Abstract
Skull base meningiomas threatening the optic nerves may require performing an extradural anterior clinoidectomy (EAC) to optimally decompress the optic pathways. The present study evaluated the functional results and morbidity after surgical resection of skull base meningiomas including EAC, focusing on visual acuity (VA) and oculomotricity. Eighty-seven consecutive patients harboring skull base meningiomas who underwent surgical resection that included an EAC between 2003 and 2020 were retrospectively analyzed (86% women, median age 53 years). Decreased visual acuity (DVA) was graded as functional (VA ≥ 5/10) and nonfunctional (VA < 5/10). Statistical analyses were performed on VA and oculomotor nerve (OcN) dysfunction. Ninety surgical procedures were performed. Meningiomas were located at the anterior clinoid process (39%), cavernous sinus (31%), and spheno-orbital (30%) levels. Patients with a preoperative functional vision (normal or functional DVA) had a 90.9% (IC95% = [84.0; 97.8]) probability of preserving it at 6 months and an 84.8% (IC95% = [76.2; 93.5]) probability at last follow-up. Patients with preoperative nonfunctional vision (nonfunctional DVA or blindness) had a 19.0% (IC95% = [2.3; 35.8]) probability of recovery of functional vision at 6 months and a 23.8% (IC95% = [5.6; 42.0]) probability at last follow-up. Preoperative DVA was significantly associated with early postoperative DVA in univariate analyses (p = 0.04). Concerning the OcN, 65% of the patients experienced a postoperative dysfunction, and 78% of those cases recovered. Our study confirms EAC as a useful technical option for skull base meningiomas threatening the optic nerve, especially relevant for patients with preoperative functional vision, and supports early surgical management for these meningiomas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The growth of a subgroup of skull base meningiomas may threaten the optic pathways, particularly those involving the optic canal. Therefore, surgical resection is recommended, even though direct manipulation of the tumor at the vicinity of the optic nerve (ON) is not without risk [18]. Extradural anterior clinoidectomy (EAC), as originally described by Dole in 1983[8], is a technical solution to overcome this issue. The rationale for this surgery is to optimize the tumor resection around the optic canal, safely open it to decompress the ON and widen the corridors between the ON, the internal carotid artery, and the oculomotor nerve (OcN) [29, 33]. Herein, we selected a subgroup of skull base meningiomas involving or threatening the optic pathways that were eligible for the EAC technique. At the time of decision-making and counselling patients, our main goal was to identify the independent predictors of the visual outcomes. Then, we chose to focus our secondary objectives on the surgical morbidity and the functional postoperative results, especially about the oculomotricity.
Methods
Inclusion and exclusion criteria
All consecutive patients who underwent surgical resection of a skull base meningioma including an EAC, between January 2003 and February 2020 in our institution, were included in this retrospective cohort study. Signed consent of the patient has routinely been registered and the protocol has been approved by the ethics committee of the French College of Neurosurgery (IRB00011687 #1: 2020/38). Every case of meningioma resection that included an EAC has been selected from a database of operated skull base meningiomas. Operative reports and postoperative imaging have been carefully reviewed for every procedure for which an EAC could be performed. Patients with follow-up shorter than 6 months have been excluded. This study has been conceived respecting the STROBE criteria designed for this kind of study [31]. The inclusion and exclusion process is detailed in a flowchart (Fig. 1).
Axial and sagittal slices of T1-weighted imaging after injection of gadolinium, of the three main meningioma locations on the left (ACP: anterior clinoid process; CS: cavernous sinus; SO: spheno-orbital), and their corresponding accessory location on the right (PCF: posterior cranial fossa) and detailed distribution in the total population (N = 90)
Collected data
Data were collected from available patient files and imaging. Three patients had a bilateral EAC performed in two different procedures and were consequently included twice, considering the ipsilateral symptoms for each procedure. Epidemiological data such as gender, age, ASA (American Society of Anesthesiologists) score [26], history of treated meningioma, location, treatment modalities (surgery, radiosurgery, or radiotherapy), and history of neurofibromatosis type 2 were routinely collected. Visual acuity (VA) was assessed by an independent ophthalmologist, and decreased visual acuity (DVA) was classified according to its severity and clinical consequence as:
-
Functional: VA ≥ 5/10;
-
Nonfunctional: VA < 5/10;
-
Blindness: VA = 0/10.
Data about the visual field (VF), oculomotricty including diplopia and ptosis (according to the affected cranial nerves: oculomotor III, trochlear IV or abducens VI), proptosis, and non-specific visual disorders (visual discomfort despite normal ophthalmological examination) were also collected. Trigeminal disorders were classified as hypoesthesia and neuralgia. Other general symptoms were registered: headache, orbital pain, epilepsy, cognitive impairment, speech disorder, and hypothalamo-hypophyseal insufficiency. The main location of the tumor (supposed primitive starting point of the meningioma) and the potential accessory location (secondary tumoral invasion) were considered from the preoperative imaging (MRI or CT scan) and operative reports. Thus, three main locations were identified, each associated to one specific accessory location, defining six types of meningiomas (Fig. 2). The first main type of tumor was anterior clinoid process (ACP) meningiomas, optionally invading the cavernous sinus (CS, either lateral wall only or full intracavernous compartment). The second identified subgroup was spheno-orbital (SO) meningiomas, with or without invasion of the CS. The last subgroup was primitive CS meningiomas, potentially invading the posterior cranial fossa (PCF). Accessory invasion of the optic canal, superior orbital fissure, and orbit were also registered for each tumor.
