Abstract
The use of antiplatelet medication is widespread as reducing risk of death, myocardial infarction, and occlusive stroke. Currently, the management of neurosurgical patients receiving this type of therapy continues to be a problem of special importance. In this paper, we present the results of an Italian survey focused on the management neurosurgical patient under antiplatelet therapy and, for any item of the investigation, the relative advices coming from literature. This survey was conducted including 129 neurosurgery units in Italy. The present paper was designed by following each question posed in the survey by a brief discussion on literature data. There is a considerable lack of consensus regarding management of antiplatelet therapy in neurosurgery, with critical impact on patient’s treatment. What is clearly evident from the present survey is the considerable variability in neurosurgical care for antiplatelet patients; it is reasonable to assume that this scenario reflects the paucity of evidence regarding this issue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of antiplatelet medication is widespread as reducing risk of death, myocardial infarction, and occlusive stroke. However, it has also been shown that usage of both antiplatelet and anticoagulant medicines increases rates of intracerebral hemorrhage. Currently, the management of neurosurgical patients receiving this type of therapy continues to be a problem of special importance. Several dilemmas in clinical practice arise when patients need neurosurgical care because of bleeding secondary to such treatment or when patients present with acute neurosurgical illnesses while taking antiplatelet or anticoagulant therapies or even when a patient with a history of intracerebral hemorrhage subsequently develop conditions for which such medicines are indicated. In addition, the consequences of reintroducing early (bleeding or rebleeding) or late (thrombotic or thromboembolic) antiplatelet therapies can be devastating. In this paper, we present the results of an Italian survey focused on the management neurosurgical patient under antiplatelet therapy and, for any item of the investigation, the relative advices coming from literature. This survey was conducted including 129 neurosurgery units in Italy. It was designed to gather information about epidemiology, management, and prophylaxis concerning patients under antiplatelet (part one) and anticoagulant (part two) therapy presenting with acute neurosurgical illnesses. The present paper was designed by following each question posed in the survey by a brief discussion on literature data.
Methods
Participants
With the aid of the secretary of the Italian Society of Neurosurgery, a mailing list, containing the email addresses of all the chief of different neurosurgical departures in Italy for a total number of 129 email addresses, was created. An email containing an Internet link to the survey (SurveyMonkey, a cloud-based online survey software) was sent to the mailing list in the September 2017; the survey link was active between September and November 2017, and then the survey was closed.
Survey
The survey consisted in 20 questions focused on the management of hemostasis and anticoagulation issues that apply to neurosurgical care practice (Table 1). In this paper, we discuss the results of the 10 questions that are focused on antiplatelet issues.
Results and discussion
On average, the number of responders for each question was 47.5 (37% of the total number of departures contacted).
Epidemiology of antiplatelet patients
Question 1 and Question 2: In the last year, indicate the approximate percentage of patients at your department with “acute” neurosurgical indication, e.g., acute/chronic epidural-subdural hematomas, intracerebral hemorrhage (ICH), subarachnoid hemorrhage (SAH), and traumatic subarachnoid hemorrhage (tSAH), in therapy with one or more antiplatelet drugs and the percentage of patient under association of antiplatelet and anticoagulant therapy.
The majority of participants (40.63%) affirmed that 25–50% of patient with acute surgical indication assumed antiplatelet medication, while 63.83% neurosurgical unit chiefs answered that concomitant therapies were assumed by less than 10% of acute neurosurgical patients(Supplementary Fig. 1 and Supplementary Fig. 2).
Antiplatelet therapy is very widespread; aspirin is used in both primary and secondary prevention of arterial thromboses. P2Y12 receptor inhibitors, such as clopidogrel, prasugrel, or ticagrelor, are frequently added in high-risk patients, for example, after arterial stent implantation [2]. Actually, it is not that uncommon to manage patient with dual antiplatelet therapy or patients on concomitant oral anticoagulants and antiplatelet therapy (up to 30% of patients with nonvalvular atrial fibrillation may receive concomitant treatments of oral anticoagulants with antiplatelets due to comorbid cardiovascular conditions) [11].
