Abstract
Even with the advent of endovascular treatment for intracranial aneurysms, microsurgical clipping continues to play a significant role in the treatment of middle cerebral artery (MCA) aneurysms. Securing perforators around unruptured intracranial aneurysms (UIAs) is essential for minimizing procedural risks in each treatment option. Therefore, we herein investigated whether the findings of high-resolution cone-beam computed tomography (HR-CBCT) have an impact on decision-making for the treatment of MCA UIAs. Patients with MCA UIAs between October 2017 and September 2021 were consecutively recruited for this study. All patients underwent HR-CBCT and 3D-DSA before treatment. The imaging quality of both modalities to visualize the microvasculature around aneurysms was evaluated. Specific findings on the microvasculature surrounding aneurysms on HR-CBCT were investigated to facilitate microsurgical clipping. Fifty-two MCA UIAs were treated, including 43 by microsurgical clipping and 9 by endovascular approaches. The overall imaging quality of HR-CBCT was superior to that of 3D-DSA. Regarding microsurgical insights, sensitivity and specificity for the visualization of small vessels around aneurysms were 79 and 100%, respectively, using HR-CBCT, and 57 and 93%, respectively, using 3D-DSA. The presence of a low-density band between adhesive vessels and aneurysm sacs was indicative of successful and safe microsurgical dissection between these structures. HR-CBCT enabled visualization of the intracranial microvasculature around MCA UIAs at the submillimeter level in vivo. In cases in which the tight adhesion of the microvasculature to the aneurysm sac is indicated by HR-CBCT, an endovascular approach may be considered in order to avoid the risks associated with securing perforators.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Unruptured intracranial aneurysms (UIAs) are a serious cause of subarachnoid hemorrhage with significant morbidity and mortality. Although the majority of UIAs in the anterior circulation (< 7 mm in size) remain asymptomatic and stable, the small potential risk of rupture represents a major concern for patients [1]. Therefore, prophylactic treatment with a minimal risk of complications and long-term durability needs to be considered. The application of endovascular treatment for UIAs has steadily increased in the last 2 decades. Recent comparative studies on UIAs suggested the superior safety and efficacy of endovascular approaches to microsurgical treatment [2]. Nevertheless, microsurgical clipping continues to play a significant role, particularly for middle cerebral artery (MCA) aneurysms, mainly because of their location and complex neck configuration. The optimal management paradigm remains controversial [3,4,5], and a novel prospective study is now underway [6]. Regarding microsurgical clipping for MCA UIAs, the majority of adverse events associated with microsurgical clipping are ischemic complications, presumably due to compromised perforating arteries [7,8,9,10]. Once a perforating artery from the MCA passing through the basal ganglia is sacrificed for neck clipping, some patients develop permanent neurological deficits. With advances in endovascular devices and techniques, including coil materials, neck bridging devices (stents and Pulserider®), flow diverters, and intrasaccular flow-disruption (WEB®) [11] treatment options are now being discussed in more detail. A better understanding of the microanatomy around an aneurysm will facilitate decision-making on the strategies selected for the endovascular treatment of intracranial aneurysms [12]. Treatment planning for UIAs mainly depends on multidisciplinary factors, such as aneurysm morphology, the presence of atherosclerosis or calcifications, age, the general condition of patients, patient requests, and institutional expertise. Recent advances in imaging technologies have allowed microvasculature with a diameter of 200 μm to be visualized [13]. Therefore, we herein performed a novel trial on the use of high-resolution cone-beam computed tomography (HR-CBCT) to visualize the microvasculature around MCA UIAs and decision-making on treatment strategies at a single center with hybrid neurosurgeons.
Subjects and methods
Institutional Review Board approval was obtained for the present study and all patients provided their informed consent before the examination.
Patients with MCA UIAs who consented to microsurgical or endovascular treatment between October 2017 and September 2021 were included. All patients underwent selective digital subtraction angiography (DSA) including the standard, 3D DSA, and HR-CBCT protocols before treatment. Based on the findings of angiography, we discussed and selected treatment options on a consensus with each patient.
