Abstract
Capillary hemangiomas (CHs) of the central nervous system represent a rare diagnosed pathology. CHs are benign vascular tumors whose most common manifestations are dermal and mucous and mainly occur during childhood or adolescence, while the involvement of the central nervous system can occur in a wider age range. We conducted a PubMed research on literature published until March 2020. We only enrolled cases with histological documented presence of intracranial CH. For every case collected, we analyzed age, sex, localization, neuroimaging studies performed, the presence of extracranial CHs, symptoms, neurological deficits, extent of surgical resection (biopsy, partial or gross total), adjunct treatment received (radiotherapy, chemotherapy, Trans-Arterial Embolization TAE), and outcome. Up to March 2020, the literature review identified 52 cases to which we added the case of our personal experience. The mean age was 26 with slightly female prevalence (28 F, 25 M). The most common presenting symptom was headache (21 cases, 40%). The surgical treatment consisted of biopsy in 7 cases (13%), partial resection in 10 cases (19%), gross total resection in 31 cases (58.5%), biopsy followed by total resection in 2 cases (3%), and partial resection followed by total resection in 1 case (1.5%), and the diagnosis was obtained from an autopsy sample in 1 case (1.5%). For symptomatic lesions, surgery is a valid option to obtain histological characterization, neurological improvement, and where possible a total resection. Stereotactic radiotherapy can be used if the lesion is not surgically approachable or as an adjuvant treatment in case of partial resection, having shown good results in terms of long-term disease control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Capillary hemangiomas (CHs) of the central nervous system represent a rare diagnosed pathology that has been receiving increasing attention for the last 10 years. CHs are benign vascular tumors whose most common manifestations are dermal and mucous and mainly occur during childhood or adolescence, while the involvement of the central nervous system can occur in a wider age range [29, 36]. Histologically, CHs are composed by single lobules of capillaries covered by a flattened endothelium, while cavernous hemangiomas are characterized by dilated hyaline vessels often associated with thrombosis, deposition of perivascular hemosiderin, and calcifications [1]. The radiological differential diagnosis often includes schwannoma, meningioma, or newly formed inflammatory granulomatous tissue (Wegener’s granulomatosis, sarcoidosis).
We present a literature review of intracranial CHs in the pediatric and adult population paying attention to clinical presentation, neuroimaging, management, and outcome, adding a case of intracranial CH in the Meckel cave of our personal experience.
Case report
A 36-year-old man arrived at the emergency department of our hospital complaining of pain and paraesthesia in the left side of the face, resistant to pharmacotherapy and with a worsening trend in the last 2 weeks. The pain started about 4 months earlier and was localized in the left maxillary and mandibular region. In the first 3 months, the pain was higher in intensity in the afternoon and then became continuous, searing, characterized by temporary exacerbations lasting a few hours, and was also associated with numbness of the left maxillary region. The pain relief therapy, initially successful, was no more effective in the last month. The patient did not report diplopia, facial weakness, otalgia, or otorrhea or any mastication disorder. The physical examination showed no deficits of cranial nerves, but the hypoesthesia in maxillary and mandibular branch regions of left trigeminal nerve. Corneal reflex, ocular motility, and pupillary function were normal. No other abnormalities were noted. Family history was irrelevant.
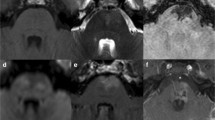
A brain CT scan showed an isodense mass in the left middle cranial fossa, adjacent to the apex of the petrous bone. An MRI of the brain with gadolinium was performed (Fig. 1) and confirmed the presence of a mass (1 cm x 1 cm x 1 cm) in the left Meckel cave extended to the cavernous sinus and ipsilateral foramen ovale. It appeared isointense in T1-weighted sequences and hyperintense in T2-weighted ones, with inhomogeneous gadolinium enhancement. Differential diagnosis included schwannoma, meningioma, metastasis, and lymphoma.
We performed a sub-temporal intradural-extradural approach. After placing a lumbar drain, the patient was placed in a supine position. During extradural dissection, the left middle meningeal artery, V3, and V2 were identified. The tumor was then exposed through a dural longitudinal incision at the level of the Meckel cave, extended along the trigeminal fibers. Intraoperatively, we found a red lobulated mass extending from the trigeminal ganglion, along V3 to the foramen ovale. The tumor was completely removed, preserving the integrity of the gasserian ganglion and its branches. Postoperative MRI showed total removal of the lesion. In the postoperative period, there was a significant reduction of pain in the left face side, no complications were observed, and the patient was discharged 8 days after surgical treatment.
