Abstract
Optimal management of intracranial pressure (ICP) among aneurysmal subarachnoid hemorrhage (aSAH) patients requiring external ventricular drainage (EVD) is controversial. To analyze predictors of delayed cerebral ischemia (DCI)-related cerebral infarction after aSAH and the influence of ICP values on DCI, we prospectively collected consecutive patients with aSAH receiving coiling and requiring EVD. Predictors of DCI-related cerebral infarction (new CT hypodensities developed within the first 3 weeks not related to other causes) were studied. Vasospasm and brain hypoperfusion were studied with CT angiography and CT perfusion (RAPID-software). Among 50 aSAH patients requiring EVD, 21 (42%) developed DCI-related cerebral infarction, while 27 (54%) presented vasospasm. Mean ICP ranged between 2 and 19 mmHg. On the multivariate analysis, the mean ICP (OR = 2, 95%CI = 1.01–3.9, p = 0.042) and the mean hypoperfusion volume on Tmax delay > 6 (OR = 1.2, 95%CI = 1.01–1.3, p = 0.025) were independent predictors of DCI. To predict DCI-related cerebral infarction, Tmax delay > 6 s presented the highest AUC (0.956, SE = 0.025), with a cutoff value of 18 ml showing sensitivity, specificity, PPV, NPV, and accuracy of 90.5% (95%CI = 69–98.8%), 86.2% (95%CI = 68.4–96%), 82.6% (95%CI = 65.4–92%), 92.5% (95%CI = 77–98%), and 88% (95%CI = 75–95%), respectively. The AUC of the mean ICP was 0.825 (SE = 0.057), and the best cutoff value was 6.7 mmHg providing sensitivity, specificity, PPV, NPV, and accuracy of 71.4% (95%CI = 48–89%), 62% (95%CI = 42–79%), 58% (95%CI = 44–70%), 75% (95%CI = 59–86%), and 66% (95%CI = 51–79%) for the prediction of DCI-related cerebral infarction, respectively. Among aSAH patients receiving coiling and EVD, lower ICP (< 6.7 mmHg in our study) could potentially be beneficial in decreasing DCI-related cerebral infarction. Brain hypoperfusion with a volume > 18 ml at Tmax delay > 6 s presents a high sensibility and specificity in prediction of DCI-related cerebral infarction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aneurysmal subarachnoid hemorrhage (aSAH) is one of the most complex acute conditions a physician may encounter. After the first bleeding, clinical outcomes are largely affected by the occurrence of a secondary injury related to neurological and systemic complications [18], and functional impairment is still common among survivors [19]. Elevated intracranial pressure (ICP) is a frequent consequence of aSAH strongly associated with a patient’s clinical outcomes [14]. Although external ventricular drainage (EVD) is the gold standard treatment of increased ICP, there are no guidelines devoted to the management of the EVD and elevated ICP after aSAH. This was recently underlined in a review by Alotaibi and Macdonald [4] that reported as most of the treatment algorithms for the management of ICP among aSAH derive from studies of traumatic brain injury (TBI), this strategy may produce suboptimal results as it fails to consider the differences in pathophysiology between the two entities. Particularly, there are no guidelines for investigating if a certain range of ICP values may correlate with better outcomes in aSAH patients. One of the most important neurological complications is the development of delayed cerebral ischemia (DCI), occurring in about 30% of surviving patients, mostly within the first 2 weeks after hemorrhage [20]. The aim of the present study was to examine prognosticators of DCI-related cerebral infarction, describing the association between ICP values and the incidence of cerebral infarction, suggesting optimal ranges of pressure among consecutive patients with aSAH requiring EVD and treatment with coiling.
Materials and methods
Patient selection
This study was approved by the Institutional Review Board of Montpellier (ID-RCB: 2018_IRB-MTP_02–11). The information was delivered with a simplified information note. Because the trial used non-invasive procedures along with standard care provided in French intensive care units, only verbal consent was required from the patient or relatives, according to French law [23]. This analysis was conducted according to the STROBE criteria. Overall, 180 consecutive patients with aSAH were prospectively collected from 2018 to 2019. Our institution adopted an endovascular first policy for ruptured aneurysms: accordingly, all consecutive patients received endovascular embolization and were hospitalized in the neurocritical care unit. Surgical indication was exclusively reserved for patients presenting parenchymal hematoma with associated mass effect. Data was reviewed by 2 and, in case of inconsistency, by 3 investigators not involved in the treatment. Treatment strategy was made by multidisciplinary consensus.
The main inclusion criteria were the following: (1) age > 18 years; (2) diagnosis of aSAH within 72 h after hemorrhage; (3) treatment with an EVD and possibility of a daily monitoring of ICP from the drainage; (4) daily monitoring of the mean arterial blood pressure (MAP), cerebral perfusion pressure (CPP), and mean flow of cerebrospinal fluid (CSF) through the EVD; and (5) patients receiving brain CT, CT perfusion (CTP), and CT angiography (CTA) imaging for diagnosis of DCI-related cerebral infarction, cerebral hypoperfusion, and cerebral vasospasm (CV).