Case of a 48-year-old female patient who presented with an epileptic seizure. A T1-weighted contrast-enhanced brain revealed a large meningioma of the left anterior clinoid process. After Simpon 1 surgical removal, the postoperative course was simple and the 1-year follow-up MRI showed no tumor recurrence
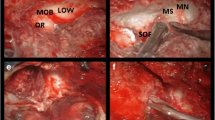
Surgical technique
All the EAC procedures were performed according to the same technique by the last author (Fig. 3) [29]. A regular pterional approach was first performed with the size of the craniotomy shaped to the target. The dura mater was gradually elevated from the orbital roof and anterior pole of the middle cranial fossa. Thus, the lesser sphenoid wing was exposed before careful drilling. The meningo-orbital band was identified in the depth and divided, allowing optimized retraction of the fronto-orbital and temporopolar dura, all the way to the superior orbital fissure and ACP. The lateral root of the ACP was removed by the drilling of the lesser sphenoid wing at the vicinity of the optic canal. A 3-mm diamond drill was used under optimal optic magnification and copious irrigation to prevent thermal lesions of the ON. The roof of the optic canal and the optic strut (respectively superomedial and inferomedial roots of the ACP) were drilled to progressively disconnect the ACP. The content inside the ACP was resected, preserving a bony shell that was elevated from the dura using a thin sharp dissector. The tip of the ACP was finally extracted with a rongeur after cautiously twisting and pulling it. The dura was opened in a standard C-shaped incision; an additional cut was done under microscope towards the falciform ligament to the optic canal for quick access and opening of the non-invaded basal cisterns to drain CSF and achieve brain relaxation. An effective extradural step with resection of the ACP allowed decompression of the ON by opening the optic canal and provided an optimized visual corridor for intradural tumor removal, including potential fragments inside the optic canal. In case of extensive CS meningiomas invading the PCF, a combined surgical approach including a Kawase anterior petrosectomy could be performed, which was noted [13]. Therefore, these interventions involved much longer extradural stages.
Intraoperative and postoperative data
The extent of the surgical resection was assessed according to the Simpson grading [28], considering the intraoperative findings and adjusted by the careful analyses of the postoperative imaging performed 3 to 6 months after the intervention (injected T1-weighted MR and bone window CT). Histological data reported included the different meningioma subtypes, their corresponding WHO grading, and the cellular marker of proliferation Ki67. The duration of hospitalization and postoperative complications were routinely recorded. Evolution of the symptoms was noted on postoperative day 1, hospital discharge, 6 months, and last follow-up. Preoperative and postoperative disorders were called primary and secondary, respectively. In cases of meningioma recurrence, we recorded the use of gamma knife radiosurgery and radiotherapy. Patients’ preoperative autonomy was assessed according to the Karnofsky performance score (KPS). A score of 70 was used as a functional threshold for the statistical analyses.
Statistics
Statical analyses were performed using the software SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). Comparisons between qualitative values were performed using Fisher’s exact test. Comparisons between quantitative and qualitative values were performed using the Student’s t-test. Multivariate analyses were based on the parameters demonstrating p < 0.20 in the univariate analyses, excluding potential irrelevant or repetitive data. Multivariate logistic regression using Firth bias correction was used to solve the phenomenon of complete or quasi-complete separation in binary logistic regression. The odds ratios are presented with their confidence interval at 95%. All tests were applied in bilateral situations and their significance was set at p < 0.05. Three patients died before 6 months follow-up and have been consequently excluded from the analyses of the functional results (87 remaining cases).
Results
Epidemiological data
From 2003 to 2020, 87 patients met the inclusion criteria, including three patients with bilateral EAC, for a total of 90 procedures. Women were largely predominant in our series, representing 86% of the cases. There were no statistical differences in age (p = 0.44) between women (56.2 ± 14.2 years old) and men (52.9 ± 10.3 years old) at the time of surgery. The mean age was 53.4 ± 10.9 years old (median 53) in the whole series. Two patients presented a neurofibromatosis type 2 (2%). The ASA score was 1 for 31 cases (34%), 2 for 42 cases (47%), and 3 for 17 cases (19%). Eighteen patients had a history of treated meningioma (including the three patients with bilateral EAC) with:
-
Thirteen surgical procedures and four radiosurgeries affecting the same location as in our study;
-
Seven surgical procedures and three radiosurgeries affecting another location;
-
One radiotherapy.
The mean delay between the EAC and the previous procedure was 44.9 ± 32.6 months. One ventriculoperitoneal shunt was implanted 1 month before the EAC for a patient presenting chronic hydrocephalus.