On histological examination, the tumor showed a lobular architecture composed by small diameter vessels coated with predominantly flat epithelium consisting of positive CD31 and CD34 endothelial cells with uniform nuclei, without cellular atypia, and low mitotic index (Fig. 2). The final diagnosis was capillary lobular hemangioma.
At 1-month follow-up, the pain in the left face side was resolved and the hypoesthesia in the left maxillary region was reported as improving. At 1-year follow-up, MRI with gadolinium showed no tumor recurrence (Fig. 3), and the patient did not report any residual pain, but left mandibular hypoesthesia did not improved.
Materials and methods
We conducted a PubMed research on literature published until March 2020. The search was undertaken using the keywords “intracranial hemangioma,” “capillary hemangioma,” and “pediatric hemangioma.” References from the retrieved reports were checked to identify other possible results. We included only articles written in English language.
We only enrolled cases with histological documented presence of intracranial CH, excluding all those in which the diagnosis was only radiological. We considered pediatric all cases with age of 21 or less and adult all cases with age 22 or more. For every case collected, we analyzed age, sex, localization, neuroimaging studies performed, the presence of extracranial CHs, symptoms, neurological deficits, extent of surgical resection (biopsy, partial or gross total), adjunct treatment received (radiotherapy, chemotherapy, Trans-Arterial Embolization TAE), and outcome.
We classified the localization of intracranial CHs in cranial convexity, deep of cerebral lobes, cerebellum, sellar region, cavernous sinus, and Meckel cave or adjacent to main vessels.
Results
Up to March 2020, the literature review identified 41 publications for a total of 52 cases with histological diagnosis of intracranial CH, to which we added the case of our personal experience. The 25 (47%) pediatric cases and the 28 (53%) adult ones are shown in separate tables (Tables 1, and 2) [1, 2, 4,5,6,7,8,9, 12, 13, 15,16,17, 19,20,21,22,23, 25,26,27,28,29,30,31, 33,34,35,36,37,38,39,40, 42,43,44,45,46,47,48,49].
The mean age was 26 ± 23 years (range 0–80) with slightly female prevalence (28 F, 25 M).
In several cases the intracranial CH’s size extended to more than one anatomical region. In 4 cases (8%), intracranial CHs were multiple. Intracranial CHs were located in the cranial convexity in 14 cases (26%), in the middle cranial fossa in 15 cases (28%), and in the posterior cranial fossa in 13 cases (25%).
In 6 cases (11%) intracranial CHs were located in the deep of cerebral lobes (4% in frontal lobe, 6% in temporal lobe, 4% in parietal lobe), 2 cases (4%) in cerebellum, 5 (9%) in sellar region, 9 (17%) in cavernous sinus and/or Meckel cave, 1 (2%) adjacent to superior sagittal sinus, and 1 (2%) adjacent to anterior choroidal artery.
The most common presenting symptom was headache (21 cases, 40%), followed by at least one cranial nerve palsy (16 cases, 30%), visual disturbance (10 cases, 19%), nausea or vomiting (9 cases, 17%), seizures (7 cases, 13%), hydrocephalus (7 cases, 13%), limb motor deficit (7 cases, 13%), and decreased level of consciousness (3 cases, 6%).
In 4 cases (8%) extracranial CHs were present. All of them presented skin CHs, and in one case there was a multi-organ dissemination.
Regarding neuroimaging, a brain CT scan was performed in 29 cases (55%), an MRI with gadolinium in 48 cases (91%), and an intracranial angiography in 13 cases (25%).
The surgical treatment consisted of biopsy in 7 cases (13%), partial resection in 10 cases (19%), gross total resection in 31 cases (58.5%), biopsy followed by total resection in 2 cases (3%), and partial resection followed by total resection in 1 case (1.5%), and the diagnosis was obtained from an autopsy sample in 1 case (1.5%), and in 1 case the data was not reported.
Among the 34 cases in which a gross total resection was achieved, 24 cases (71%) did not received any adjuvant treatment, in 2 cases (6%) a preoperative partial TAE of the tumor was performed, in 5 cases (14%) systemic steroid therapy was administered of which only one case (3%) accompanied by IFNα administration, and in 3 cases (9%) the data was not reported. In 27 cases (77%) no recurrence of disease was observed at follow-up (ranging from 2 months to 15 years), while in 8 cases (23%) the data was not reported. After total resection, an improvement of neurological status was observed in 23 cases (66%); in 2 cases (6%) it remained unchanged, and in 10 cases the data was not reported.
