Abstract
As the incidental diagnosis of unruptured intracranial aneurysms has been increasing, several scores were developed to predict risk of rupture and growth to guide the management choice. We retrospectively applied these scores to a multicenter series of patients with subarachnoid hemorrhage to test whether they would have predicted the risk of bleeding in the event of aneurysm discovery previous to its rupture. Demographical, clinical, and radiological information of 245 adults were retrieved from two neurovascular centers’ database. Data were pooled and PHASES, UCAS, and ELAPSS scores were retrospectively calculated for the whole population and their performances in identifying aneurysms at risk of rupture were compared. Mean PHASES, UCAS, and ELAPSS scores were 5.12 ± 3.08, 5.09 ± 2.62, and 15.88 ± 8.07, respectively. Around half (46%) of patients would have been assigned to the low- or very low-risk class (5-year rupture risk < 1%) in PHASES. Around 28% of patients would have been in a low-risk class, with a probability of 3-year rupture risk < 1% according to UCAS. Finally, ELAPSS score application showed a wider distribution among the risk classes, but a significant proportion of patients (45.5%) lie in the low- or intermediate-risk class for aneurysm growth. A high percentage of patients with ruptured aneurysms in this multicenter cohort would have been assigned to the lower risk categories for aneurysm growth and rupture with all the tested scores if they had been discovered before the rupture. Based on these observations, physicians should be careful about drawing therapeutic conclusions solely based on application of these scores.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The incidence of unruptured intracranial aneurysms (uIAs) has progressively increased over the past decades, but this observation might be only due to an increasing availability of diagnostic imaging.
At the same time, neurosurgeons around the world daily cope with two apparent paradoxes regarding IAs: firstly, the prevalence of uIAs appears higher than the incidence of subarachnoid hemorrhage (SAH) [10]; secondly, most of ruptured intracranial aneurysms (rIAs) are smaller than 5 mm [5], although the ISAT trial set a risk of rupture cutoff at 7 mm [13].
Possible explanations can be that rIAs and uIAs have different characteristics; small IAs are probably much more numerous than large IAs in general; not all IAs tend to grow over time; some IAs present weak points in their wall; etc. However, none of these theories provided strong enough criteria to decide whether to treat a patient or not.
Two main scores predicting the risk of rupture of IAs have been designed: PHASES, which is based on data collected from European, American, Canadian, and Japanese databases, identifying predictors for aneurysms rupture and providing a 5-year risk-prediction chart [9]; and UCAS, which was developed on the Japanese Unruptured Cerebral Aneurysm Study data and externally validated on 3 prospective Japanese cohorts (Supplementary Tables 1, 2) [16].
A third score, named ELAPSS, was designed based on data from 10 cohorts in Europe, Canada, China, and Japan with the aim of predicting the risk of aneurysm growth at 3 and 5 years, that is, in turn, considered a major risk for rupture (Supplementary Table 3) [1].
All these scores consider the possible role of demographics, clinical risk factors, and morphological characteristics of uIA, but assign them different weights.
In this study, we retrospectively applied these 3 scores to a multicenter series of SAH patients with rIA to test whether they would have predicted the risk of bleeding in the event of aneurysm discovery previous to its rupture.
Material and methods
Population
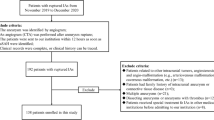
A retrospective consecutive multi-institutional cohort of SAH patients admitted from 2015 to 2019 to two Italian tertiary referral neurosurgical centers was retrieved from data-warehouse by using the appropriate ICD and analyzed according to the STROBE criteria [17]. We limited the timespan of the research to 5 years to assure the complete availability of all necessary clinical and radiological data. Inclusion criteria were as follows: age > 18 years old and complete clinical-radiological documentation. Demographic, clinical, and radiological data, were searched, anonymized, and entered into an operative database.