Tumor location
Sixty-nine patients underwent CT and MRI prior to surgery (77%). Eighteen patients had only an MRI (20%) and three only a CT scan (3%) because of MRI contraindications. The meningiomas were located as follows (Fig. 2):
-
ACP: 35 cases (39%);
-
CS: 28 cases (31%);
-
SO: 27 cases (30%).
Our series had no preferred side, with 46 meningiomas on the right side (51%) and 44 on the left side (49%). When comparing the age of the patients between the location subgroups, SO meningiomas patients were significantly older (p = 0.01) and CS meningiomas patients were younger (p = 0.01) than the total population (Appendix 1).
Preoperative clinical presentation
Preoperative signs and symptoms were classified as neurological (including neuroendocrine), ophthalmological, oculomotor, and other cranial nerve dysfunctions (Fig. 4). DVA was predominant in our series, affecting 43 cases (47%). Patients suffered from visual deficits prior to surgery for a mean of 125 ± 230 days. CS meningiomas were responsible for more visual symptoms (64%) visually than ACP and SO meningiomas (46% and 48% respectively). Most SO meningiomas cases presented a proptosis (22 of the 27 cases, 81%). Few presented headache (2 of 27 cases, 7%) compared to the ACP (12 of the 35 cases, 34%) and CS (11 of the 28 cases, 39%) meningiomas cases. Blindness affected three cases of ACP meningioma and one case of SO meningioma. The remaining visual dysfunctions equally affected the three main location subgroups. CS meningiomas cases presented most cranial nerve dysfunction, affecting 10 of 19 OcN dysfunction (53%), seven of the 10 cases of ptosis (70%), and 10 of the 14 cases of trigeminal hypoesthesia (71%). Trochlear (two cases) and abducens (two cases) dysfunction and hypothalamo-hypophyseal insufficiency (five cases) were exclusively CS meningiomas cases. Finally, ACP meningioma cases presented the most epilepsy (seven of nine cases, 78%), cognitive impairment (four of five cases, 80%), and speech disorder (two of two cases, 100%) cases.
Evolution of the visual acuity (VA) through time according to the preoperative status: normal VA in green; functional decreased visual acuity (DVA) in yellow; nonfunctional DVA in orange; blindness in red. Each line represented the evolution of the AV status in each preoperative subgroups, using pie charts (number of cases between brackets)
Operative data
As assessed by the Simpson scale, the surgical resection was grade 4 in 71% of the cases (grade 1: 6%; grade 2: 17%; grade 3: 6%). Non-invasive ACP meningioma cases presented lower Simpson grading (p < 0.01) and CS meningiomas invading the PCF cases presented higher Simpson grading (p < 0.01) than the total population. Among the 64 Simpson grade 4 cases, residual tumor mostly affected the CS (51 cases; 80%), the orbit (12 cases; 19%), and other locations (6 cases, 9%). Ten of the 18 CS meningioma invading the PCF cases have justified an anterior petrosectomy (56%).
Histology
Eighty-two cases corresponded to WHO grade I tumors (91%, Ki67 3.1 ± 1.8%), and eight cases corresponded to WHO grade II (9%, 12.4 ± 7.3%, Appendix 2).
Duration of hospitalization and follow-up
The median duration of hospitalization was 9 days (Appendix 3). Compared to the total population, the duration of hospitalization was shorter for non-invasive ACP meningiomas cases (7.5 ± 3.5 days, p < 0.01) and longer for CS meningiomas invading the PCF cases (22.0 ± 23.2 days, p < 0.01). The median duration of follow-up was 53 months (maximum 196 months).
Postoperative complications
Three CSF leakages occurred (3%; two from the frontal sinuses and one unidentified), and two were treated conservatively (oral administration of acetazolamide and repeated depleting lumbar punctures). One was treated surgically (packing of the frontal sinus). There were two cases of meningitis (2%) without an identified bacterial germ. Two chronic hydrocephalus cases required a ventriculoperitoneal shunt at 2 and 7 months after the surgery (2%). One case has required a cranioplasty for esthetic reasons (1%). One patient presented a severe postoperative hemiparesis resulting from an intraoperative ischemia of the anterior choroid artery, which persisted throughout the follow-up. Four months after the surgery, another patient presented an ischemic stroke caused by the tumor stenosis of the cavernous portion of the internal carotid artery, leading to initial hemiplegia followed by partial recovery.
Deaths
There were 3 postoperative deaths in our series. The first patient was 77 years old (ASA score 3), featuring a SO meningioma (atypical WHO grade II) and presented a postoperative status epilepticus with quick unfavorable evolution. The two other patients displayed complex and extensive CS meningiomas invading the PCF and were operated on again. The first (56 years old, ASA 3, atypical meningioma WHO grade II) presented massive intraoperative brain edema related to venous bleeding and unfavorable evolution with refractory intracranial hypertension. The second (67 years old, ASA 3, meningothelial meningioma WHO grade I) featured a drug-resistant status epilepticus, 4 months after the surgery.