Among the 11 cases of partial resection, 6 (55%) underwent radiation therapy, in one case (9%) systemic steroid therapy was administered, one case (9%) went to a surgical procedure of total resection, and in 3 cases (37%) no adjuvant treatment was used. At follow-up, stability of the residual tumor was observed in 4 cases (36%), in 4 cases (36%) there was a reduction in volume of the residual mass, and in 1 case (9%) there was an increased size of residual mass at follow-up. After partial resection, the neurological status improved in 6 cases (55%), while it remained unchanged in 3 cases (27%), and the data was not reported in 1 case.
Among the biopsies, 2 cases (22%) went to a surgical procedure of total resection, in one case (11%), the biopsy sampling was followed by radiotherapy with resolution of the tumor and stability of the neurological status at follow-up, in one case (11%) followed by treatment with propanolol (1 mg/kg twice per day) a reduction in size with stability of the neurological status was found, in another case (11%), the adjunct treatment with Thalidomide 4 mg/kg was associated with the volumetric reduction of the lesion and worsening of the neurological status, while in 2 cases (22%) in which no adjuvant treatment was used, the lesion appeared unchanged at a distance; in 2 cases follow-up data are not reported.
Discussion
CHs are rare benign vascular tumors that usually occur at birth or in early infancy in 1.1– 2.6% of cases with a frequency of 10% within the first year of life [3, 14, 18, 21, 38]. Most commonly they occur in the skin or in the oral mucosa, but they can occur in any organ [1, 38]. These lesions go through distinctive phases: a proliferative phase during the first year of life, a stable period, and a phase of involution, which occurs over months or years even in the absence of therapy; a rule of thumb is that 50% of them completely regress within 5 years and 70% within 7 years, and the rest continue to fade until the age of 12 [14]. No sufficient data on intracranial CHs are available to evaluate the grow rate in the natural history of this pathology. When these lesions do not regress and cause symptoms, they are usually treated with systemic steroid therapy, interferon, laser, cryotherapy, embolization, radiotherapy, and/or surgically [17, 38, 42]. A women prevalence has been observed particularly in reproductive age indicating an hormone-sensitive mechanism [32, 38].
CHs of the central and peripheral nervous system are very rare especially for the intracranic localization. Slightly more common CNS localization are spinal nerve roots and cauda equina [1, 2, 38]. Histologically they are composed of lobules separated by variable degree of fibrous bands. These lobules are cellular due to the plump endothelial cells lining the vascular spaces and poorly defined capillary channels. Differently, cavernous hemangiomas are composed of dilated blood vessels with walls entirely made of collagen with evidence of previous bleeding in the form of organized thrombus and macrophages loaded with hemosiderin and cholesterol, although some may have capillary components [2].
Some authors describe that CH could represent the first stage of cavernous hemangioma, which however does not present any type of spontaneous involutional phase [42]. According to our literature review, the first histologically proven case of intracranial CH was reported by Suss et al. in 1984 and concerned a 13-year-old boy with right temporal headache, right eye visual impairment and optic atrophy for an injury extended to sphenoid bone and sella turcica [40]. In about half of the cases, the diagnosis was made in infant or adolescent age (range 0–20 years) with a prevalence for the male sex, while in adulthood (range 21-80 years) we found a general prevalence to the female sex in accordance with what already reported in the literature [38]. Usually the mechanism of injury is the mass effect on adjacent anatomical structures. So the clinical presentation depends on the site of the lesion and varies from headache, seizure, cranial nerve disturbance to central hypotonia, hemiparesis, and behavioral abnormalities in adults, while in the childhood signs and symptoms are often related to the increase in intracranial pressure (Table 1). John S.G. et al. reported the case of a 59-year-old man who manifested behavioral abnormalities in the form of dissociative disorder mimicking Ganser’s syndrome, decreased levels of orientation, motor, and sensory and visual neglect on the left side due to a large cystic temporo-parietal intracranial CH [20]. Morace et al. reported the case of a 26-year-old woman with a ICH in the sellar region, the clinical picture consisted of endocrine disorders such as galactorrhea, irregular menstrual cycles, and high serum prolactin levels, which remained unchanged after a year despite the reduction of the intracranial CH after partial removal and radiotherapy [30].