Clinical data collection
Clinical and radiological data were retrieved from medical records and summarized in:
(a) Demographical: age, sex, ethnicity;
(b) Risk factors: hypertension, previous SAH;
(c) Neuroimaging: aneurysm topography, size, morphology.
Neuroimaging
CT angiographies, MR angiographies, and DSAs archived in the institutional PACS systems were analyzed by two neuro-radiologists, blinded to patients’ clinical data. Aneurysm size, morphology, topography, and multiplicity were analyzed. Blebs, lobulations, and daughter sacs were used as criteria to define aneurysm irregularity.
Score calculation
UCAS, PHASES, and ELAPSS scores (Supplementary Tables 1, 2, 3) were individually calculated for each patient. Scores’ parameters were sub-categorized into demographics, radiological, and clinical data.
Score comparison
The relative contribution (%) of demographic, clinical, and angio-architectural variables was calculated for each score and compared with one another.
The UCAS and PHASES high-risk of rupture (> 1%) categories were also compared with each other to test the scores’ performance in recognizing aneurysms that eventually ruptured.
Statistical analysis
Data were individually extracted for each patient and pooled. Percentage of incidence was calculated for all the score variables. Quantitative data were expressed as mean ± standard deviation. Two-sided Fisher’s exact test was used to compare categorical variables and a p < 0.05 was considered significant.
Ethical statement
IRB review was not necessary as we only collected anonymous data archived in institutional databases. All included patients gave their informed consent to the use of their clinical data for scientific purposes at the time of treatment.
Results
Characteristics of the study population
Data from 245 SAH patients were retrieved and included in the study. Patients’ characteristics are reported in Table 1.
In this cohort, 180 patients (73.5%) were females, and 65 (26.5%) males. Mean age was 59.42 ± 13.87. Overall, 183 patients (74.7%) were younger than 70 years, and 63 (25.2%) were older. Among them, 119 (48.6%) suffered from hypertension, and 4 (1.6%) had family history of SAH.
In general, regarding aneurysm size, 193 (78.8%) were small (< 10 mm), 48 (19.6%) were large (10–25 mm), and 4 (1.6%) giant (> 25 mm), with a mean size of 6.91 ± 4.17 mm. We further stratified aneurysm size according to the cutoffs of PHASES (58% < 7 mm; 20.8% between 7 and 9.9 mm; 19.2% between 10 and 19.9 mm; 2% ≥ 20 mm), UCAS (58% between 3 and 7 mm; 20.8% between 7 and 10 mm; 19.2% between 11 and 20 mm; 2% > 20 mm), and ELAPSS (7% between 1 and 2.9 mm; 26.5% between 3 and 4.9 mm; 24.5% between 5 and 6.9 mm; 20.8% between 7 and 9.9 mm; 21.2% ≥ 10 mm).
About one-fourth of patients (64; 26.1%) had multiple aneurysms.
Concerning topography, 82 aneurysms (33.5%) originated from the anterior communicating artery (ACom); 43 (17.6%) from the internal carotid artery (ICA); 29 (11.8%) at the posterior communicating artery origin (PCom); 55 (22.4%) from the middle cerebral artery (MCA); 13 (5.3%) from the anterior cerebral artery (ACA); 12 (4.9%) from the basilar artery (BA); 9 (3.7%) from the posterior inferior cerebellar artery (PICA); and 2 (0.8%) from the vertebral artery (VA). Overall, 222 (90.6%) rIAs originated from the anterior circulation, and 23 (9.4%) from the posterior circulation.
More than half of these rIAs (137; 55.9%) showed the presence of wall irregularity such as blebs, daughter sacs, or polylobulations.
Score application
Patient distribution
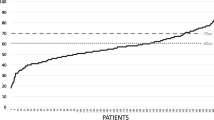
We retrospectively applied PHASES, UCAS, and ELAPSS scores and calculated the number of patients assigned to the different risk classes as reported in Table 2. Patient distribution is illustrated in Fig. 1a, b, and c.