Visual function
The assessment of the visual function (and of all functional data) has focused on cases with 6 months follow-up, that is 87 cases (excluding the three deaths occurring before 6 months). Seventy-nine patients had an ophthalmological examination before the procedure (91%) and 69 patients 6 months after the surgery (79%). Forty cases presented a preoperative DVA (Fig. 5).
There were 10 cases of early postoperative VA deterioration (day 1: 12%, including two patients with a postoperative VA within the same range as their preoperative VA):
-
Three functional DVA cases;
-
Four nonfunctional DVA cases;
-
Three blindness cases.
Among them, two patients presented a deterioration 12 and 24 h after the intervention. In contrast, eight cases featured a late VA deterioration a few months after the surgical intervention (9%).
Preoperative DVA was the only setting significantly associated with early postoperative DVA in univariate analyses (Appendix 4; p = 0.04, OR = 5.63) but not in the multivariate analyses (p = 0.09). An ASA score equal or superior to 2 was significant in multivariate analyses (p < 0.05, OR = 5.52). Concerning late postoperative DVA, a KPS ≤ 70 appeared to be significant in both univariate (p < 0.01, OR = 23.33) and multivariate analyses (p < 0.01, OR = 62.89). Age was only significant in multivariate analyses (p = 0.01, OR = 0.88).
Twelve of the 40 cases featuring a preoperative DVA presented a postoperative improvement of their VA (30%). Six of the 19 cases featuring a preoperative functional DVA recovered a normal VA (32%). Among the 18 preoperative nonfunctional DVA cases, four recovered a functional DVA (22%), and one case had a normal postoperative VA (6%).
Patients with a preoperative functional vision (normal or functional DVA) have a 90.9% (IC95% = [84.0; 97.8]) probability of preserving it at 6 months (p < 0.01, OR = 42.5 [10.7; 168.1]) and an 84.8% (IC95% = [76.2; 93.5]) probability at last follow-up (p < 0.01, OR = 17.9 [5.4; 60.0]). However, patients with preoperative nonfunctional vision (nonfunctional DVA or blindness) have a 19.0% (IC95% = [2.3; 35.8]) probability of recovery of functional vision at 6 months (p = 0.02, OR = 0.03 [0.001; 0.59]) and a 23.8% (IC95% = [5.6; 42.0]) probability at last follow-up (p = 0.01, OR = 0.02 [0.001; 0.44]). There was no statistically significant association between the sequential number of procedures divided by median and the occurrence of an early DVA (p = 0.09) or late DVA (p = 0.71).
Concerning the VF, three of the 50 preoperative non-symptomatic cases had VF loss during the follow-up (6%; Fig. 6). In contrast, 12 of the 37 symptomatic cases postoperatively recovered their VF (32%).
Charts of the evolution of the symptomatic cases concerning the oculomotor, trochlear, and abducens nerve dysfunction, diplopia, and ptosis. Blue and orange lines respectively indicated preoperative and postoperative symptomatic cases (orange for the trochlear nerve dysfunction and grey for the abducens nerve dysfunction)
Oculomotricity
Concerning the OcN, there were 18 preoperative (21%) and 45 postoperative dysfunctions (52%; Fig. 7). Seven of the 18 primitive dysfunction cases (39%) and 10 of the 45 secondary dysfunction cases persisted during the follow-up (22%; Fig. 8). Among these 10 cases of secondary dysfunction at the long-term follow-up, one had isolated ptosis, three had diplopia, and six had both ptosis and diplopia.
CS meningioma invading the PCF (Appendix 5; univariate: p = 0.02; multivariate: p = 0.01) and residual tumors in the CS (univariate: p < 0.01; multivariate: p < 0.01) appeared to be associated with persisting secondary OcN dysfunction. Statistically significant sources of the persisting primitive dysfunction were CS meningioma invading the PCF (univariate: p = 0.01; multivariate: p = 0.02) residual tumors in the CS (univariate: p = 0.03; multivariate: p = 0.03) and ASA score of 2 or 3 (multivariate: p = 0.01).
One case presented a simultaneous dysfunction of the trochlear and abducens nerves, persisting during the follow-up (Fig. 7). There were 10 new postoperative dysfunctions of the trochlear nerve (11%), four recovering during the follow-up (40%). There were three new postoperative dysfunctions (3%) of the abducens nerve, one recovered during the follow-up (33%).
CS meningiomas invading the PCF (Appendix 6; p < 0.01), CS invasion (p = 0.03), CS residual tumor (p = 0.02), and anterior petrosectomy (p < 0.01) were significantly associated with a risk of postoperative dysfunction of the trochlear nerve. CS meningiomas invading the PCF (p < 0.01) and anterior petresectomy (p < 0.01) were associated with a risk of postoperative dysfunction of the abducens nerve.
There was no statistically significant association between the sequential number of procedures divided by median and the occurrence of secondary oculomotor nerve dysfunction (p = 0.62).