The main exclusion criteria were the following: (1) patients with aSAH and intracerebral hematoma and mass effect; (2) EVD accidentally removed or occluded; (3) mycotic aneurysms; and (4) CSF shunt infection. Patients with severe cardiac and respiratory dysfunction were excluded due to the potential influence of the hypoxia and the impaired cardiac output on the brain tissue oxygenation and cerebral blood flow [15]. With the aim to analyze variations of the cerebral perfusion imaging exclusively related to CV, DCI-related cerebral infarction, or impaired microcirculation, patients with intracerebral hematoma and mass effect were excluded because of the hypoperfused hemorrhagic and peri-hemorrhagic tissue. Figure 1 shows the flowchart for patient selection.
Primary investigated outcome
The primary investigated outcome was on the development of DCI-related cerebral infarction that was defined as the presence of cerebral infarction on a brain CT scan within 3 weeks after aSAH, which was not present between 24 and 48 h after aneurysm occlusion, and not attributable to other causes such as aneurysm treatment [26]. Hypodensities on CT imaging resulting from ventricular catheter were not classified as DCI-related cerebral infarction.
Design of the study
The following factors were analyzed as potential predictors of DCI-related cerebral infarction: (1) age; (2) sex; (3) smoking; (4) arterial hypertension; (5) WFNS (World Federation of Neurosurgical Society) score at presentation; (6) intraventricular hemorrhage; (7) diameter of the clot at the basal cisterns; (8) presence of CV; (9) mean ICP; (10) mean volume of the CSF drained through the EVD; (11) MAP; (12) CPP; and (13) volume of the cerebral hypoperfusion at the CTP imaging. All these factors were recorded until day 21 after hemorrhage.
Diagnosis and severity of CV was based on CTA performed between days 0–4, 5–9, 10–14, and 15–21 after aSAH. Transcranial Doppler (TCD) was performed every day by a trained neurocritical care physician to help in the monitoring of CV. In case of anomalies of the mean flow velocities (MFV) in the TCD, a CTA was performed to confirm CV. A total of six arterial locations were examined in CTA images: proximal locations (suprasellar ICA, M1 segment, A1 segment, and basilar artery) and distal locations (A2 and M2 segments). Using calipers and a finely calibrated ruler, the investigators categorized the degree of CV as none/mild (0–33%), moderate (34–66%), or severe (> 67%) compared with the initial diameter of the vessel [25].
In the TCD, the MFVs were determined in the middle and anterior cerebral arteries and internal carotid artery (MCA, ACA, ICA) on both sides through a temporal window; the MFV in the basilar artery was examined via the foramen magnum. Definition of the “sonographic vasospasm” was a MFV > 140 cm/s in the MCA, ACA, and/or PCA or a MFV of > 90 cm/s in the basilar artery [27].
ICP was monitored through the EVD (Codman ICP monitor) with patients in clinostatism with 30° head elevation. MAP was obtained noninvasively, and CPP was calculated as follows: CPP = MAP − ICP.
ICP, MAP, CPP, and mean volume of CSF were recorded every 2 h for 3 weeks after aSAH.
Imaging protocol and analysis
CT imaging was conducted on a 64 slice CT scanner (Lightspeed; General Electric Healthcare, Waukesha). For the CTP protocol, 40 ml contrast agent (Xenetix 350 mg Iode/ml) was injected at 4 ml/s through an antecubital vein. CTP images were processed with a fully automated, software platform (RAPID version 2017; iSchemaView [3]).
The color-coded perfusion parameter maps for cerebral blood flow (CBF), cerebral blood volume (CBV), mean transit time (MTT), and time-to-maximum (Tmax) of the residue function were produced for qualitative and quantitative CTP analysis. Cerebral hypoperfusion was evaluated based on Tmax delay automatically generated by the RAPID software. The prespecified Tmax delays used to quantify hypoperfusion on perfusion weighted imaging were > 4, > 6, and > 8 s. The volume of the brain hypoperfusion on Tmax > 4, > 6, and > 8 s was analyzed as predictors of DCI-related cerebral infarction. CTP was performed between day 0–4, 5–9, 10–14, and 15–21 after aSAH.
CTA was performed injecting 4 ml/s of contrast agent, for a total volume of 60 ml. Image processing consisted of axial, coronal, and sagittal multiplanar volume reformatted MIP (maximum intensity projection) images.
Intervention and management of aSAH patients
All patients were treated under general anesthesia with coiling within the first 24 h after aSAH.