Mean PHASES score for the entire population was 5.12 ± 3.08. Patient distribution according to risk classes showed that 46.1% patients were assigned to very low- and low-risk classes, a similar proportion (43.3%) to the intermediate-risk class, and only a minority (about 10.6%) to the high-risk one.
Mean UCAS score was 5.09 ± 2.62. Most of the patients were assigned to the first two risk classes, and in particular, 68 (27.8%) to the first (0–3 points), and 88 (35.9%) to the second (4–5 points); instead, 59 patients (24.1%) were assigned to the third class (from 6 to 8 points), and 30 (12.2%) to the fourth (higher than 9 points).
Finally, mean ELAPSS score was 15.88 ± 8.07. Its retrospective application showed a wide patient distribution in the different risk classes, with a higher number in the middle ones. In particular, 21 patients (8.6%) would have obtained less than 5 points, 36 (14.7%) a score comprised between 5 and 9, 55 (22.4%) between 10 and 14, 58 (23.7%) between 15 and 19 points, 28 (11.4%) from 20 to 24, and 47 (19.2%) more than 25.
Aneurysm rupture and growth prediction
The retrospective application of PHASES to our multicenter cohort of rIA showed that almost half (46.1%) of patients would have been assigned to low or very low rupture risk classes (5-year risk < 1%). A similar proportion (43.3%) would have been classified as having an intermediate risk. Likewise, a similar proportion of patients (63.6%) would have been considered having low to intermediate rupture risk (5-year risk ≤ 3%) with UCAS. (Table 3).
The application of ELAPSS showed that about 35% would have had a 5-year high growth risk (from 28.1 to 39.9%), about 23% a 5-year low risk (≤ 13%), about 22% an intermediate risk (~ 19%), and less than 3% a very elevated risk (> 60%) (Table 4).
Score comparison
A synoptic comparison of the contribution of each variable to the 3 scores is reported in Table 5 and Fig. 1d. We distinguished demographics, aneurysmal angio-architectural features, and clinical risk factors.
Aneurysm size represents the most influential variable in the 3 scores, ranging from 45.5% in PHASES to more than 53% in UCAS.
Aneurysm topography is the second most weighting variable among the scores, ranging from ≈ 11% for ELAPSS to 20% for UCAS.
Concerning demographics, ethnicity is considered important in PHASES and ELAPSS (22.7% and 15.6%, respectively), but not in UCAS that was specifically designed for Japanese patients. Similarly, UCAS includes gender, which is instead unobserved in the other scores. All three scores consider age as a risk factor, but its contribution to ELAPSS is twice greater when compared with the other two scores. Finally, the presence of hypertension contributes in small part to PHASES and UCAS, so does a history of previous SAH to PHASES and ELAPSS.
The patient distribution in the different risk classes of the three scores according to their demographic and clinical characteristics as well as their aneurysmal angio-architectural features is reported in Supplementary Table 4.
The highest number of patients appeared concentrated in the intermediate-risk categories of all the three scores, and this was observed in both older and younger patients.
With regard to aneurysm size and morphology, scores’ application showed that the larger the sac, the higher the risk of bleeding/growth category and that the occurrence of irregularity was directly related to a higher risk of rupture/growth.
Concerning topography, instead, most ruptured aneurysms in our series originated from the ACom (82/245; 33.4%), followed by those originating from the ICA (72/245 included PCom; 29.3%), MCA (55/245; 22.44%), and ACA (13/245; 5.3%); a minority originated from the posterior circulation (overall 23/245; 9.3%).
ICA aneurysms would have been assigned to the lowest risk class in 42% of cases according to PHASES, and in > 60% according to UCAS; these aneurysms would be instead almost equally divided among the four risk groups according to ELAPSS growth risk prediction.
MCA aneurysms would have been mostly assigned to the two intermediate-risk classes according to PHASES and UCAS, whereas ELAPSS score would have assigned to most of them a high/very high growth risk.