Discussion
Visual function
Preservation of visual function is a key concern in meningiomas threatening the ON. In this context, the main goal of the EAC is therefore to achieve early and complete release of the ON. Nevertheless, postoperative DVA still occurred despite satisfying anatomical release. We have tried to determine which factors potentially predict unfavorable visual evolution. Preoperative visual status appeared to be the main prognostic factor of postoperative visual function recovery. Functional vision before undergoing surgery was associated with better postoperative visual outcomes, whereas patients presenting with nonfunctional vision have a low probability of restoring it in the postoperative period.
There were two kinds of postoperative visual loss: early deterioration related to surgical iatrogeny and late visual worsening related to tumor progression. Preoperative DVA was associated with early postoperative DVA in univariate analyses (OR = 5.63) and was close to significance in the multivariate analyses (p = 0.09). The ASA score was the only significant parameter in the multivariate analyses (OR = 5.52). A KPS ≤ 70 was associated with late postoperative DVA in both univariate (OR = 23.33) and multivariate analyses (OR = 62.89). The lack of significance in our statistical analyses is probably explained by the small size of the subgroups, but it seems to highlight that impaired general condition and preoperative DVA might be associated with a worse visual prognosis.
Prior to surgery, the deterioration of the visual function is induced by the compression of the ON by the meningioma, leading to interruption of the axoplasmic flow and demyelination [15]. Intraoperative drilling of the optic canal during the early stages of the EAC exposes the ON to high temperatures (potentially reaching 80 °C) with a risk of thermal nervous lesions [27]. Thus, it is essential to use generous irrigation and frequently suspend the drilling in the vicinity of the ON to avoid injury [19]. Trauma to the ON can occur during the intradural and extradural stages, related to direct or indirect surgical manipulations.
Later in the procedure, the presence of an arachnoid plane between the meningioma and the ON conditions the quality of the tumor resection and decreases the risk of intraoperative injury [3]. Vascularization of the intracranial and intracanalar portions of the ON is provided by the superior hypophyseal and ophthalmic arteries [23]. Consequently, these vessels are directly threatened by tumor invasion and surgical dissection, leading to optic nerve ischemia and low retinal flow [15].
In our series, two cases of sudden postoperative blindness were slightly delayed from the awakening (12 and 24 h), supporting the hypothesis of vascular etiologies (ischemia or micro-bleeding). One of these two cases partially recovered a nonfunctional VA during the follow-up, consistent with a transitory retinal low flow.
Some (24%) patients presenting a preoperative DVA improved their VA during the follow-up, mostly recovering one functional grade compared to the initial status. Only 6% of the patients presenting a normal preoperative visual function experienced deteriorated VA during the follow-up (Fig. 5). In other words, patients with preoperative functional vision (normal or functional DVA) have a 90.9 ± 6.9% probability of retaining it at 6 months and an 84.8 ± 8.7% probability at last follow-up, while patients with preoperative nonfunctional vision (nonfunctional DVA or blindness) had only a 19.0 ± 16.8% probability of recovery to a functional vision at 6 months and a 23.8 ± 18.2% probability at last follow-up.
Regarding the VF, 6% of the non-symptomatic cases in the preoperative period had a VF loss during the follow-up, while 32% of the symptomatic preoperative cases recovered in the long term (Fig. 6). These results were congruent with the recovery of VA in the subgroup of preoperative functional DVA (32%, Fig. 5).
A literature review is informative on this topic, highlighting the different strategies and outcomes depending on the tumor location and preoperative disorders. The intradural approach for decompression of the optic canal, reported by Unteroberdörster et al. [30] in a retrospective study of 31 patients, displayed favorable outcomes with preservation or improvement of preoperative VA in 92% of the patients. Lehmberg et al. [16] investigated the use of EAC for skull base meningiomas in the vicinity of the optic canal in a retrospective series of 46 patients, concluding a more frequent improvement of the postoperative visual function after clinoidectomy. In a systematic review of the literature about meningiomas compressing the ON, Hénaux et al. [12] concluded that the potential of visual recovery fluctuated according to the location of the conflict. The intraorbital and intracanalar compressions demonstrated the most unfavorable results (11% and 31% of recovery, respectively), whereas intracranial compressions had a better outcome (49%). In a retrospective study of medial sphenoid wing meningiomas, Nakamura et al. [22] found better visual recovery for cases without invasion of the CS (56% and 30%, respectively).
Despite important methodological changes between our work and these different reports, our conclusions can be considered consistent with the current literature, confirming that visual recovery is variable and mostly moderate. Nonetheless, all these results corroborate with the current therapeutic strategy, aiming at surgically treating meningiomas as soon as they threaten the ON and especially before damage occurs, because it appears that patients with preoperative functional vision had very good results. In contrast, patients with preoperative nonfunctional vision seem to have both a limited chance of visual recovery and an increased risk of further deterioration. In our opinion, the single intradural approach is insufficient to optimally decompress the ON and remove the intracanal portion of the meningioma, putting the vision at risk in case of subtotal resection and tumoral regrowth, without a safe radiosurgical option for the optic pathways. In the case of preoperative blindness, the challenge may affect the contralateral ON [34].