To date, there is no clear association between intracranial CHs and genetical syndromes. In particular, phacomatoses like Sturge-Weber syndrome are not associated with the development of intracranial CHs. Beside this, a probable relationship between POEMS syndrome and intracranial CHs is not to be excluded; in fact in support of this hypothesis, Maurer et al. reported the case of a 44-year-old woman with POEMS syndrome, and they observed that all skin lesions and two of the intracranial tumors were CHs while one intracranial hemangioma displayed both capillary and glomeruloid features. However even if skin glomeruloid hemangiomas are considered POEMS-specific, the reason for developing intracranial hemangiomas in this patient remains unclear [27, 41].
In literature, among the cases with a radiological involvement of the Meckel cave, only three are similar to that of our personal experience, in which the intracranial CH was located in the Meckel cave, and the patient was symptomatic for the V cranial nerve [29, 30, 36]. Intracranial CHs involving the trigeminal ganglion are uncommon: tumors of the fifth cranial nerve constitute only 0.2% of all intracranial neoplasms [10]. Furthermore, the commonest tumor for this location, the trigeminal schwannoma, accounts for only 1.5% of all intracranial schwannomas [11]. In this anatomical region, cavernous hemangiomas, although rare, are more frequent than the capillary variant [5, 36]. The involvement of the gasserian ganglion and the branches of the V cranial nerve led to the same clinical presentation in the four cases, characterized by pain in the ipsilateral face side. In the case reported by Brazis et al., this did not produce evidence of trigeminal-distribution sensory loss, but a horizontal diplopia caused by compression of the abducens nerve. It is not rare for masses within Meckel cave to cause subjective trigeminal symptoms such as pain or paresthesia, without significant trigeminal distribution sensory loss [5].
No single imaging modality was diagnostic for capillary hemangioma [28]. In the MRI study of the brain, the intracranial CH appears T1-isointense and T2-hyperintense with enhancement after administration of gadolinium; the detection of the flow-voids signal indicates a high flow vascular nature [1, 19, 38, 42, 44]. Angiography was used in a few cases, where they are characterized by sharp margins and intense persistent staining in a lobular pattern, and supplied by slightly enlarged branches of systemic arteries [21]. In our case, MRI showed the presence of a solid, non-hemorrhagic, non-calcified, fusiform mass with inhomogeneous contrast enhancement, in the context of the left gasser ganglion with perineural extension along ipsilateral V3 branch, through the oval foramen up to the infratemporal fossa, with involvement of the medial and lateral pterygoid muscles and the upright branch of the left jaw, following the trigeminal alveolar course of the upper dental arch. The differential diagnosis initially concerned schwannoma, meningioma, or newly formed inflammatory granulomatous tissue (Wegener’s granulomatosis, sarcoidosis). Generally, these tumors, when they occur in the soft tissue or skin, are managed conservatively. However, there is no evidence to support this strategy for intracranial CHs as they are extremely rare. In the cases reported in the literature, the most used treatment strategy for intracranial lesions, both in adults and in children, was total or partial surgical resection. In addition to the obvious advantage of providing a histological diagnosis, this has shown to have good long-term results both for the control of the pathology and for the improvement of neurological disorders.
We only found one asymptomatic case in literature, but a possible bias is that it is rare to find asymptomatic cases in a surgical court. Because the radiological diagnosis of intracranial CHs is still inconclusive, and the condition is very rare, often asymptomatic cases are treated as asymptomatic meningiomas. In our opinion, considering the benignity of the condition, in asymptomatic cases, a “wait and see” strategy can be adopted.
In our case, a sub-temporal extradural-intradural approach, introduced by Dolenc and Hakuba, was performed to achieve total surgical removal, which allowed for a good exposure of the trigeminal complex [24].
Radiation therapy can be used if the intracranial CH cannot be surgically treated or as an adjuvant treatment in case of partial resection. In the cases reported by Tsao et al. [42] with VI cranial nerve paralysis, the involvement of the cavernous sinus prevented a total resection. At 18-month follow-up, after stereotactic fractional radiotherapy (SFRT), in one case MRI showed the stability of the residual disease, while in the remaining case (in which only a biopsy was performed) 21 months after radiotherapy, there was no residual disease; in both cases, ophthalmological surgical treatment was necessary to compensate for the paralysis of the VI cranial nerve. Morace et al. [30] and Grosu et al. [16] described 4 cases in which partial resection was followed by SFRT with good local control of the mass. In all the 7 cases described in literature, intracranial CHs seemed to be responsive to radiotherapy.