Finally, patients with familial history of SAH would have been mainly distributed in the lower and intermediate-risk category according to all scores, while those harboring multiple aneurysms would have been homogenously divided among the four risk groups.
We also compared with each other the UCAS and PHASES intermediate + high-risk of rupture categories (> 1% per year; Table 3) to test their performance in recognizing aneurysms that eventually bled. In fact, these two groups are those on which clinicians mainly agree toward intervention rather than observation. Therefore, according to their designs, we compared the PHASES intermediate- and high-risk classes with the UCAS classes II, III, and IV.
The two groups were not different for sex, age, aneurysm morphology, and topography as well as for other familial risk factors, but UCAS high-risk classes performed significantly better in identifying a larger number of at risk of rupture aneurysms (Table 6). ELAPSS was not included in this comparison as it is aimed at estimating the risk of aneurysm growth rather than rupture.
Discussion
In this study, the retrospective application of the currently available risk scores to a multicenter cohort of rIAs showed that patients appeared mainly distributed into the two lowest risk classes according to PHASES and UCAS, and fairly equally distributed among the four risk classes according to ELAPSS.
Therefore, at first glance, these results seem to suggest a limited use of these scores in guiding the decision-making process regarding uIA treatment. Accordingly, physicians should take into account that, despite these score systems constitute additional tools to the clinical experience, they may fail in exhaustively predicting rupture in a subpopulation of aneurysms at high risk. Accordingly, management decisions should always include a comprehensive evaluation of the individual patients, also including their preference, and the specialist’s and institutional experience.
In fact, if on one hand surgical and endovascular treatments are nowadays considered safe and effective procedures, on the other the decision to suggest only observation still represents a viable strategy in some circumstances.
When managing incidental uIAs, practitioners and patients have to deal with two opposite issues: one is related to the risks of treatment, the other is connected to the natural history. In particular, physicians are asked to answer the question “how high are these risks?”, while patients need to answer the question “are these risks acceptable?”. This last query may have diverse responses for different patients, especially because, to date, some uncertainties remain regarding the natural history of incidental uIAs, as we do not yet possess longitudinal patient cohorts of strictly untreated uIAs.
For this reason, several attempts have been made to design risk scores in order to assist doctors in clinical practice. Among them, the purpose of PHASES [9] and UCAS [16] is to estimate the rupture risk of IAs, whereas ELAPSS [1] aims to predict the growth risk, which is in turn associated with increased risk of rupture (Supplementary Tables 1, 2, 3).
Interestingly, in our study, when compared with PHASES, UCAS recognized 45 more aneurysms (+ 34.1%) as having a rupture risk > 1%, which would have been likely addressed toward interventional management had they been discovered before their rupture (p < 0.001).
Hence, based on our data, UCAS outperformed PHASES in identifying a larger number of patients as having a higher risk of rupture in our series. Of note, Tominari et al. had already warned in their original work about UCAS’ possible overperformance in case of future validation studies on non-Japanese patients. Specifically, calibration and discrimination of the model were calculated on a Japanese population, who is known to possess a 3-fold increased risk of aneurysm rupture compared with Caucasians other than Finnish [16].
Although PHASES considers similar variables, being it developed on multicenter international cohorts, it assigns them diverse weights which differently affect the final score result and the relative risk of rupture prediction. In addition, the timing of the prediction is different as UCAS provides a 3-year risk prediction, while PHASES a 5-year one.
Moreover, when compared with PHASES, UCAS lacks prior SAH as a risk factor. However, according to Tominari et al., this could not reasonably affect risk prediction on Japanese patients as most of the aneurysms are incidentally detected by means of screening and as a result almost all of the patients did not have history of SAH [16]. Conversely, PHASES does not include female sex and daughter sac as risk factors, despite being these well-associated to IA rupture.
On the other hand, PHASES was developed using data from cohorts also including Japanese patients. Accordingly, considering the increased risk in this population, ethnicity was included in the variables to be considered [9].