Intraoperative visual-evoked potentials’ (VEP) monitoring may represent the most promising future option for preserving the visual function, but is rarely used in the daily routine at present. Several studies including a large cohort of various intracranial procedures have reported the feasibility and potential added value of this neurophysiological technique. The association between preserved VEP and preserved visual function and the sensitivity to detect vascular damage and mechanical manipulation of the anterior visual pathways have been notably emphasized [14, 17]. In the specific field of skull base surgery, its use during endoscopic transsphenoidal procedures demonstrated a significant correlation between changes in VEP amplitude and postoperative visual function [9]. Permanent VEP loss is associated with severe postoperative visual dysfunction, whereas transient VEP changes do not indicate postoperative visual disturbance [20]. The predictive value of VEP for the VF remains a matter of debate. In their study of 53 surgical procedures, including 36 parasellar tumors, Kodama et al. [14] concluded that VF defect without VA decrease might not be predicted by VEP monitoring. At the same time, Gutzwiller et al. [11] illustrated the reliability of intraoperative VEP for VF changes in 29 patients undergoing brain tumor resection. Therefore, neurophysiological monitoring of the visual function should be specifically investigated for resection of meningiomas in the vicinity of the optic pathways.
Based on these results and considerations, three points seem essential to improve the functional outcome of these patients. The mastery of microsurgery techniques learned in books, acquired in the anatomy laboratory, and perfected in the operating room is an essential and mandatory prerequisite before considering any intervention of this type. Then, considering the increased risk of worsening once optic nerve damage has occurred, it is crucial to propose surgery as soon as possible, and in the best case, before any visual deficit. Finally, intraoperative VEP represents a promising area of study that could become a standard in the future.
Oculomotricity
One of the most striking features of our series was the rate of postoperative OcN dysfunction (Figs. 7 and 8). Forty-five of the 69 initially non-symptomatic cases were affected right immediately after surgery (65%), but most recovered during the follow-up (78%).
The statistical analyses showed that primary and secondary persistent OcN dysfunctions were mainly associated with CS meningiomas invading the PCF and CS residual tumors. Although rarer, dysfunctions of the trochlear and abducens nerves were associated with the same location. Postoperative trochlear nerve dysfunction was more frequent (11%) than abducens nerve dysfunction (3%). Both nerve dysfunctions also displayed less effective recovery than the OcN (78% compared to 40% for the trochlear nerve and 33% for the abducens nerve). Nevertheless, we are careful about drawing conclusions, considering the different tumor locations, involved segments of the cranial nerves (cisternal, intracavernous, intraorbital) and the limited size of the subgroups.
In their retrospective study of surgically treated medial sphenoid wing meningiomas, Nakamura et al. [22] found slightly more frequent postoperative OcN dysfunction (12% against 8%) and reduced recovery (83% against 100%) in cases of CS invasion by the meningioma. In a retrospective study of 63 patients with CS meningiomas, Gozal et al. [10] highlighted that 12% of patients experienced postoperative OcN dysfunction, of which 6% demonstrated permanent dysfunction. In a retrospective series of 63 patients with SO meningiomas, Ringel et al. [24] identified 32% who experienced postoperative OcN dysfunction, of whom 13% had permanent dysfunction.
As a result of the risk of causing injury to the oculomotor nerves during intracavernous tumoral resection, the surgical strategy progressively evolved towards a more conservative perspective [1, 4, 5, 7]. Current multimodal approaches are aimed at limiting the functional consequences of the surgery and reducing the tumoral volume for further complementary treatment, generally using radiosurgery as a first choice [2, 6, 21, 25, 32].
To conclude, our results are partially consistent with the literature, presumably because of the singularity of our population [10, 22, 24]. These discrepancies are derived from our main inclusion criteria, based on the surgical technique rather than the location of the tumor. In particular, CS meningiomas implied higher rates of functional and oculomotor consequences, making it difficult to compare our series and these studies.
Therefore, our observations confirm the intraoperative vulnerability of the OcN, which poorly tolerates surgical manipulations in this context. Most postoperative OcN dysfunctions recover over time but are at risk of persisting in the case of tumor invasion of the CS.
Limits and perspectives
This was an observational retrospective monocentric study corresponding to a low level of scientific evidence. Additionally, the limited size of the subgroups could explain the lack of significance of the statistical analyses.
Because of the absence of an objective evaluation of the visual function in some patient files, VA and VF could be approximate and based on simple clinical exams and reported patient impressions.
Radiological parameters did not include volumetric analyses because some exams were missing (old files) and measurement difficulties and approximations, particularly for SO meningiomas. There were no analyses of the tumor control in the long term, which should be explored including meningiomas operated on without EAC. Finally, we could not completely separate the morbidity resulting from the specific EAC and from the overall meningioma resection.
Conclusion
EAC appears to be a useful technical option to operate on skull base meningiomas threatening the ON, especially relevant for patients with preoperative functional vision. The method supports early surgical management for these tumors before symptom onset or further deterioration. The current therapeutic strategy is associated with favorable visual outcomes at the cost of frequent postoperative OcN dysfunction, which recovered in most cases.