3D DSA and HR-CBCT protocols
Angiography was performed under local anesthesia through a 4 French diagnostic catheter (Terumo, Terumo Medical, Tokyo, Japan). The examination was conducted on a biplane, high-resolution angiographic system (AlluraClarity, Philips Healthcare, Best, Netherlands) using Iopamiron 300 (Iopamidol, Bracco, Italy) for vessel opacification. The 3D DSA and HR-CBCT protocols using Philips AlluraClarity were described below. To create a circumferential run, the C-arm rotated around the patient over 220° in 4 s for 3D DSA and in 20 s for HR-CBCT. The injector was coupled to the acquisition system, with a fixed 2-s delay for 3D DSA and 5-s delay for HR-CBCT. To achieve good vessel opacification during the run, a non-ionic contrast agent was injected at a flow rate of 3 mL/s (18 mL in total) for 3D DSA and 1 mL/s (25 mL in total) for HR-CBCT. 3D DSA acquired 122 images of the region of interest (a 13-inch field of view), while HR-CBCT acquired 622 images (an 8-inch field of view). Acquired pixel sizes were 308 μm in 3D DSA and 154 μm in HR-CBCT. Mean radiation exposure for diagnostic angiography was 225 mGy.
Data reconstruction and imaging analysis
Transverse flat-detector CT reconstructions (the arterial mode) were performed at the discretion of the operator. Multiplanar reconstructions with a slice thickness of 0.5 mm and maximum intensity projections with section thicknesses of between 5 and 10 mm as well as volume rendering reconstructions were sent to the local Picture Archiving and Communicating System (R11.4.1, 2009; Philips, Best, Netherlands; Sectra, Linkoping, Sweden; or Coral, Toronto, Canada) and used for a perforator analysis. Clinical information was retrieved from locally maintained databases, and all imaging findings were independently evaluated by board-certified neuroradiologists with 10 years of experience who were blinded to clinical data and operative findings. The following features were qualitatively assessed: the aneurysm, parental and major branching arteries, and other small vessels from the M1 to M2 segments. The quality of each image was rated using a five-point scale (5 = excellent, 4 = good, 3 = moderate, 2 = poor, and 1 = non-diagnostic). Overall imaging quality was calculated as the average of these 3 factors. Vessels adhering to aneurysms were classified by the presence of a low-density band along the attaching aneurysm sac. Discrepancies were resolved by a third reviewer with more than 20 years of neuroimaging experience. Intraoperative views were reviewed by a board-certificated neurosurgeon for the presence of adhesive vessels and whether microsurgical dissection between the microvasculature and aneurysm sac was successful. Regarding aneurysms treated by microsurgical clipping, imaging correlations with intraoperative insights were evaluated.
Patient outcome and microsurgical result
Postoperative diffusion-weighted imaging (DWI) to evaluate ischemic lesions was performed 1-day after the surgery in all patients. Immediate aneurysmal closure was evaluated by CT angiography at 1 week after the microsurgical clipping and MR angiography or DSA at 6 months after the endovascular treatment. Patient outcome was evaluated at 30-day follow-up in our outpatient clinic.
Statistical analysis
Statistical analyses were performed using the JMP statistical package (JMP 14, SAS Institute Inc., Cary, NC). Continuous variables were summarized as means ± standard deviations (range), and categorical variables were presented as percentages. Cohen’s κ coefficient was calculated to assess inter-observer agreement in evaluations of the adherence of the microvasculature to the aneurysm sac. The intraclass correlation coefficient with the 95% confidence interval (CI) was analyzed to assess the interobserver variabilities.
Intergroup comparisons were performed using a univariate analysis with Fisher’s exact test for categorical variables and the Mann-Whitney U test for continuous variables. A two-tailed p value < 0.05 was considered to be significant.
Results
Patient backgrounds
Fifty-two patients with MCA UIAs (38 females, 14 males) and a mean age of 67 ± 11 years (range 43–83 years) were examined in the present study. After preoperative conferences with our hybrid neurosurgeon team and consultations with patients, 43 patients were treated by microsurgical clipping and 9 by an endovascular approach (including 6 by coiling alone and 3 by coiling with an adjunctive technique). In discussions on the microanatomy detected by HR-CBCT, there were 6 crossover patients: the treatment strategy changed from microsurgical clipping to endovascular treatment for 2 patients and vice versa for 4. There were no hemorrhagic or permanent ischemic complications associated with either procedure.
Imaging quality
A detailed comparison of the quality of images is summarized in Table 1. The imaging quality of HR-CBCT was superior to that of 3D DSA. The mean overall image quality was 4.2 (SEM 0.1, range 3–5) for HR-CBCT and 3.9 (SEM 0.1, range 2–5) for 3D DSA (p < 0.01). The visualization of aneurysm morphology was equivalent, with a score of 3.7 (SEM 0.1, range 3–5) for HR-CBCT and 3.9 (SEM 0.1, range 3–5) for 3D DSA (p = 0.11). The visualization of parental and branching arteries was similar, with a score of 4.0 (SEM 0.1, range 3–5) for 3D DSA scoring and 3.9 (SEM 0.1, range 3–5) for HR-CBCT (p = 0.65). Scores for the visualization of perforating arteries were significantly higher for HR-CBCT at 4.3 (SEM 0.1, range 4–5) than for 3D DSA at 3.4 (SEM 0.1, range 2–5) (p < 0.001).