Tominari et al. suggested that if PHASES was to be applied to a homogeneous Japanese cohort, it could have produced lower estimates when predicting rupture risks for aneurysms associated with daughter sacs, as these are reported in approximately 20% of cases in Japanese cohorts. On the contrary, PHASES and ELAPSS may fail in recognizing female patients as having a higher rupture risk, as they do not consider sex a risk factor. In fact, in their original work, they tested PHASES on the UCAS II study cohort, which has not been used to develop the PHASES score, confirming its underperformance compared with the UCAS-based score [16].
To the best of our knowledge, UCAS was instead never externally validated in a non-Japanese population. Thus, our study is the first to retrospectively apply the UCAS score to a non-Japanese SAH cohort, confirming its relative overperformance compared with PHASES, but still with low sensitivity in recognizing as at high risk a large number of patients with rIA. This difference in the predictive performance between uIA and rIA seems to suggest the existence of different populations of IAs according to the length of their natural history from their formation to the bleeding.
Interestingly, we recently observed in our institution that the age of SAH patients was higher than in the past decades with a mean of about 59 years, as reported in this study. We suppose that this may result from the increased number of patients discovering incidental uIAs in the last decades due to the more extensive use of brain MRI for minor neurological problems. Among them, younger patients are usually more prone to treatment, while older ones often prefer a conservative approach.
We chose not to test the UIATS score [6] in this study. In fact, differently from the other 3 risk scores, UIATS was designed through a Delphi consensus among a panel of specialists not to predict the risk of aneurysms rupture or growth, but to assist the decision-making process in controversial cases that may or may not require treatment.
UIATS also takes into account patients’ comorbidities and technical risk factors associated with the difficulty of treatment. Differently from the other scores, it provides a straightforward recommendation on treat vs do-not-treat vs no-clear-indication, which is not directly comparable with the result of a score predicting the rupture/growth risk and leaving the decision to the physician in a second step.
Moreover, UIATS includes variables such as reduced quality of life due to fear of rupture and aneurysm de novo formation or growth on serial imaging, which cannot be retrospectively collected, introducing an additional bias to the scores’ comparison reliability.
Previous scores’ validation studies
Several validation studies of PHASES and ELAPSS have been published so far.
Biljienga et al. in 2017 analyzed 841 patients with IAs admitted to their institution to investigate whether PHASES matched decisions taken by their multidisciplinary team at the time of diagnosis. Patients were divided into 4 groups: stable uIA, growing uIA, immediately treated uIA, and rIA. The authors observed a reliable risk progression along these groups, with higher odds of SAH when PHASES score was > 3. Instead, a score ≤ 3 appeared associated with a lower likelihood of bleeding [3].
Foreman et al. in 2018 tested PHASES score in a prospective double-center US cohort of 149 patients with rIA finding that up to 61.7% presented a score ≤ 5. In particular, more than 52% of them had a score of 4 or 5 points with a 5-year bleeding risk of 0.9% and 1.3%, respectively [7]. These risk classes, if applied to uIAs, would have suggested a conservative treatment, highlighting that even a low risk cannot be neglected.
Analogous results were found by Brinjikji et al. in 2018, who tested PHASES and ELAPSS on 352 patients harboring 431 uIA. They confirmed that ELAPSS appeared reliable when applied to their cohort, and that, as reported by Backes et al. [2], higher PHASES scores were also able to predict the growth risk [2, 4]. At the multivariate analysis, they showed that aneurysm size was the sole independent predictor of aneurysm growth among the variables included in the scores. Extending the analysis to generic risk factors, instead, only smoking status correlated with increased growth risk, which is not contemplated in the two scores [4].
Similarly, in 2018, Hilditch et al. retrospectively tested PHASES and ELAPSS on 700 rIAs. They found that most patients had a PHASES score > 4, and only 17% had a score ≤ 3. On the other hand, more than 35% of patients were assigned to a 5-year low risk according to ELAPSS (< 13%), and 23% to an intermediate-risk category (< 19%). Therefore, a significant proportion of patients who eventually presented SAH would have received a suggestion toward a conservative management if the aneurysms had been discovered before rupture [10].