Availability of data and material
Stored on a professional computer with encrypted, anonymized, and password-protected data.
Code availability
Only known by the first author.
References
Abdel Aziz KM, Froelich SC, Dagnew E, Jean W, Breneman JC, Zuccarello M, van Loveren HR, Tew JM (2004) Large sphenoid wing meningiomas involving the cavernous sinus: conservative surgical strategies for better functional outcomes. Neurosurgery 54:1375–1384. https://doi.org/10.1227/01.NEU.0000125542.00834.6D
Akyoldaş G, Hergünsel ÖB, Yılmaz M, Şengöz M, Peker S (2020) Gamma Knife radiosurgery for anterior clinoid process meningiomas: a series of 61 consecutive patients. World Neurosurg 133:e529–e534. https://doi.org/10.1016/j.wneu.2019.09.089
Al-Mefty O (1990) Clinoidal meningiomas. J Neurosurg 73:840–849. https://doi.org/10.3171/jns.1990.73.6.0840
Al-Mefty O, Ayoubi S, Smith RR (1991) Direct surgery of the cavernous sinus: patient selection. In: Koos W, Richling B (eds) Processes of the Cranial Midline. Springer Vienna, Vienna, pp 117–121
Al-Mefty O, Smith RR (1988) Surgery of tumors invading the cavernous sinus. Surg Neurol 30:370–381. https://doi.org/10.1016/0090-3019(88)90200-5
Azar M, Kazemi F, Jahanbakhshi A, Chanideh I, Jalessi M, Amini E, Geraily G, Farhadi M (2017) Gamma Knife radiosurgery for cavernous sinus meningiomas: analysis of outcome in 166 patients. Stereotact Funct Neurosurg 95:259–267. https://doi.org/10.1159/000478024
Cusimano MD, Sekhar LN, Sen CN, Pomonis S, Wright DC, Biglan AW, Jannetta PJ (1995) The results of surgery for benign tumors of the cavernous sinus. Neurosurgery 37:1–10. https://doi.org/10.1227/00006123-199507000-00001
Dolenc V (1983) Direct microsurgical repair of intracavernous vascular lesions. J Neurosurg 58:824–831. https://doi.org/10.3171/jns.1983.58.6.0824
Feng R, Schwartz J, Loewenstern J, Kohli K, Lenina S, Ultakan S, Iloreta A-M, Govindaraj S, Bederson J, Banik R, Shrivastava R (2019) The predictive role of intraoperative visual evoked potentials in visual improvement after endoscopic pituitary tumor resection in large and complex tumors: description and validation of a method. World Neurosurg 126:e136–e143. https://doi.org/10.1016/j.wneu.2019.01.278
Gozal YM, Alzhrani G, Abou-Al-Shaar H, Azab MA, Walsh MT, Couldwell WT (2020) Outcomes of decompressive surgery for cavernous sinus meningiomas: long-term follow-up in 50 patients. J Neurosurg 132:380–387. https://doi.org/10.3171/2018.10.JNS181480
Gutzwiller EM, Cabrilo I, Radovanovic I, Schaller K, Boëx C (2019) Intraoperative monitoring with visual evoked potentials for brain surgeries. J Neurosurg 130:654–660. https://doi.org/10.3171/2017.8.JNS171168
Hénaux P-L, Bretonnier M, Le Reste P-J, Morandi X (2018) Modern management of meningiomas compressing the optic nerve: a systematic review. World Neurosurg 118:e677–e686. https://doi.org/10.1016/j.wneu.2018.07.020
Kawase T, Shiobara R, Toya S (1991) Anterior transpetrosal-transtentorial approach for sphenopetroclival meningiomas: surgical method and results in 10 patients. Neurosurgery 28:869–875 (discussion 875-876)
Kodama K, Goto T, Sato A, Sakai K, Tanaka Y, Hongo K (2010) Standard and limitation of intraoperative monitoring of the visual evoked potential. Acta Neurochir 152:643–648. https://doi.org/10.1007/s00701-010-0600-2
Le HM, Boch A-L, Gerber S, Cornu P, Bodaghi B, Lehoang P, Touitou V (2019) Troubles visuels aigus associés aux méningiomes sphénoïdaux. J Français d’Ophtalmol 42:485–491. https://doi.org/10.1016/j.jfo.2018.12.006
Lehmberg J, Krieg SM, Mueller B, Meyer B (2013) Impact of anterior clinoidectomy on visual function after resection of meningiomas in and around the optic canal. Acta Neurochir 155:1293–1299. https://doi.org/10.1007/s00701-013-1741-x
Luo Y, Regli L, Bozinov O, Sarnthein J (2015) Clinical utility and limitations of intraoperative monitoring of visual evoked potentials. PLoS One 10:e0120525. https://doi.org/10.1371/journal.pone.0120525
Margalit NS, Lesser JB, Moche J, Sen C (2003) Meningiomas involving the optic nerve: technical aspects and outcomes for a series of 50 patients. Neurosurgery 53:523–533. https://doi.org/10.1227/01.NEU.0000079506.75164.F4