Systemic heparinization was performed only during the procedure, and it was started after the first coil or first set of coils had been placed (targeted activated coagulation time of 2 times baseline). Anti-platelet therapy was not systematically given because simple coiling was performed for all patients. In case of thromboembolic complication during treatment, intraoperative tirofiban (Aggrastat, Merck and Company, West Point, PA) was adopted with the following protocol: a bolus of 0.6 μg/kg/min of tirofiban was intravenously administrated. If a remnant thrombus was identified via angiography after tirofiban infusion, a maintenance infusion of 0.1 μg/kg/min was continued for a maximum 18 h [28].
The following standard protocol of care was applied: (1) oral (for conscious patients without swallowing dysfunction) or intravenous (for intubated patients) nimodipine (360 mg/d); (2) MAP and CPP were maintained above to 90 mmHg and 75 mmHg, respectively; (3) analgesic treatment with intravenous paracetamol or opioids; (4) anti-epileptic treatment was given if required; and (5) fluid management was adopted to maintain normovolemia.
Based on our standard operating procedures, in case of anatomical moderate/severe CV (or in case of clinical deterioration potentially related to CV), escalating doses of intravenous milrinone (0.1–0.2 mg/kg bolus + infusion of 1 mcg/kg/min) were added to the nimodipine therapy. Because milrinone is an inhibitor of phosphodiesterase III with a positive inotropic effect, the aim was to decrease vasospasm without significant changes in systemic hemodynamics [1, 12]. In case of moderate/severe CV and associated hypoperfusion on the CTP imaging, intraarterial infusion of nimodipine (3 mg) + milrinone (4 mg) was performed through a 4 French femoral access via a diagnostic catheter placed in the target internal carotid artery.
Treatment of raised ICP and management of EVD
Indication for EVD was made by an experienced neurosurgeon and was based on the following criteria: (1) diagnosis of acute hydrocephalus on CT scan defined on the basis of the “bicaudate index” [24]; (2) Glasgow Coma Scale (GCS) < 12 and ventricular volume at the upper limit of the normal size (based on the bicaudate index); and (3) the presence of intraventricular hemorrhage and increased ventricular volume. Ventricular catheter was placed at the anterior horn of the lateral ventricle. The height of the EVD was adjusted to maintain a normal ICP. Normal values of ICP were considered ≤ 15 mmHg, and EVD was lowered when ICP values were at 15 mmHg or more [2]. Accordingly, in about 90% of patients, the height of the EVD (calculated from the external acoustic meatus) was between 5 and 10 cm, with only 4% of patients having the EVD at 10–15 cm.
Statistical analysis
Categorical data were described by frequency. The normality of the distribution of the qualitative variables was assessed using the Shapiro-Wilk test. A variable was Gaussian distributed if p was > 0.05 at the Shapiro-Wilk test. For normal variables, mean and standard deviation (SD) was reported. The primary outcome was the development of DCI-related cerebral infarction. Chi-square or Fisher exact tests were used to evaluate qualitative factors: sex, smoking, arterial hypertension, intraventricular hemorrhage, and CV. The t test (two-tailed) was applied to assess quantitative factors: age, diameter of the clot, mean ICP, mean volume of CSF, MAP, CPP, and hypoperfusion volume on Tmax > 4, > 6, and > 8 s. Variables significantly associated (p ≤ 0.1) to the development of DCI-related cerebral infarction were analyzed together in a binary logistic regression (Wald test). Results were expressed using p value and odds ratio. Receiver-operating characteristic (ROC) curves were generated for ICP and CTP values (Tmax > 4, > 6, > 8 s). Parameters with areas under the curve (AUC) > to 0.5 were selected. The optimal threshold values were derived from the ROC curves that were used to find the optimum ICP and Tmax values to distinguish patients with and without DCI-related cerebral infarction. The cutoff values obtained in ROC space were calculated using the Youden’s index. All statistical analyses were performed with SPSS version 24 (SPSS Inc. SPSS® Chicago, IL, USA).
Results
Baseline population characteristics
Among 180 aSAH patients (treated between 2018 and 2019), 50 patients (Fig. 1) met our above reported inclusion criteria (33 women, 17 men; mean age, 55.3 years, SD ± 11.5; range, 20–74). Overall, 60% of them presented a WFNS score of 4–5 and 90% were Fisher 3–4, whereas 76% showed intraventricular hemorrhage. Table 1 shows that the DCI and no-DCI groups were comparable for all the reported variables, except for the proportion of aneurysms ≤ 5 mm that was higher in the no-DCI-related cerebral infarction group.
All included patients received EVD treatment (Supplemental Table 1): 52% before and 48% after aneurysm treatment. The EVD level (calculated from the external acoustic meatus) was between 0–5 cm, 6–10 cm, and 11–15 cm among 64%, 32%, and 4% of patients, respectively (Supplemental Table 1). The mean daily volume of CSF drained from the EVD was 225 ml (85–325, SD = ± 60). The mean duration of the EVD was 15 days (6–25 days, SD = ±5.8).