Another ELAPSS multicenter external validation study was conducted by Van Kammen et al. in 2019 on 1072 patients with 1452 uIA. They showed that ELAPSS possesses good calibration and modest discrimination at 3 and 5 years for aneurysm growth prediction. A subtle underestimation of growth risk was instead observed at 3 and 5 years especially for low-risk categories, and overestimation at 5 years for the highest risk category [12].
Finally, Pagiola et al. in 2019 tested PHASES score on 155 patients with rIAs finding that roughly 70% of them would have received a score < 5 with a 5-year rupture risk of 1.3%, and up to 79% of them would have been scored as having a 5-year risk below 2% [14].
No additional validation studies regarding the score designed on the UCAS cohort were instead published [16].
Limitations
We recognize several limitations to our study.
First of all, we collected a multicenter cohort of rIAs and retrospectively applied 3 score systems (PHASES, UCAS, and ELAPSS) that were primarily designed for a prospective application to uIA.
Secondly, when evaluating aneurysm size, we could not exclude that this may decrease after rupture.
Thirdly, we could not apply a specific protocol regarding the imaging used in radiological assessment and its timing: in fact, aneurysm morphology was evaluated with various imaging modalities, including CT angiography, DSA, and MR angiography, introducing possible, although minimal, errors in the measurements. In addition, we do not have a control group to compare our study population with.
Finally, we have to recognize caution in extending clinical application of the UCAS-derived score to the non-Japanese cohort of patients in our study.
Future perspectives
In the next future, more refined scores including other proven risk factors such as smoking [8], genetic factors such as single nucleotide polymorphism profiles or specific genes’ expression [15], and sophisticated techniques of neuroimaging able to visualize at high resolution the vessel wall [11] will result in an improved predictive performance.
Conclusions
This study showed that a high percentage of ruptured aneurysms of our multicenter cohort would have been assigned to the lowest risk categories by all of the evaluated scores. Moreover, risk factors for aneurysm rupture did not show their particular concentration in the high-risk groups according to the three scores. Therefore, despite its methodological limitations, this study underlines the critical aspects of applying risk scores to multifactorial pathologies such as cerebral aneurysms, whose rupture leads to high risk of mortality and a very high risk of morbidity.
References
Backes D, Rinkel GJE, Greving JP, Velthuis BK, Murayama Y, Takao H, Ishibashi T, Igase M, terBrugge KG, Agid R, Jääskeläinen JE, Lindgren AE, Koivisto T, von und zu Fraunberg M, Matsubara S, Moroi J, Wong GKC, Abrigo JM, Igase K, Matsumoto K, Wermer MJH, van Walderveen MAA, Algra A, Vergouwen MDI (2017) ELAPSS score for prediction of risk of growth of unruptured intracranial aneurysms. Neurology 88:1600–1606. https://doi.org/10.1212/WNL.0000000000003865
Backes D, Vergouwen MDI, Tiel Groenestege AT, Bor ASE, Velthuis BK, Greving JP, Algra A, Wermer MJH, van Walderveen MAA, terBrugge KG, Agid R, Rinkel GJE (2015) PHASES score for prediction of intracranial aneurysm growth. Stroke 46:1221–1226. https://doi.org/10.1161/STROKEAHA.114.008198