Traumatic brain injury (TBI) in patients on antiplatelet therapy
In a cohort of 10.010 patients with subdural hematoma retrieved from the Danish National Patient Register, more than 18.9% of patients were on single antiplatelet therapy (mostly on low-dose aspirin), 6.7% were on dual antiplatelet therapy, and 4% were on dual antithrombotic therapy [15]. Authors stated that low-dose aspirin was associated with a small increased risk of subdural hematoma and that concomitant use of more than one antithrombotic drug was related to substantially higher subdural hematoma risk, in particular for combined treatment of clopidogrel with a vitamin K antagonist (VKA) [15]. Similarly, in a single-center, retrospective study, authors analyzed 116 consecutive cases involving patients with acute subdural hematoma, and they found that 24% of the patient were taking thrombocyte inhibitor; author concluded that thrombocyte inhibitor treatment was associated with increased mortality in those patients [36]. In a series of 2773 consecutive patients with mild traumatic brain injury that underwent a head computed tomography (CT) scan, 21% were on antiplatelet therapy (and 4.2% of these patients were on dual antiplatelet therapy) [33]; antiplatelet therapy was related to a higher incidence of positive head CT scans, especially in patients assuming P2Y12 receptor inhibitors [33]. Patients on antiplatelet therapy seem to have a worst outcome after TBI [32], but data about the influence of thrombocyte inhibitor on hematoma expansion, morbidity, and mortality are conflicting.
Spontaneous intracerebral hemorrhage (sICH) in patients on antiplatelet therapy
Lovelock et al. identified all cases of sICH from April, 2002, to March, 2006in Oxfordshire, England; they found that 27% of patients were taking antiplatelet therapy [21]. Thrombocyte inhibitor might slightly increase (~0.08%/year) the incidence of intracerebral hemorrhage [7]. It is established that antithrombotic-associated ICH carries a worse clinical outcomes as compared with spontaneous intracerebral hemorrhage in non-anticoagulated patients [9, 13]; however, the effects of antiplatelet therapy on sICH outcomes are less evident. Antiplatelet therapy has been associated with hematoma expansion, higher rates of mortality, and worse functional outcomes in some studies [1, 6, 10, 24], while other studies have failed to detect differences in outcome or hematoma growth [16, 23, 29].
Management of antiplatelet patients
Question 3: In the case of the abovementioned acute neurosurgical pathologies, in which conditions do you apply a “forced” emergency reversal of antiplatelet agents?
The answer “only if a surgical treatment is planned” was chosen by 89% of responders, while “also if a conservative treatment is planned” was answered by the remnants.
Hematoma expansion is an independent predictor of early neurological deterioration and poor long-term clinical outcomes in both trauma and ICH patients [5], and correcting for platelet inhibition may reduce hematoma expansion. Platelets are irreversibly inhibited by aspirin and P2Y12 receptor inhibitors for the life of the platelet; there is no reversal agent available; consequently the only way to reverse platelet inhibition would be to create new platelets. Quick replenishment with new platelets appears to be a reasonable option to reverse the platelet inhibition, but studies addressing the ability of platelet transfusion to improve laboratory metrics of platelet function have yielded mixed results [14]. Additionally, correlations among platelet function assay measures and functional outcomes are largely understudied. Observational studies have reported variable associations with outcome after platelet transfusion for intracerebral hemorrhage in people taking antiplatelet therapy, with several small cohort studies showing slightly improved mortality rates [8, 12]. However, the first randomized trial of platelet transfusions in patients with ICH without an indication for neurosurgery (PATCH) showed significantly greater odds of death or dependence at 3 months in patients receiving transfusions compared with patients who did not receive transfusions [1]. The investigators also found an increase in hematoma growth at 24 h in patients treated with platelet transfusion. The mechanisms underlying the higher probability of bad clinical outcome and complications of intracerebral hemorrhage in patients treated with platelet transfusion have not been clarified, but authors advised physicians against platelet transfusions in patients with antiplatelet-associated sICH. Moreover, platelet transfusion is not a procedure to be taken lightly because it is associated with inherent risk. One of the most feared complications is transfusion-related acute lung injury (TRALI) that has been reported to have an incidence closer to 1 in every 1000 recipients [20]. Other serious risks include thrombosis, disseminated intravascular coagulopathy, hemolytic transfusion reactions, and transfusion-associated sepsis, among others [20].