EVD-related hematoma was documented in 26% of patients. Volumes of the hematoma (calculated with the rule ABC/2) [11] were as follows: < 5 ml, 6–10 ml, and 15–30 ml among 14%, 6%, and 6% of patients, respectively. The overall rate of shunt dependency was 12%.
DCI-related cerebral infarction, cerebral vasospasm, and clinical outcome
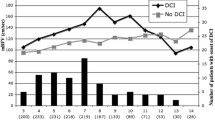
Overall, 21 patients (42%) developed DCI-related cerebral infarction that was detected between days 11–15, 16–21, and within the first week among 14 (66.5%), 5 (24%), and 2 (9.5%) patients, respectively.
The incidence of anatomical CV was 54% (27 patients). The highest incidence of CV occurred between days 8 and 12 (17 patients, 34%); five patients (10%) developed CV within the first week and 2 subjects (4%) after day 15. The severity of CV was as follows: mild, moderate, and severe among 9 (18%), 10 (20%), and 8 patients (16%), respectively. Vasospasm was predominantly located in the anterior circulation (24 patients, 48%): M1 and A1 segments were the most common locations (16 patients, 32%), followed by ICA (8 patients, 16%). Bilateral CV was diagnosed among 7 patients (14%).
Overall, 15 patients (71%) developing DCI-related cerebral infarction presented CV, whereas 6 patients with DCI-related cerebral infarction did not have CV (29%). Of the 29 subjects without DCI-related cerebral infarction, 41% presented CV (12 patients). All of the 27 patients with anatomical CV were treated by adding intravenous milrinone, whereas 20 patients presenting CV and hypoperfusion on Tmax > 6 s received endovascular intraarterial infusion of milrinone + nimotop.
At 6-month clinical follow-up, 27 patients (54%) presented an mRS0-2. Functional independence was achieved among 6/21 (28.5%) and 21/29 (72%) subjects with and without diagnosis of DCI-related cerebral infarction, respectively.
Cerebral hypoperfusion
Hypoperfusion on Tmax > 4, > 6, and > 8 s was detected among 46 (92%), 35 (70%), and 27 (54%) of patients, respectively. The higher incidence of hypoperfusion was around days 8–10, and the maximum hypoperfused cerebral volume at Tmax > 4, > 6, and > 8 s was 767 ml, 302 ml, and 75 ml, respectively.
The mean volume of the brain hypoperfusion among patients with and without DCI-related cerebral infarction was 363 ml (SD ± 236) and 82.5 ml (SD ± 108) for the Tmax > 4 s; 154 ml (SD ± 174) and 9.1 ml (SD ± 17) for the Tmax > 6 s; and 72 ml (SD ± 145) and 3 ml (SD ± 8.7) for the hypoperfusion Tmax > 8 s.
Mean values of ICP
ICP values ranged between 2 (one patient) and 19 mmHg (one patient) (Table 2) (mean = 8.4 mmHg; SD ± 3.6). Overall, 91% of them ranged between 3 and 15 mmHg.
Predicting factors of DCI-related cerebral infarction
At the univariate analysis, the presence of CV (p = .035), mean ICP (p = 0.0001), CPP (0.106), diameter of the clot (p = 0.008), and the mean volume of the hypoperfusion on Tmax > 4 (p = 0.031), Tmax > 6 s (p = 0.0001), and Tmax > 8 s (p = 0.012) were associated with significantly higher incidence of DCI-related cerebral infarction (Table 3).
On the multivariable logistic regression, the mean ICP (OR = 2, 95%CI = 1.01–3.9, p = 0.042) and the mean volume of hypoperfusion Tmax delay > 6 s (OR = 1.2, 95%CI = 1.01–1.3, p = 0.025) were independent predictors of DCI-related cerebral infarction.
Cutoff of ICP and brain hypoperfusion for the prediction of DCI-related cerebral infarction
The AUC of the mean ICP values was 0.825 (standard error = 0.057) (Fig. 2); the best cutoff value was 6.7 mmHg providing sensitivity, specificity, positive (PPV) and negative predictive values (NPV), and accuracy of 71.4% (95%CI = 48–89%), 62% (95%CI = 42–79%), 58% (95%CI = 44–70%), 75% (95%CI = 59–86%), and 66% (95%CI = 51–79%) for the prediction of DCI-related cerebral infarction, respectively (Table 4).
Hypoperfusion Tmax > 6 s presented the highest AUC (0.956) (standard error = 0.025) (Fig. 3), and the best cutoff value was 18 ml showing sensitivity, specificity, PPV, NPV, and accuracy of 90.5% (95%CI = 69–98.8%), 86.2% (95%CI = 68.4–96%), 82.6% (95%CI = 65.4–92%), 92.5% (95%CI = 77–98%), and 88% (95%CI = 75–95%), respectively.