Mariniello G, de Divitiis O, Bonavolontà G, Maiuri F (2013) Surgical unroofing of the optic canal and visual outcome in basal meningiomas. Acta Neurochir 155:77–84. https://doi.org/10.1007/s00701-012-1485-z
Mazzeo A, Gupta D (2018) Intraoperative visual evoked potential monitoring for a safer endoscopic transsphenoidal surgery
Mostafapour E, Nikoobakht M, Azar M, Pakpour AH (2015) Combination of surgery and Gamma Knife in the management of en plaque meningioma. Neurosurg Q 25:562–564. https://doi.org/10.1097/WNQ.0000000000000114
Nakamura M, Roser F, Jacobs C, Vorkapic P, Samii M (2006) Medial sphenoid wing meningiomas: clinical outcome and recurrence rate. Neurosurgery 58:626–639. https://doi.org/10.1227/01.NEU.0000197104.78684.5D
Overbeeke JJ, Sekhar LN (2003) Microanatomy of the blood supply to the optic nerve. Orbit 22:81–88. https://doi.org/10.1076/orbi.22.2.81.14316
Ringel F, Cedzich C, Schramm J (2007) Microsurgical technique and results of a series of 63 spheno-orbital meningiomas. Oper Neurosurg 60:214–222. https://doi.org/10.1227/01.NEU.0000255415.47937.1A
Roche P-H, Régis J, Dufour H, Fournier H-D, Delsanti C, Pellet W, Grisoli F, Peragut J-C (2000) Gamma knife radiosurgery in the management of cavernous sinus meningiomas. J Neurosurg 93:68–73. https://doi.org/10.3171/jns.2000.93.supplement_3.0068
Saklad M (1941) Grading of patients for surgical procedures. Anesthesiology 2:281–284. https://doi.org/10.1097/00000542-194105000-00004
Shih AJ, Tai BL, Zhang L, Sullivan S, Malkin S (2012) Prediction of bone grinding temperature in skull base neurosurgery. CIRP Ann 61:307–310. https://doi.org/10.1016/j.cirp.2012.03.078
Simpson D (1957) The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry 20:22–39. https://doi.org/10.1136/jnnp.20.1.22
Troude L, Bernard F, Baucher G, De La Rosa MS, Roche P-H (2017) Extradural resection of the anterior clinoid process: how I do it. Neurochirurgie 63:336–340. https://doi.org/10.1016/j.neuchi.2017.03.001
Unteroberdörster M, Müller O, Özkan N, Pierscianek D, Hadamitzky M, Kleist B, Sure U, El Hindy N (2020) Impact of optic canal decompression on visual outcome in subtotal resected skull base meningiomas. J Neurosurg Sci 64. https://doi.org/10.23736/S0390-5616.17.04020-6
Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M, for the STROBE Initiative (2007) Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Med 4:e297. https://doi.org/10.1371/journal.pmed.0040297
Williams BJ, Yen CP, Starke RM, Basina B, Nguyen J, Rainey J, Sherman JH, Schlesinger D, Sheehan JP (2011) Gamma Knife surgery for parasellar meningiomas: long-term results including complications, predictive factors, and progression-free survival: clinical article. J Neurosurg 114:1571–1577. https://doi.org/10.3171/2011.1.JNS091939
Yonekawa Y, Ogata N, Imhof H-G, Olivecrona M, Strommer K, Kwak TE, Roth P, Groscurth P (1997) Selective extradural anterior clinoidectomy for supra- and parasellar processes: technical note. J Neurosurg 87:636–642. https://doi.org/10.3171/jns.1997.87.4.0636
Zweckberger K, Unterberg AW, Schick U (2013) Pre-chiasmatic transection of the optic nerve can save contralateral vision in patients with optic nerve sheath meningioms. Clin Neurol Neurosurg 115:2426–2431. https://doi.org/10.1016/j.clineuro.2013.08.027
Author information
Authors and Affiliations
Contributions
Guillaume Baucher: collecting data, writing, figure and table design;
Lucas Troude: reviewing, correction;
Alexandre Roux: reviewing, correction;
Anderson Loundou: statistical analyses;
Mohamed Boucekine: statistical analyses;
Torstein Meling: reviewing, correction;
Pierre-Hugues Roche: main operator, conception, reviewing, correction.
Corresponding author
Ethics declarations
Ethics approval
French College of Neurosurgery (IRB00011687 #1: 2020/38).
Consent to participate
Signed consent for each patient.
Consent for publication
Signed consent for each patient.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1
Appendix 2
Appendix 3
Appendix 4
Appendix 5
Appendix 6
Rights and permissions
About this article
Cite this article
Baucher, G., Troude, L., Roux, A. et al. Predictors of visual function after resection of skull base meningiomas with extradural anterior clinoidectomy. Neurosurg Rev 45, 2133–2149 (2022). https://doi.org/10.1007/s10143-021-01716-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-021-01716-w