HR-CBCT findings and adherent microvasculature
Among the 43 aneurysms treated by microsurgical clipping, intraoperative insights showed 27 adherent vessels on the aneurysm sac. Comparison of imaging quality for the visualization of structures around middle cerebral artery aneurysms was summarized in Table 2 regarding microsurgical insights, sensitivity and specificity for the visualization of small vessels around aneurysms were 79 and 100%, respectively, for HR-CBCT, 57 and 93%, respectively, for 3D-DSA. HR CBCT demonstrated a large AUC compared with 3D DSA (p = 0.007). Regarding HR-CBCT, the relationship between microsurgical success for dissection and visualization of the microvasculature is shown in Table 3. Twenty-two out of 27 small vessels were detected on HR-CBCT, and 17 showed a low-density band between the two structures. Twelve out of the 17 small vessels were successfully exfoliated in their entirety, while the other 5 were too challenging to completely detach. Five small vessels without a low-density band on the sac were not detached from the aneurysm sac.
Patient outcome and microsurgical result
In microsurgical clipping, 2 of 43 (5%) patients showed a small DWI positive lesion, though both patients demonstrated no neurological deficit. In endovascular treatment, 3 of 9 patients (33%) demonstrated multiple DWI positive lesions. At 3-month follow-up, no patient showed neurological deterioration nor worsened the daily activity. Complete aneurysmal obliteration was achieved in 42 of 43 patients (98%) in microsurgical clipping. A neck remnant was observed in 1 patient to secure a branching artery from the aneurysm neck. In endovascular treatments, 6 of 9 (67%) showed complete aneurysmal obliteration. Additional treatment was required for one patient by microsurgical clipping.
Discussion
The present study showed that the image quality of HR-CBCT was superior to that of 3D DSA, and it clearly visualized the adherence of the microvasculature to the aneurysm sac. Moreover, the present results suggest the potential of HR-CBCT as a valuable preoperative imaging for the preservation of small, but indispensable vessels with microsurgical clipping. Submillimeter vessels around MCA UIAs, particularly perforating arteries, may be compromised, which results in the most critical ischemic complications in the treatment of UIAs. This microvasculature is generally not visualized in routine work-ups. Therefore, we conducted a study that focused on the visualization of the microanatomy around MCA UIAs using HR-CBCT. This novel concept of preoperative evaluations to select a treatment strategy, either microsurgical clipping or an endovascular approach, will improve treatment outcomes, and only 6 out of 52 patients required cross-over treatment in the present study.
The current of treatment strategies for UIAs has markedly changed with advances in endovascular devices and techniques. Recent studies on the sites of UIAs suggested that endovascular treatment was preferred to microsurgical clipping due to its procedural safety and efficacy. However, the optimal treatment of UIAs in the territory of MCAs remains a highly debated topic mainly due to their location and neck configuration. According to the latest systematic reviews and a meta-analysis of MCA UIAs, microsurgical clipping was performed with less than 3% periprocedural risks and exhibits long-term durability [3,4,5]. Data obtained from high-volume centers confirmed that the ischemic complications leading to permanent morbidity were caused by compromised perforators [7, 9, 10]. The presumable cause of this unfavorable, but unavoidable, complication appears to be the sacrifice of small vessels adhering to aneurysms or hidden behind aneurysms, which cannot be appraised as 3% before surgery.
HR-CBCT in combination with selective angiography allows for microvasculature with a diameter of 200 μm to be visualized before craniotomy [13]. According to the present results, HR-CBCT provides superior imaging quality with high sensitivity and specificity for the detection of vessels around MCA UIAs. Regarding the visualization of perforators, HR-CBCT was superior to 3D DSA. Imaging findings on HR-CBCT may show adherence around MCA UIAs. Vascular structures on HR-CBCT were visualized by the intraluminal opacification of contrast materials. Therefore, the low-density space between the aneurysm sac and vascular structure may include each wall structure. Based on actual intraoperative observations, this low-density band suggests sufficient space, including the presence of fibrous tissue, to be exfoliated by a microscissors, microspatula, or the spearing of tiny needles (Fig. 1). In contrast, the adherence of vessels without a low-density band on the aneurysm sac may be too strong to safely detach (Fig. 2). The forcible dissection of adhesive small vessels may result in injury or severe vasospasm, which ultimately leads to brain ischemia.