ROC analysis illustrating the AUCs of the hypoperfusion Tmax > 4, > 6, and > 8 s for the prediction of DCI. The highest AUC was 0.956 for the Tmax >6 s. The best cutoff value for the hypoperfusion Tmax > 6 s was 18 ml (red lines and yellow arrowhead) showing sensitivity and specificity at 90.5% (95%CI = 69–98.8%) and 86.2% (95%CI = 68.4–96%), respectively
In patients presenting both hypoperfusion on Tmax > 6 s (cutoff = 18 ml) and mean ICP with a cutoff of 6.7 mmHg, sensitivity, specificity, PPV, NPV, and accuracy for the prediction of DCI-related cerebral infarction were 94.5% (95%CI = 73–99.8%), 94.1% (95%CI = 71.3–99.8%), 94.4% (95%CI = 71.6–99.1%), 94.1% (95%CI = 70.3–99.1%), and 94.2% (95%CI = 90.5–99.3%), respectively.
Discussion
In this study, ICP values and brain hypoperfusion on Tmax delay predicted DCI-related cerebral infarction occurrence on a multivariable logistic regression analysis. Hypoperfusion on Tmax >6 s, with a threshold of 18 ml, showed the highest sensitivity and specificity in predicting cerebral infarction. In addition, ICP significantly influenced the occurrence of DCI-related cerebral infarction, indicating that values < 6.7 mmHg were likely protective of cerebral ischemia among aSAH patients.
Optimal ICP values among aSAH patients
The American Heart Association guidelines for the management of aSAH patients reported that acute symptomatic hydrocephalus should be treated with CSF diversion, without adding recommendations about ICP values, EVD levels, or CSF volume [8]. In addition, most of the algorithms for the treatment of raised ICP after aSAH derived from guidelines for TBI [4]. Thus, optimal EVD management remains controversial. A recent multi-institutional survey underlined an important practice variance on the management of EVD among aSAH patients, with centers adopting a more (0 cmH2O to 5 cmH2O) or less “aggressive” (10 cmH2O to 20 cmH2O) drainage based on the practitioner preferences [7].
In our population, ICP independently influenced the occurrence of DCI-related cerebral infarction (OR = 2, p = 0.042). The mean ICP value among patients developing DCI-related cerebral infarction was 11 mmHg compared with 6 mmHg of those without ischemic lesions. In addition, the ROC analysis identified a threshold of ICP close to 7 mmHg: 75% of patients with an ICP < 7 mmHg had a lower probability to develop cerebral infarction. This is the first study describing an association between optimal ICP values and a lower incidence of DCI-related cerebral infarction among aSAH patients.
The Mayo Clinic group reported a patient with aSAH and normal size ventricles showing substantially clinical and cerebral perfusion improvement after lowering the EVD from 15 to 5 mmHg. The authors concluded that CSF drainage at low levels of ICP (5 mmHg) could improve blood flow in the microcirculation and tissue perfusion [11]. Several studies underlined the importance of the microcirculation in the development of DCI, showing that the pathophysiological mechanism is likely related to multiple factors (cortical spreading depression, endothelial and autoregulation dysfunctions, inflammation, microthrombosis, microvascular spasm, and blood brain barrier disruption [6, 16]). A lower ICP (in our study < 6.7 mmHg) may reduce the pressure surrounding the impaired capillary vessels, improving nutritive exchanges with brain parenchyma.
Samuelsson et al. [22] studied the relationship between ICP variation and brain tissue energy metabolism assessed by microdialysis samplings. Interestingly, they found a threshold of ICP at 10 mmHg: when ICP was lowered to ≤ 10 mmHg, there was an instantaneous sharp increase in interstitial Glt and pyruvate, indicating a favorable brain tissue metabolism. Ryttlefors et al. [21] have shown that periods with ICP close to or higher than 20 mmHg following aSAH were associated with clinical worsening, whereas periods with CPP > 100 mmHg were associated with clinical improvement. Our patient population presented ICP values in a range of 2 mmHg and 19 mmHg, with 91% of patients having an ICP from 3 to 15 mmHg. Despite these values not being pathological, in our opinion, accepting ICP at the upper limit of the normality (15–19 mmHg) may expose an already vulnerable brain to additional stress, especially during vasospasm [22].
Predictive value of the hypoperfusion on Tmax delay
The complex pathophysiology of DCI is associated with different pathways leading at some point in a reduction of cerebral perfusion which may lead to cerebral infarction. Accordingly, cerebral perfusion is an important target for the prediction of DCI. A meta-analysis of about 600 aSAH patients showed that, for diagnosing DCI, quantitative thresholds for CBF ranged from 25 to 36 ml/100 g per minute and for MTT from 5.0 to 6.5 s, with high sensitivity and specificity [9]. Another recent study underlined that the mean Tmax delay was the most powerful parameter, and a cutoff value of 2.24 s provided sensitivity of 74% and specificity of 72% for the early prediction of DCI development [10].