Bijlenga P, Gondar R, Schilling S, Morel S, Hirsch S, Cuony J, Corniola M-V, Perren F, Rüfenacht D, Schaller K (2017) PHASES score for the management of intracranial aneurysm: a cross-sectional population-based retrospective study. Stroke 48:2105–2112. https://doi.org/10.1161/STROKEAHA.117.017391
Brinjikji W, Pereira VM, Khumtong R, Kostensky A, Tymianski M, Krings T, Radovanovich I (2018) PHASES and ELAPSS scores are associated with aneurysm growth: a study of 431 unruptured intracranial aneurysms. ssWorld Neurosurg 114:e425–e432. https://doi.org/10.1016/j.wneu.2018.03.003
Dolati P, Pittman D, Morrish WF, Wong J, Sutherland GR (2015) The frequency of subarachnoid hemorrhage from very small cerebral aneurysms (< 5 mm): a population-based study. Cureus. https://doi.org/10.7759/cureus.279
Etminan N, Brown RD, Beseoglu K, Juvela S, Raymond J, Morita A, Torner JC, Derdeyn CP, Raabe A, Mocco J, Korja M, Abdulazim A, Amin-Hanjani S, Al-Shahi Salman R, Barrow DL, Bederson J, Bonafe A, Dumont AS, Fiorella DJ, Gruber A, Hankey GJ, Hasan DM, Hoh BL, Jabbour P, Kasuya H, Kelly ME, Kirkpatrick PJ, Knuckey N, Koivisto T, Krings T, Lawton MT, Marotta TR, Mayer SA, Mee E, Pereira VM, Molyneux A, Morgan MK, Mori K, Murayama Y, Nagahiro S, Nakayama N, Niemelä M, Ogilvy CS, Pierot L, Rabinstein AA, Roos YBWEM, Rinne J, Rosenwasser RH, Ronkainen A, Schaller K, Seifert V, Solomon RA, Spears J, Steiger H-J, Vergouwen MDI, Wanke I, Wermer MJH, Wong GKC, Wong JH, Zipfel GJ, Connolly ES, Steinmetz H, Lanzino G, Pasqualin A, Rüfenacht D, Vajkoczy P, McDougall C, Hänggi D, LeRoux P, Rinkel GJE, Macdonald RL (2015) The unruptured intracranial aneurysm treatment score: a multidisciplinary consensus. Neurology 85:881–889. https://doi.org/10.1212/WNL.0000000000001891
Foreman PM, Hendrix P, Harrigan MR, Fisher WS, Vyas NA, Lipsky RH, Walters BC, Tubbs RS, Shoja MM, Griessenauer CJ (2018) PHASES score applied to a prospective cohort of aneurysmal subarachnoid hemorrhage patients. J Clin Neurosci 53:69–73. https://doi.org/10.1016/j.jocn.2018.04.014
Giordan E, Lanzino G, Rangel-Castilla L, Murad MH, Brinjikji W (2019) Risk of de novo aneurysm formation in patients with a prior diagnosis of ruptured or unruptured aneurysm: systematic review and meta-analysis. J Neurosurg 131:14–24. https://doi.org/10.3171/2018.1.JNS172450
Greving JP, Wermer MJH, Brown RD, Morita A, Juvela S, Yonekura M, Ishibashi T, Torner JC, Nakayama T, Rinkel GJE, Algra A (2014) Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol 13:59–66. https://doi.org/10.1016/S1474-4422(13)70263-1
Hilditch CA, Brinjikji W, Tsang AC, Nicholson P, Kostynskyy A, Tymianski M, Krings T, Radovanovic I, Pereira VM (2018) Application of PHASES and ELAPSS scores to ruptured cerebral aneurysms: how many would have been conservatively managed? J Neurosurg Sci. https://doi.org/10.23736/S0390-5616.18.04498-3
Hsu CC-T, Suthiphosuwan S, Huynh T, Murphy A, Li Y, Bharatha A (2019) High-resolution MRI vessel wall imaging in acute aneurysmal subarachnoid hemorrhage: spatiotemporal pattern and clinicoradiologic implications. Clin Neuroradiol. https://doi.org/10.1007/s00062-019-00843-8
van Kammen MS, Greving JP, Kuroda S, Kashiwazaki D, Morita A, Shiokawa Y, Kimura T, Cognard C, Januel AC, Lindgren A, Koivisto T, Jääskeläinen JE, Ronkainen A, Pyysalo L, Öhman J, Rahi M, Kuhmonen J, Rinne J, Leemans EL, Majoie CB, Vandertop WP, Verbaan D, Roos YBWEM, van den Berg R, Boogaarts HD, Moudrous W, van den Wijngaard IR, ten Hove L, Teo M, George EJS, Hackenberg KAM, Abdulazim A, Etminan N, Rinkel GJE, Vergouwen MDI (2019) External validation of the ELAPSS score for prediction of unruptured intracranial aneurysm growth risk. J Stroke 21:340–346. https://doi.org/10.5853/jos.2019.01277