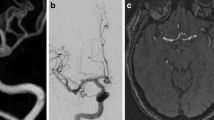
A 78-year-woman with a left unruptured middle cerebral artery (MCA) aneurysm treated by microsurgical clipping. A The irregularly shaped MCA aneurysm on 3D DSA (arrow). A perforating artery branching from the superior trunk is hardly detected on 3D DSA (arrowhead). B High-resolution cone-beam CT (HR-CBCT) shows a perforating artery adhering to the aneurysm sac (arrow). C Oblique image on maximum intensity projection demonstrates a low density band between the perforator and aneurysm sac (arrowhead). D An intraoperative view shows the adherent perforating artery on the aneurysm sac (arrow). E The perforating artery is completely exfoliated along the thin connective tissue (arrow) using a microscissor. F After exfoliating and preserving the perforating artery (arrow), the MCA aneurysm is clipped completely using two clips
A 50-year-woman with a right unruptured middle cerebral artery (MCA) aneurysm treated by microsurgical clipping. A The irregularly shaped MCA aneurysm on 3D DSA (arrow). Adherent vessels to the aneurysm sac are not clearly identified. B High-resolution cone-beam CT (HR-CBCT) showing a perforating artery adhering to the aneurysm sac (arrow). C Oblique image on maximum intensity projection shows no low density band between the perforator and aneurysm sac (arrowhead). D Intraoperative view shows strongly adherent two perforating arteries on the aneurysm sac (arrow). E An injured and coagulated perforating branch is demonstrated (arrowhead). The other preserved perforator shows partially spastic (arrow). F The MCA aneurysm is clipped using two clips without peeling off the perforating artery
The advent of endovascular treatment has allowed for the safe treatment of MCA UIAs. With the development of novel devices, such as stents, flow-diverting stents, and intrasaccular flow-disruption, MCA UIAs may be treated without interventions for these adhesive vessels. In this study, treatments for 2 patients were crossed over from microsurgical clipping to endovascular treatment due to the detection of adhesive perforators (Fig. 3). Visualization of the microvasculature around MCA UIAs may provide important insights for endovascular treatment, particularly the use of flow-diverting stents. Previous studies suggested that aneurysms involving small vessels may result in incomplete occlusion using flow diverting stents [12]. The presence of small vessels around aneurysms was the final decisive factor in 4 patients in the present study for whom treatment options were changed from endovascular treatment to microsurgical clipping. Decisions regarding treating options for MCA UIAs are still made on an individual basis. Patient profiles, aneurysm morphologies, and patient preferences are major factors contributing to the selection of treatment strategies, so far. The authors suggested that our favorable treatment results and smaller complication rate compared with those in previous studies [3,4,5] might depend on the assessment of microvasculature around the aneurysm and the choice of more advantageous treatment in this two-way physician era. By using this state-of-the-art imaging modality, centers may identify additional risks in the treatment of MCA UIAs and discuss alternative options to reduce these risks. In the near future, visualization of the microanatomy will be pivotal for the selection of treatment strategies for UIAs.
A 74-year-old man with a right unruptured middle cerebral artery (MCA) aneurysm, whose treatment strategy was a cross-over from microsurgical clipping to endovascular coiling. A An irregular shaped MCA aneurysm on 3D DSA (arrow). B High-resolution cone-beam CT (HR-CBCT) shows an artery adhering to the aneurysm sac (arrow) that is not detected on 3D DSA. C Orthogonal cross section to the adhesive artery on maximum intensity projection shows no low density band between the microvasculature and the aneurysm sac (arrowhead). D Angiography before coil embolization shows the bi-lobulated MCA aneurysm. E Craniogram shows the optimal positioning of PULSERIDER® (arrow). F The aneurysm is completely obliterated by coils with the assist of PULSERIDER® (arrow)
Limitations
The present study was conducted at a single center with a team consisting of hybrid neurosurgeons. Expert neurosurgeons may safely perform the exfoliation of tightly adhering vessels. Nevertheless, HR-CBCT allows neurosurgeons to detach adherent vessels for microsurgical clipping when they evaluate UIAs by HR-CBCT. We do not recommend the detachment of adhering perforators without a low-density band on the aneurysm sac on HR-CBCT. We herein focused on MCA UIAs in the present study. This novel concept may be applied to all sites of UIAs. UIAs close to the skull base may be unsuitable because vascular structures may be invisible beside bone structures using this CT modality. The adherence of perforating arteries to the aneurysm sac is one of the factors being debated in treatment planning. The present study was performed from a neurosurgical perspective; therefore, endovascular experts may obtain other insights on the microanatomy around UIAs. Since endovascular approaches are changing with constant innovation, it is important to establish the safety and efficacy of microsurgical clipping and endovascular treatment. State-of-the-art imaging modalities will boost on-site debates on treatment strategies for MCA UIAs beyond morphology.