In our study, we used the Tmax volume hypoperfusion with different thresholds automatically estimated with the RAPID software, which is nowadays largely adopted for the detection of the infarct core and the ischemic penumbra among large vessel occlusion patients [3].
Interestingly, hypoperfusion with a Tmax delay > 6 s presented the highest AUC (0.956), and a volume of 18 ml predicted more than 80% of cerebral infarction. This result can be used to carry out a more aggressive endovascular intraarterial treatment (as infusion of vasodilator or angioplasty) earlier, in patients at risk of DCI-related cerebral infarction.
CV has been reported as the main treatment target to prevent secondary brain injury. However, in the literature, while up to 70% of patients demonstrate a degree of arterial narrowing on DSA imaging, only 20–30% develop clinical symptoms [6]. In addition, approximately a third of patients with DCI have no CV [9, 25]. The phase 3 CONSCIOUS-2 trial [13], assessing the effect of clazosentan (an endothelin receptor antagonist) on morbidity and mortality after aSAH, showed that patients in the clazosentan group, despite improvement of CV, had a non-significant reduction in vasospasm-related morbidity at 3 months compared with the placebo. The AHA/ASA guidelines reported that oral nimodipine should be administrated to all patients with aSAH, but nimodipine has been shown to improve neurological outcome but not CV [8]. These results underlined as cerebral infarction after aSAH can be associated with vasospasm-dependent and independent effects, mainly related to the impaired microcirculation. In our study, about 54% of patients presented CV, while approximately 40% developed DCI-related cerebral infarction. Interestingly, one third of patients with DCI-related cerebral infarction did not have CV, and about 40% of subjects without DCI presented CV. The presence of CV significantly influenced the occurrence of DCI-related cerebral infarction at the univariate analysis. However, when CV was pooled together with the Tmax hypoperfusion, vasospasm was not an independent factor of DCI-related cerebral infarction at the multivariate model. It is likely that Tmax hypoperfusion delay > 6 s, having been able to detect perfusion impairment related to the macro- and microcirculation, is more powerful to predict cerebral infarction than the CV (Fig. 4).
(A) A 68-year-old patient with a modified Fisher III aSAH related to a ruptured anterior communicating artery aneurysm (B and C) treated with coiling (D). The patient has been treated with EVD and the mean ICP was 6 mmHg. Left (E) and right (F) anteroposterior ICA angiograms performed at day 9 depicting vasospasm of the ICA, MCA, and A1 with approximately 50% reduction of the vessel diameter. Patient was under full dose of oral nimodipine. (G and H) CTP imaging showing the absence of a significant hypoperfusion on Tmax delay. (I and L) CT scan after 30 days from hemorrhage showing no ischemic lesions, despite the presence of a bilateral vasospasm. The patient received a ventriculoperitoneal shunt
Investigating the literature, the PPV of vasospasm for DCI is only 67%, and up to 25% of DCI are not located in the territory of the spastic artery or are found in patients who did not demonstrate vasospasm [17, 5]. In our study, in about 20% of patients developing DCI-related cerebral infarction, CV was located (or was more severe) at the contralateral side, whereas hypoperfusion Tmax > 6 s was always located in the area of the infarction (Fig. 5).
(A) A 55-year-old patient with a modified Fisher II aSAH due to a ruptured fusiform right vertebral artery (B). The aneurysm has been treated with the occlusion of the parent artery (C and D), without complications. The patient was treated with an EVD and the mean ICP was 11 mmHg. After 10 days from hemorrhage, the patient developed a bilateral vasospasm. Vasospasm was mild on the left side (E) and severe on the right side (F). Despite vasospasm being severe on the right side, patient developed hypoperfusion (Tmax > 6 s) on the left parieto-occipital lobe (G). Patient received intraarterial infusion of nimodipine and milrinone. (H) CT scan performed on day 15 showed a delayed ischemic lesion localized on the same area of the hypoperfusion (I)
Finally, about 9 out of 10 patients presenting both ICP values > 6.7 mmHg and hypoperfusion volume > 18 ml on Tmax >6 s were at risk DCI-related cerebral infarction.
These results underlined important associations between ICP, microcirculation, and DCI-related cerebral infarction, raising relevant research perspectives. However, they should be interpreted with caution because, while statistical methods showed an association between lower ICP values, hypoperfusion volumes, and the occurrence of DCI-related cerebral infarction, bias related to the relatively small sample size and patient selection should be underlined. Most of the included patients presented a high-grade aSAH since low-grade patients required EVD much less frequently. Accordingly, in a high clinical grade aSAH population, the frequency of raised ICP, intraventricular hemorrhage, volume of the aSAH, and neurological severity (WFNS score) are quite frequent, and this might be a reason why these factors were not significant predictors in our analysis.