Molyneux AJ, Kerr RSC, Yu L-M, Clarke M, Sneade M, Yarnold JA, Sandercock P (2005) International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. 366:9
Pagiola I, Mihalea C, Caroff J, Ikka L, Chalumeau V, Iacobucci M, Ozanne A, Gallas S, Marques M, Nalli D, Carrete H, Caldas JG, Frudit ME, Moret J, Spelle L (2019) The PHASES score: to treat or not to treat? Retrospective evaluation of the risk of rupture of intracranial aneurysms in patients with aneurysmal subarachnoid hemorrhage. J Neuroradiol:S0150986119302603. https://doi.org/10.1016/j.neurad.2019.06.003
Samuel N, Radovanovic I (2019) Genetic basis of intracranial aneurysm formation and rupture: clinical implications in the postgenomic era. Neurosurg Focus 47:E10. https://doi.org/10.3171/2019.4.FOCUS19204
Tominari S, Morita A, Ishibashi T, Yamazaki T, Takao H, Murayama Y, Sonobe M, Yonekura M, Saito N, Shiokawa Y, Date I, Tominaga T, Nozaki K, Houkin K, Miyamoto S, Kirino T, Hashi K, Nakayama T, for the Unruptured Cerebral Aneurysm Study Japan Investigators (2015) Prediction model for 3-year rupture risk of unruptured cerebral aneurysms in Japanese patients: cerebral aneurysm rupture risk. Ann Neurol 77:1050–1059. https://doi.org/10.1002/ana.24400
Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, Initiative STROBE (2007) Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 335:806–808. https://doi.org/10.1136/bmj.39335.541782.AD
Acknowledgments
We would like to thank all colleagues of the neurovascular teams of Fondazione Policlinico Universitario Agostino Gemelli IRCCS of Rome (Enrico Marchese, MD, PhD; Alessandro Olivi, MD; Alessandro Pedicelli, MD; Alfredo Puca, MD) and Santo Spirito Hospital of Pescara (Annunziato Mangiola, MD) who contributed to treating these patients with ruptured intracranial aneurysms.
Author information
Authors and Affiliations
Contributions
Conception and design: CLS, VS, LR, AA. Data collection: CLS, VS, LR, GT, IV, KL, SD, PB. Data analysis: CLS, VS, SD, GT, IV, AA. Helping with data analysis: PB, LR, KL. Drafting: CLS, LR, VS, AA. Helping with drafting: SD, GT, PB, IV, KL. Approval of final version: CLS, VS, FT, GT, IV, SD, KL, PB, AA
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethical approval was not needed for analysis of retrospective anonymous data.
Informed consent
All included patients gave their informed consent to the treatment of their personal data for scientific purposes at the time of treatment.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Table 1
PHASES risk score (DOCX 14 kb)
Supplementary Table 2
UCAS-based risk score (DOCX 14 kb)
Supplementary Table 3
ELAPSS score for aneurysm growth (DOCX 15 kb)
Supplementary Table 4
Percentages of distribution of the patients’ characteristics (tot. 245) in the risk (DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Sturiale, C.L., Stumpo, V., Ricciardi, L. et al. Retrospective application of risk scores to ruptured intracranial aneurysms: would they have predicted the risk of bleeding?. Neurosurg Rev 44, 1655–1663 (2021). https://doi.org/10.1007/s10143-020-01352-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-020-01352-w