As the PATCH study did not include patients undergoing neurosurgery, management of patients requiring urgent surgery during antiplatelet therapy is still an unsolved problem. A prospective study showed a benefit of platelet transfusions in patients treated with documented aspirin-related platelet dysfunction who required emergency surgery [31]. According to the same study, there does not appear to be a benefit of platelet transfusion for patients with aspirin resistance or normal platelet function undergoing surgery. If platelet function tests are not available, it may be reasonable to transfuse platelets in patients on antiplatelet therapy if emergency surgery is required [20].Moreover the PATCH study mainly included patients treated with acetylsalicylic acid, so only little information about the role of platelet transfusion in patients receiving P2Y12 receptor inhibitors are supplied. In vitro and in vivo studies have shown only a modest effect of platelet transfusions to reverse P2Y12 inhibitors [1, 19, 31]. One of the possible explanations is that the persistence of active metabolites in the blood could lead to inhibition of the transfused platelet; for this reason ideally, the platelet transfusion should be started 4–6 h after the last administration of the P2Y12 receptor inhibitors (10–12 h in patients assuming ticagrelor) [28].
In conclusion, in patients with intracranial hemorrhage that are under antiplatelet therapy, cessation of antiplatelet therapy is recommended. If a neurosurgical procedure is required, platelet transfusion is suggested (at least one pool of platelets for patients treated with acetylsalicylic acid and at least two pools in patients on P2Y12 inhibitors), possibly after the execution of a platelet function testing addressing a real aspirin-related platelet dysfunction. In patients with laboratory-documented platelet function within normal limits or documented antiplatelet resistance, platelet transfusion is not recommended.
Reversal therapy
Question 4: In antiplatelet patients in whom surgery cannot be postponed over 12–24 h, do you routinely perform platelet transfusion?
The majority of responders (40%) answered “yes, in any case”, while the answer “only in case of proven platelet disfunction” was chosen by 27% of responders. “No” was selected by 34% of responders.
Aspirin irreversibly inhibits both Cyclooxygenase Enzymes 1 and 2 (COX-1 and COX-2), leading to platelet dysfunction. Aspirin use is associated with ICH after TBI, even when the aspirin therapy is considered to be low dose. Use of this antiplatelet agent increases the rate of morbidity and mortality by 5 to 6 times the rate in those not taking aspirin. Clopidogrel use also carries with it serious consequences; in the context of head injury, preinjury use is associated with a nearly 15-fold increase in frequency of ICH. According to Watson [34], when head-injured patients are known to be on an antiplatelet drug at the time of presentation, they should receive a PFA, because of its widespread and rapidity to give results. In the case of platelet function assay (PFA) > 115, which is considered to be the upper limit of normal, the treatment should be initiated based on CT findings. In light with the substantial risk of morbidity and mortality associated with platelet transfusion, nonoperative patients should be treated with a single dose of 0.4 mcg/kg intravenous desmopressin (DDAVP). The effects of aspirin can be reversed by 1 dose of platelet transfusion. Clopidogrel and other drug effect lasts until new platelets are generated, and their half-life is generally considered to be about 4 days. The level of platelet dysfunction can be monitored with PFA, and the treatment consists of a single dose of 0.4 mcg/kg of desmopressin for nonoperative head-injured patients. For patients who are candidate to surgery, 2 or 3 doses of platelets can help to restore platelet reactivity in case of concomitant therapy with clopidogrel or clopidogrel and aspirin, respectively.
Question 5: In antiplatelet patients in whom surgery cannot be postponed over 12–24 h, do you routinely utilize desmopressin?
The vast majority of responders (91%) answered “No” while the others answered “Yes.”
Desmopressin is a synthetic analogue of arginine vasopressin and increases plasma levels of factor VIII and von Willebrand factor, shortening bleeding time in patients who were taking an ADP antagonist. Desmopressin has demonstrated efficacy in restoring platelet function in patients taking antiplatelet medications [27]. Side effects of DDAVP include facial flushing, peripheral edema, hypervolemia, decreased urine output, and hyponatremia. Particular care should be applied in patients with recent ischemic stroke or acute myocardial infarction due to the risk of cerebrovascular or cardiac thrombosis (< 1%) with DDAVP use [14, 20]. Some authors [14, 34] suggest consideration of a single dose of desmopressin (0.4 mcg/kg) in intracranial hemorrhage patients exposed to antiplatelet agents. In antiplatelet patients undergoing neurosurgical procedure, desmopressin can be used in addition to platelet transfusion; however in the recent Australian consensus statement, authors stated that they cannot provide clear recommendation for or against the use of DDAVP in hemorrhagic TBI patients because of the paucity of supporting data [35].