Conclusion
HR-CBCT provides images of the microvasculature around an aneurysm sac at the submillimeter level and a low-density band between these structures indicates the potential for successful microsurgical dissection. Current state-of-the-art imaging modalities will facilitate discussions on appropriate treatment planning for MCA UIAs and change neurosurgeons’ or interventional neuroradiologists’ approaches to minimize unexpected complications.
References
Morita A, Kirino T, Hashi K et al (2012) The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med 366:2474–2482
McDonald JS, McDonald RJ, Fan J et al (2013) Comparative effectiveness of unruptured cerebral aneurysm therapies: Propensity score analysis of clipping versus coiling. Stroke 44:988–994
Alreshidi M, Cote DJ, Dasenbrock HH et al (2018) Coiling versus microsurgical clipping in the treatment of unruptured middle cerebral artery aneurysms: A meta-analysis. Neurosurgery 83:879–889
Smith TR, Cote DJ, Dasenbrock HH et al (2015) Comparison of the efficacy and safety of endovascular coiling versus microsurgical clipping for unruptured middle cerebral artery aneurysms: A systematic review and meta-analysis. World Neurosurg 84:942–953
Zijlstra IA, Verbaan D, Majoie CB et al (2016) Coiling and clipping of middle cerebral artery aneurysms: A systematic review on clinical and imaging outcome. J Neurointerv Surg 8:24–29
Darsaut TE, Keough MB, Boisseau W et al (2022) Middle cerebral artery aneurysm trial (MCAAT): A randomized care trial comparing surgical and endovascular management of MCA aneurysm patients. World Neurosurg 160:e49–e54
Chung J, Hong CK, Shim YS et al (2015) Microsurgical clipping of unruptured middle cerebral artery bifurcation aneurysms: Incidence of and risk factors for procedure-related complications. World Neurosurg 83:666–672
Lee HS, Kim M, Park JC et al (2021) Clinical features of ischemic complications after unruptured middle cerebral artery aneurysm clipping: Patients and radiologically related factors. Neurosurg Rev 44:2819–2829
Nussbaum ES, Madison MT, Goddard JK et al (2018) Microsurgical treatment of unruptured middle cerebral artery aneurysms: A large, contemporary experience. J Neurosurg 130:1–7
Matsukawa H, Kamiyama H, Miyazaki T et al (2019) Comprehensive analysis of perforator territory infarction on postoperative diffusion-weighted imaging in patients with surgically treated unruptured intracranial saccular aneurysms. J Neurosurg 132:1088–1095
Lee KS, Zhang JJY, Nguyen V et al (2022) The evolution of intracranial aneurysm treatment techniques and future directions. Neurosurg Rev 45:1–25
Lylyk I, Scrivano E, Lundquist J et al (2021) Pipeline embolization devices for the treatment of intracranial aneurysms, single-center registry: Long-term angiographic and clinical outcomes from 1000 aneurysms. Neurosurgery 89:443–449
Dobrocky T, Piechowiak EI, Goldberg J et al (2021) Absence of pontine perforators in vertebrobasilar dolichoectasia on ultra-high resolution cone-beam computed tomography. J Neurointerv Surg 13:580–584
Data availability
Data and pictures shown in the manuscript will be available on demand.
Author information
Authors and Affiliations
Contributions
Toshinori Matsushige and Shigeyuki Sakamoto contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Yukishige Hashimoto, Taichi Ogawa, Gosuke Makimoto, Michitsura Yoshiyama, Takeshi Hara, and Shohei Kobayashi. The draft of the manuscript was written by Toshinori Matsushige, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the institutional ethical committee (ethics authorization #2018-02-20). All patients consented their data to be used for research according to the information and consent forms.
Human and animal ethics
Research procedures were in accordance with the Helsinki Declaration of the World Medical Association.
Consent for publication
Patients signed informed consent regarding publishing their data and photographs.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Matsushige, T., Hashimoto, Y., Ogawa, T. et al. The impact of high-resolution cone-beam CT findings on decision-making for the treatment of unruptured middle cerebral artery aneurysms. Neurosurg Rev 46, 26 (2023). https://doi.org/10.1007/s10143-022-01933-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-022-01933-x