Conclusion
Capillary hemangiomas (CHs) are rare benign vascular tumors that usually occur at birth or in early infancy most commonly affecting the skin and soft tissues. Localization in the central and peripheral nervous system is very rare and even less common is its intracranial presentation since spinal nerve roots and cauda equina are more common locations. For symptomatic lesions, surgery is a valid option to obtain histological characterization, neurological improvement, and where possible a total resection. Stereotactic radiotherapy can be used if the lesion is not surgically approachable or as an adjuvant treatment in the case of partial resection, having shown good results in terms of long-term disease control.
References
Abe M, Tabuchi K, Tanaka S, Hodozuka A, Kunishio K, Kubo N, Nishimura Y (2004) Capillary hemangioma of the central nervous system. J Neurosurg 101:73–81. https://doi.org/10.3171/jns.2004.101.1.0073
Almaghrabi NA, Almaghrabi A, Al-Maghrabi H (2018) A unique case of benign intracranial hemangioma mimicking malignant transformation. Radiol Case Rep 13:1058–1062. https://doi.org/10.1016/j.radcr.2018.04.016
Batsakis JG, Rice DH (1981) The pathology of head and neck tumors: vasoformative tumors, part 9B. Head Neck Surg 3:326–339. https://doi.org/10.1002/hed.2890030408
Benvenisti H, Ben-Sira L, Constantini S, Roth J (2015) Giant cranial and cerebellar hemangioma treated with propranolol. Childs Nerv Syst 31:805–808. https://doi.org/10.1007/s00381-014-2603-4
Brazis PW, Wharen RE, Czervionke LF, Witte RJ, Jones AD (2000) Hemangioma of the mandibular branch of the trigeminal nerve in the Meckel cave presenting with facial pain and sixth nerve palsy. J Neuroophthalmol 20:14–16
Brotchi J, Baleriaux D, Kalangu KKN, Morelli D, Rodesch G, Rorive S, Pirotte B (2005) Capillary hemangioma in the superior sagittal sinus as a rare cause of intracranial hypertension in a child: case report. Neurosurgery 57:E815. https://doi.org/10.1093/neurosurgery/57.4.E815
Dabdoub CB, Chavez M, Ferrufino JL, Claros E, Silveira Edo N, Dabdoub CF (2016) Hemangioma capilar intracraniano assemelhando-se a um meningioma. Arq Neuropsiquiatr 74:356–357
Daenekindt T, Weyns F, Kuan HK, Peuskens D, Engelborghs K, Wuyts J (2008) Giant intracranial capillary hemangioma associated with enlarged head circumference in a newborn: Case report. J Neurosurg Pediatr 1:488–492. https://doi.org/10.3171/PED/2008/1/6/488
Dalsin M, Silva RS, Chaves JPG, Oliveira FH, Antunes ÁCM, Vedolin LM (2016) Intracranial extra-axial hemangioma in a newborn: a case report and literature review. Surg Neurol Int 7:S314–S316
de Benedittis G, Bernasconi V, Ettorre G (1977) Tumours of the fifth cranial nerve. Acta Neurochir 38:37–64. https://doi.org/10.1007/BF01401542
Fehlings MG, Tucker WS (1988) Cavernous hemangioma of Meckel’s cave. J Neurosurg 68:645–647. https://doi.org/10.3171/jns.1988.68.4.0645
Fierek O, Laskawi R, Kunze E (2004) Large intraosseous hemangioma of the temporal bone in a child. Ann Otol Rhinol Laryngol 113:394–398. https://doi.org/10.1177/000348940411300510
Frei-Jones M, McKinstry RC, Perry A, Leonard JR, Tae SP, Rubin JB (2008) Use of thalidomide to diminish growth velocity in a life-threatening congenital intracranial hemangioma: Case report. J Neurosurg Pediatr 2:125–129. https://doi.org/10.3171/PED/2008/2/8/125
Gold R, O’Keefe M, Langer P (2006) Management of capillary hemangiomas. In: Journal of Pediatric Ophthalmology and Strabismus. pp 326–330