Limitations
There are several limitations to our study. First, this is a small, single-center study performed in a high-volume hospital adopting specific treatment algorithms. In our center, more than 90% of aSAH are treated by endovascular means; accordingly, all the included patients received coiling: this can represent a different treatment strategy compared with other centers. The use of CT perfusion, although performed in specified time periods, was not standardized. Patients with cerebral hematoma and mass effect, or not requiring EVD, were excluded. Accordingly, these results are likely not applicable to all patients with aSAH.
Conclusions
This study provides further information about the management of patients at risk of DCI-related cerebral infarction after aSAH. Important questions were raised about the association between DCI-related cerebral infarction and microcirculation, values of ICP, and volume and severity of the brain tissue hypoperfusion. Although limited to selected aSAH patients who underwent coiling and requiring EVD, our results suggest that lower ICP values could potentially be beneficial in decreasing DCI-related cerebral infarction. In addition, in our practice, the whole-brain CTP allows a reliable estimation of the risk of DCI-related cerebral infarction, with a particularly high sensibility and specificity of the hypoperfusion at Tmax delay > 6 s.
References
Abulhasan YB, Ortiz Jimenez J, Teitelbaum J, Simoneau G, Angle MR (2020) Milrinone for refractory cerebral vasospasm with delayed cerebral ischemia. J Neurosurg 27:1–12
Albeck MJ, Borgesen SE, Gjerris F, Schmidt JF, Sorensen PS (1991) Intracranial pressure and cerebrospinal fluid outflow conductance in healthy subjects. J Neurosurg 74:597–600
Albers GW, Goyal M, Jahan R, Bonafe A, Diener HC, Levy EI, Pereira VM, Cognard C, Cohen DJ, Hacke W, Jansen O, Jovin TG, Mattle HP, Nogueira RG, Siddiqui AH, Yavagal DR, Baxter BW, Devlin TG, Lopes DK, Reddy VK, de Rochemont RM, Singer OC, Bammer R, Saver JL (2016) Ischemic core and hypoperfusion volumes predict infarct size in SWIFT PRIME. Ann Neurol 79:76–89
Alotaibi NM, Wang JZ, Pasarikovski CR, Guha D, Al-Mufti F, Mamdani M, Saposnik G, Schweizer TA, Macdonald RL (2017) Management of raised intracranial pressure in aneurysmal subarachnoid hemorrhage: time for a consensus? Neurosurg Focus 43:E13
Brown RJ, Kumar A, Dhar R, Sampson TR, Diringer MN (2013) The relationship between delayed infarcts and angiographic vasospasm after aneurysmal subarachnoid hemorrhage. Neurosurgery 72:702–707 discussion 707-708
Budohoski KP, Guilfoyle M, Helmy A, Huuskonen T, Czosnyka M, Kirollos R, Menon DK, Pickard JD, Kirkpatrick PJ (2014) The pathophysiology and treatment of delayed cerebral ischaemia following subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry 85:1343–1353
Chung DY, Leslie-Mazwi TM, Patel AB, Rordorf GA (2017) Management of external ventricular drains after subarachnoid hemorrhage: a multi-institutional survey. Neurocrit Care 26:356–361
Connolly ES Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, Hoh BL, Kirkness CJ, Naidech AM, Ogilvy CS, Patel AB, Thompson BG, Vespa P, American Heart Association Stroke C, Council on Cardiovascular R, Intervention, Council on Cardiovascular N, Council on Cardiovascular S, Anesthesia, Council on Clinical C (2012) Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke 43:1711–1737
Cremers CH, van der Schaaf IC, Wensink E, Greving JP, Rinkel GJ, Velthuis BK, Vergouwen MD (2014) CT perfusion and delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. J Cereb Blood Flow Metab 34:200–207
Dong L, Zhou Y, Wang M, Yang C, Yuan Q, Fang X (2019) Whole-brain CT perfusion on admission predicts delayed cerebral ischemia following aneurysmal subarachnoid hemorrhage. Eur J Radiol 116:165–173
Fugate JE, Rabinstein AA, Wijdicks EF, Lanzino G (2012) Aggressive CSF diversion reverses delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage: a case report. Neurocrit Care 17:112–116
Lannes M, Teitelbaum J, del Pilar CM, Cardoso M, Angle M (2012) Milrinone and homeostasis to treat cerebral vasospasm associated with subarachnoid hemorrhage: the Montreal neurological hospital protocol. Neurocrit Care 16:354–362
Macdonald RL, Higashida RT, Keller E, Mayer SA, Molyneux A, Raabe A, Vajkoczy P, Wanke I, Bach D, Frey A, Marr A, Roux S, Kassell N (2011) Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: a randomised, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2). Lancet Neurol 10:618–625
