required.
Neuroanatomical models of various structures have immense scope in neurosurgical training, which is not utilized even to a minimum. Physical models of skull base for preoperative planning are being made using 3-dimensional (3-D) printer which is technically challenging and resource-driven and takes 6–24 h to make one model based on the type of 3-D printer [7-9]. Virtual neurosurgery training models are available but physical models will be much more helpful in the early part of the neurosurgical career. Virtual models are currently not accessible to all and are very costly [10]. Physical models, except saw bone models of bones, available in other disciplines, are of poor quality and cannot be used currently for teaching and training purposes.
The authors feel that despite the availability of cadaver dissection facilities for undergraduate students at most medical colleges and institutions, they are rarely being used for super-specialty training and teaching. Neuroanatomy teaching in undergraduates is too basic and most of the specimens of the brain and skull base are left unused despite its immense utility for neurosurgical training. Among various reasons, poor coordination between the departments of anatomy and neurosurgery seems the most obvious. Integration of anatomy departments in various aspects of teaching and research activities of neurosurgery may greatly enhance the quality of neurosurgery and skull base surgery teaching and training.
Variety of materials like silicon, alginate, and resins are used for making dental impressions and may also be used for the purpose. However, these materials are much costlier than glue. Putty too is used for making dental impressions, but making a thin layer of it is not feasible mainly due to its fragility. The consistency of silicon is ideal, whereas alginate is too soft and resins are too hard. The authors do use silicon material for making molds for other purposes [11].
It is important to use “transparent” glue sticks, because other types of non-transparent glue adhere to the bone and it becomes difficult to separate it and may break the thin bone of the skull base. Molten glue, when used with 100-Watt glue gun, is a gel like flowable liquid and special care is needed to spread it in continuity; otherwise, air pockets will be entrapped leading to errors in the model. These models cannot be used for hands-on training; however, the feasibility of visual neuroanatomy teaching of otherwise inaccessible skull base dura, its cost-effectiveness, and simple reproducible technique is quite useful, especially in the early part of neurosurgery teaching. The authors are not aware of any other technique or material except automated 3-dimensional printing using silicon and other artificial materials for models related to neurosurgery [12, 13]. The authors do understand that there is the necessity of neurosurgery hands-on training models, which will immensely help in skull base training. A number of patients and number of surgeries done in India, and many other LMICs, are far more than many western countries which help trainees to observe, assist, and train reasonably and satisfactorily. Many centers in various parts of the world including India are using cost-effective models for hands-on training for endoscopic surgeries which is encouraging [14, 15].
Conclusion
We conclude that glue models are inexpensive, reproducible, and simple to make and can be widely used for visual neuroanatomy teaching especially for endoscopic skull base surgical approaches. These models may be an important teaching tool in LMICs where resources like cadaver dissection facilities are limited.
Data availability
There is no dataset for this study. A short video of the procedure is being uploaded with the manuscript as “Related file.”
References
Tschabitscher M, Di Ieva A (2013) Practical guidelines for setting up an endoscopic/skull base cadaver laboratory. World Neurosurg 79(2 Suppl):S16.e1-S16.e7. https://doi.org/10.1016/j.wneu.2011.02.045
Garg K, Deora H, Mishra S et al (2020) Quality of neurosurgery training in India when compared to UK- are we there yet? Neurol India 68(4):950–951. https://doi.org/10.4103/0028-3886.293439
Tan L, Wang Z, Jiang H et al (2022) Full color 3D printing of anatomical models. Clin Anat 35(5):598–608. https://doi.org/10.1002/ca.238754
Oostra A, van Furth W, Georgalas C (2012) Extended endoscopic endonasal skull base surgery: from the sella to the anterior and posterior cranial fossa. ANZ J Surg 82(3):122–130. https://doi.org/10.1111/j.1445-2197.2011.05971.x
Rosahl SK, Gharabaghi A, Hubbe U, Shahidi R, Samii M (2006) Virtual reality augmentation in skull base surgery. Skull Base 16(2):59–66. https://doi.org/10.1055/s-2006-931620
Lee SC, Senior BA (2008) Endoscopic skull base surgery. Clin Exp Otorhinolaryngol 1(2):53–62. https://doi.org/10.3342/ceo.2008.1.2.53
Sekhar LN, Juric-Sekhar G, Qazi Z et al (2020) The future of skull base surgery: a view through tinted glasses. World Neurosurg 142:29–42. https://doi.org/10.1016/j.wneu.2020.06.172
Kilinc MC, Basak H, Çoruh AG et al (2021) Endoscopic anatomy and a safe surgical corridor to the anterior skull base. World Neurosurg 145:e83–e89. https://doi.org/10.1016/j.wneu.2020.09.106
Narang P, Raju B, Jumah F et al (2021) The evolution of 3D anatomical models: a brief historical overview. World Neurosurg 155:135–143. https://doi.org/10.1016/j.wneu.2021.07.133
Abe M, Tabuchi K, Goto M, Uchino A (1998) Model-based surgical planning and simulation of cranial base surgery. Neurol Med Chir (Tokyo) 38(11):746–751. https://doi.org/10.2176/nmc.38.746
Jha DK, Garg M, Bhaskar S, et al (2021) Methylmethacrylate inter-facetal and inter-vertebral body spacers for cranio-vertebral junction and various spine surgeries: technical note. Asian J Neurosurg 16(3):648–654. Published 2021 Sep 14. https://doi.org/10.4103/ajns.AJNS_443_20
Neuro 3D trainer. https://3dneurotrainer.us10.list-manage.com/track/click?u=ebc108b4959fb030e4b6e1f95&id=72969258a3&e=68c6153199. Accessed on 21st February 2023
Baby B, Singh R, Singh R et al (2020) A review of physical simulators for neuroendoscopy skills training. World Neurosurg 137:398–407. https://doi.org/10.1016/j.wneu.2020.01.183
Bajaj J, Yadav YR, Pateriya A, Parihar V, Ratre S, Dubey A (2017) Indigenous inexpensive practice models for skill development in neuroendoscopy. J Neurosci Rural Pract 8(2):170–173. https://doi.org/10.4103/jnrp.jnrp_495_16
Deopujari CE, Karmarkar VS, Shaikh ST, Gadgil US (2019) Developing a dynamic simulator for endoscopic intraventricular surgeries. Childs Nerv Syst 35:621–627. May137:398–407. https://doi.org/10.1016/j.wneu.2020.01.183
Author information
Authors and Affiliations
Contributions
Jha DK: conception or design of the work, data collection, data analysis and interpretation, drafting the article, critical revision of the article, final approval of the version to be published.
Janu V: data collection, data analysis and interpretation, drafting the article, critical revision of the article, final approval of the version to be published.
Bhaskar S: conception or design of the work, data analysis and interpretation, critical revision of the article, final approval of the version to be published.
Gosal JS: data collection, data analysis and interpretation, critical revision of the article, final approval of the version to be published.
Ghatak S: data collection, data analysis and interpretation, critical revision of the article.
Corresponding author
Ethics declarations
Ethical approval
This study does not involve human or animal subjects; therefore, ethical approval was not required.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Supplementary file1 (MOV 174638 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jha, D.K., Janu, V., Bhaskar, S. et al. Skull base dural reflection models: tool for teaching neuroanatomy at resource-scarce centers. Neurosurg Rev 46, 105 (2023). https://doi.org/10.1007/s10143-023-02008-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-023-02008-1