Grabb PA (2016) Surgical management of intracranial capillary hemangiomas in children: Report of 2 cases. J Neurosurg Pediatr 17:310–317. https://doi.org/10.3171/2015.7.PEDS14627
Grosu AL, Nieder C (2006) Stereotactic fractionated radiotherapy for recurrent capillary hemangioma of the cavernous sinus. Strahlenther Onkol 182:179–182. https://doi.org/10.1007/s00066-006-1473-4
Haine E, Sevely A, Boetto S, Delisle MB, Cances C (2017) Infantile Hemangioma of the Posterior Fossa in a Newborn: Early Management and Long-Term Follow-up. Neuropediatrics 48:378–381. https://doi.org/10.1055/s-0037-1599235
Jacobs AH, Walton RG (1976) The incidence of birthmarks in the neonate. Pediatrics 58:218–222
Jalloh I, Dean AF, O’Donovan DG, Cross J, Garnett MR, Santarius T (2014) Giant intracranial hemangioma in a neonate. Acta Neurochir 156:1151–1154. https://doi.org/10.1007/s00701-014-2007-y
John SG, Pillai U, Lacasse A (2012) Intracranial capillary hemangioma mimicking a dissociative disorder. Clin Pract 2:35. https://doi.org/10.4081/cp.2012.e35
Karikari IO, Selznick LA, Cummings TJ, George TM (2006) Capillary hemangioma of the fourth ventricle in an infant: Case report and review of the literature. J Neurosurg 104 PEDIAT:188–191. https://doi.org/10.3171/ped.2006.104.3.188
Khanam H, Lipper MH, Wolff CL, Lopes MB (2001) Calvarial hemangiomas: report of two cases and review of the literature. Surg Neurol 55:63–67 discussion 67
Le Bihannic A, Michot C, Heckly A, Loget P, Beucher A, Brassier G, Hamlat A (2005) Capillary haemangioma arising from the anterior choroidal artery. Childs Nerv Syst 21:265–271. https://doi.org/10.1007/s00381-004-1085-1
Lee KS (2001) Extradural approach to the lateral sellar compartment. Yonsei Med J 42:120–127. https://doi.org/10.3349/ymj.2001.42.1.120
Lee YH, Park YS, Kim DS, Park YG, Shim KW (2010) 59-year old female with suprasellar mass: Com july 09 case 2. Brain Pathol 20:257–260. https://doi.org/10.1111/j.1750-3639.2009.00345.x
Low JCM, Maratos E, Kumar A, King A, Al-Sarraj S, Barazi S (2019) Adult Parasellar Capillary Hemangioma with Intrasellar Extension. World Neurosurg 124:184–191. https://doi.org/10.1016/j.wneu.2018.12.185
Maurer GD, Schittenhelm J, Ernemann U, Kempf VAJ, Ritz R, Weller M, Schmidt F (2010) Intracranial hemangiomas in a patient with POEMS syndrome. J Neurol 257:484–487. https://doi.org/10.1007/s00415-009-5398-6
Mirza B, Shi WY, Phadke R, Holton JL, Turner C, Plant GT, Brew S, Kitchen N, Zrinzo L (2013) Strawberries on the brain - Intracranial capillary hemangioma: Two case reports and systematic literature review in children and adults. World Neurosurg 80:900.e13–900.e21
Montibeller GR, Nakamura M, Brandis A, Krauss JK (2008) Capillary hemangioma of the Meckel cave in an adolescent: Case illustration. J Neurosurg Pediatr 1:170. https://doi.org/10.3171/PED/2008/1/2/170
Morace R, Marongiu A, Vangelista T, Galasso V, Colonnese C, Giangaspero F, Innocenzi G, Esposito V, Cantore G (2012) Intracranial capillary hemangioma: A description of four cases. World Neurosurg 78:191.e15–191.e21
Nepute J, Lai J, Zhou Y (2016) Intracranial Capillary Hemangioma in the Posterior Fossa of an Adult Male. Case Rep Radiol 2016:1–4. https://doi.org/10.1155/2016/6434623
Nichols GE, Gaffey MJ, Mills SE, Weiss LM (1992) Lobular capillary hemangioma. An immunohistochemical study including steroid hormone receptor status. Am J Clin Pathol 97:770–775
Okamoto A, Nakagawa I, Matsuda R, Nishimura F, Motoyama Y, Park YS, Nakamura M, Nakase H (2015) Intracranial capillary hemangioma in an elderly patient. Surg Neurol Int 6:S539–S542. https://doi.org/10.4103/2152-7806.168066