Macdonald RL, Schweizer TA (2017) Spontaneous subarachnoid haemorrhage. Lancet 389:655–666
Meng L, Hou W, Chui J, Han R, Gelb AW (2015) Cardiac output and cerebral blood flow: the integrated regulation of brain perfusion in adult humans. Anesthesiology 123:1198–1208
Ostergaard L, Aamand R, Karabegovic S, Tietze A, Blicher JU, Mikkelsen IK, Iversen NK, Secher N, Engedal TS, Anzabi M, Jimenez EG, Cai C, Koch KU, Naess-Schmidt ET, Obel A, Juul N, Rasmussen M, Sorensen JC (2013) The role of the microcirculation in delayed cerebral ischemia and chronic degenerative changes after subarachnoid hemorrhage. J Cereb Blood Flow Metab 33:1825–1837
Rabinstein AA, Friedman JA, Weigand SD, McClelland RL, Fulgham JR, Manno EM, Atkinson JL, Wijdicks EF (2004) Predictors of cerebral infarction in aneurysmal subarachnoid hemorrhage. Stroke 35:1862–1866
Rabinstein AA, Lanzino G, Wijdicks EF (2010) Multidisciplinary management and emerging therapeutic strategies in aneurysmal subarachnoid haemorrhage. Lancet Neurol 9:504–519
Rinkel GJ, Algra A (2011) Long-term outcomes of patients with aneurysmal subarachnoid haemorrhage. Lancet Neurol 10:349–356
Roos YB, de Haan RJ, Beenen LF, Groen RJ, Albrecht KW, Vermeulen M (2000) Complications and outcome in patients with aneurysmal subarachnoid haemorrhage: a prospective hospital based cohort study in the Netherlands. J Neurol Neurosurg Psychiatry 68:337–341
Ryttlefors M, Howells T, Nilsson P, Ronne-Engstrom E, Enblad P (2007) Secondary insults in subarachnoid hemorrhage: occurrence and impact on outcome and clinical deterioration. Neurosurgery 61:704–714 discussion 714-705
Samuelsson C, Howells T, Kumlien E, Enblad P, Hillered L, Ronne-Engstrom E (2009) Relationship between intracranial hemodynamics and microdialysis markers of energy metabolism and glutamate-glutamine turnover in patients with subarachnoid hemorrhage. Clinical article. J Neurosurg 111:910–915
Toulouse E, Masseguin C, Lafont B, McGurk G, Harbonn A, Roberts JA, Granier S, Dupeyron A, Bazin JE (2018) French legal approach to clinical research. Anaesth Crit Care Pain Med 37:607–614
van Gijn J, Hijdra A, Wijdicks EF, Vermeulen M, van Crevel H (1985) Acute hydrocephalus after aneurysmal subarachnoid hemorrhage. J Neurosurg 63:355–362
Vergouwen MD, Ilodigwe D, Macdonald RL (2011) Cerebral infarction after subarachnoid hemorrhage contributes to poor outcome by vasospasm-dependent and -independent effects. Stroke 42:924–929
Vergouwen MD, Vermeulen M, van Gijn J, Rinkel GJ, Wijdicks EF, Muizelaar JP, Mendelow AD, Juvela S, Yonas H, Terbrugge KG, Macdonald RL, Diringer MN, Broderick JP, Dreier JP, Roos YB (2010) Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke 41:2391–2395
Westermaier T, Pham M, Stetter C, Willner N, Solymosi L, Ernestus RI, Vince GH, Kunze E (2014) Value of transcranial Doppler, perfusion-CT and neurological evaluation to forecast secondary ischemia after aneurysmal SAH. Neurocrit Care 20:406–412
Wright RS, Anderson JL, Adams CD, Bridges CR, Casey DE Jr, Ettinger SM, Fesmire FM, Ganiats TG, Jneid H, Lincoff AM, Peterson ED, Philippides GJ, Theroux P, Wenger NK, Zidar JP, Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE Jr, Chavey WE 2nd, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS (2011) 2011 ACCF/AHA focused update of the Guidelines for the Management of Patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction (updating the 2007 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the American College of Emergency Physicians, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 57:1920–1959
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
This study was approved by the Institutional Review Board of Montpellier (ID-RCB: 2018_IRB-MTP_02-11). The information was delivered with a simplified information note. Because the trial used non-invasive procedures along with standard care provided in French intensive care units, only verbal consent was required from the patient or relatives, according to French law [23].
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 15 kb).
Rights and permissions
About this article
Cite this article
Cagnazzo, F., Chalard, K., Lefevre, PH. et al. Optimal intracranial pressure in patients with aneurysmal subarachnoid hemorrhage treated with coiling and requiring external ventricular drainage. Neurosurg Rev 44, 1191–1204 (2021). https://doi.org/10.1007/s10143-020-01322-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-020-01322-2