Phi JH, Kim SK, Cho A, Kim DG, Paek SH, Park SH, Wang KC (2012) Intracranial capillary hemangioma: extra-axial tumorous lesions closely mimicking meningioma. J Neuro-Oncol 109:177–185. https://doi.org/10.1007/s11060-012-0884-6
Philpott C, Wray A, MacGregor D, Coleman L (2012) Dural infantile hemangioma masquerading as a skull vault lesion. Am J Neuroradiol 33:E85–E87. https://doi.org/10.3174/ajnr.A2752
Saliba I, El Fata F, Berthelet F, Moumdjian R (2009) Trigeminal nerve haemangioma eroding the petrous carotid canal. J Laryngol Otol 123:1258–1261. https://doi.org/10.1017/S0022215109004654
Shakir HJ, McBride P, Reynolds RM (2016) Dural-based infantile hemangioma of the posterior fossa: case report. Surg Neurol Int 7. https://doi.org/10.4103/2152-7806.181827
Simon SL, Moonis G, Judkins AR, Scobie J, Burnett MG, Riina HA, Judy KD (2005) Intracranial capillary hemangioma: case report and review of the literature. Surg Neurol 64:154–159. https://doi.org/10.1016/j.surneu.2004.10.025
Smith IF, Skelton V (2007) An unusual intracranial tumour presenting in pregnancy. Int J Obstet Anesth 16:82–85. https://doi.org/10.1016/j.ijoa.2006.04.016
Suss RA, Kumar AJ, Dorfman HD, Miller NR, Rosenbaum AE (1984) Capillary hemangioma of the sphenoid bone. Skelet Radiol 11:102–107
Tsai CY, Lai CH, Chan HL, Kuo T (2001) Glomeruloid hemangioma--a specific cutaneous marker of POEMS syndrome. Int J Dermatol 40:403–406
Tsao MN, Schwartz ML, Bernstein M, Halliday WC, Lightstone AW, Hamilton MG, Jaywant S, Laperriere N (2003) Capillary hemangioma of the cavernous sinus: Report of two cases. J Neurosurg 98:169–174. https://doi.org/10.3171/jns.2003.98.1.0169
Uyama A, Kawamura A, Akiyama H, Nakamizo S, Yamamoto K, Nagashima T, Uetani T, Takeda H, Yoshida M (2008) A case of cerebellar capillary hemangioma with multiple cysts. Pediatr Neurosurg 44:344–349. https://doi.org/10.1159/000138375
Willing SJ, Faye-Petersen O, Aronin P, Faith S (1993) Radiologic-pathologic correlation. Capillary hemangioma of the meninges. Am J Neuroradiol 14:529–536
Xia X, Zhang H, Gao H, Yang Y, Dai Y, Jiao Y, He J (2017) Nearly asymptomatic intracranial capillary hemangiomas: A case report and literature review. Exp Ther Med 14:2007–2014
Yang G, Li C, Chen X, Liu Y, Han D, Gao X, Kawamoto K, Zhao S (2014) Large capillary hemangioma of the temporal bone with a dural tail sign: A case report. Oncol Lett 8:183–186. https://doi.org/10.3892/ol.2014.2143
Youn I, Kim JK, Byun JS, Park ES (2012) Intracerebral Capillary Hemangioma: A Case Report. J Korean Soc Radiol 66:1. https://doi.org/10.3348/jksr.2012.66.1.1
Younas F, Durrani Q, Shahzad MA, Mushtaq A, Imlay S (2011) Multiple intracranial capillary hemangiomas and transient cerebrovascular insufficiency. Neurol Sci 32:963–966. https://doi.org/10.1007/s10072-011-0569-5
Zheng SP, Ju Y, You C (2012) Giant intracranial capillary hemangioma in a 3-year-old child: Case report and literature review. Clin Neurol Neurosurg 114:1270–1273. https://doi.org/10.1016/j.clineuro.2012.02.052
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
No need of ethical committee approval
Informed consent
Patient is informed and agrees for publication
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Santoro, G., Piccirilli, M., Chiarella, V. et al. Intracranial capillary hemangiomas: literature review in pediatric and adult population. Neurosurg Rev 44, 1977–1985 (2021). https://doi.org/10.1007/s10143-020-01419-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-020-01419-8