Point of care in neurosurgery
Question 6: In antiplatelet patients in whom surgery cannot be postponed over 12–24 h, which platelet function assay do you perform and how frequently (Fig. 1)? For every option listened (“we do not perform any evaluation,” “PFA-100,” “Vasodilator-Stimulated Phosphoprotein Phosphorylation Assay (VASP assay),” “VerifyNow® assay,” “Multiplate® platelet function analysis,” and “platelet aggregometry test), responders had to choose among “routinely,” “frequently,” “rarely,” and “never.”
Question 7: In antiplatelet patients in whom surgery can be postponed, which platelet function assay do you perform and how frequently (Fig. 2)? For every option listened (“we do not perform any evaluation,” “PFA-100 (Platelet Function Assay),” “VASP assay,” “VerifyNow® assay,” “Multiplate® platelet function analysis,” and “platelet aggregometry test), choices among “routinely,” “frequently,” “rarely,” and “never” were provided.
Interestingly, for the question 6, the answer “we do not perform any evaluation” was chosen as “routinely” by 41% of responders. The platelet aggregometry test was most common “routinely” used (20%), but 31% and 46% of responders utilize this test “rarely” and “never,” respectively. All other platelet function analysis was “never “utilized in situation of urgent surgery by at least 69% of responders (Fig. 3).
Question 8: In antiplatelet patients (aspirin) in whom surgery can be postponed, how long do you wait before planning surgery? Question 9: In patients on antiplatelet therapy other that aspirin (P2Y12 receptor inhibitors) in whom surgery can be postponed, how long do you wait before planning surgery?
Similarly, for planned surgery (question 7), the answer “we do not perform any evaluation” was chosen as “routinely” by 39% of responders. The platelet aggregometry test was “routinely” used by 3% and “frequently” used by 16%), but 22% and 59% of responders utilize this test “rarely” and “never,” respectively. All other platelet function analysis was “never” utilized in situation of planned surgery by the vast majority of responders.
Standard laboratory analyses are used in patients with TBI to evaluate the degree of anticoagulation due to vitamin K antagonists (i.e., warfarin) as well as guide goal-directed reversal of the anticoagulatory effects of these medications to acceptable subtherapeutic levels. Unfortunately, these routine coagulation tests are insufficient to evaluate platelet activity and guide reversal therapy in TBI patients on antiplatelet therapy.
Differently from routine coagulation assays, thromboelastography provides a kinetic assessment of coagulation and clot formation, reflecting the contribution of clotting factors, platelet function, and red blood cells [4].
Thromboelastography is clinically used to accurately characterize coagulopathy, but limited data exist regarding the utility of traumatic brain injury. According to Rao et al. [26], admission thromboelastography values did not predict progression of traumatic intracranial hemorrhage, and caution has to be paid against relying on thromboelastography to guide clinical decisions related to progression of traumatic intracranial hemorrhage.
Platelet function analyzer (PFA-100; Siemens Healthcare Diagnostics, Deerfield, IL) is a test that detects quantitative and qualitative platelet anomalies. This test simulates platelet adhesion/aggregation in a blood vessel. The PFA-100 system is sensitive to detect aspirin therapy. Karger et al. [18] found that the correction of a platelet function deficit did not reduce bleeding complications and transfusion requirements. Which patients may actually benefit from routine preoperative testing of platelet function has remained an open question.
VerifyNow (Accumetrics, San Diego, CA) is an assay that provides rapid measurement of platelet responsiveness of antiplatelet therapies. The assays enable platelet function testing for patients taking aspirin, P2Y12 inhibitors (clopidogrel, ticlopidine, or prasugrel), and IIb/IIIa inhibitors (abciximab or eptifibatide) and have demonstrated nearly 100% sensitivity and 96% specificity for the detection of antiplatelet [3].
In general population, approximately 1 out 4 patients taking aspirin and 1 out 5 patients taking clopidogrel have been reported to show biochemical resistance [17, 30]. Conversely, platelet dysfunction in patients without a history of antiplatelet use in the setting of traumatic brain injury is not uncommon. Actually, the majority of traumatic brain injured patient have VerifyNow results compatible with antiplatelets regardless of a history of antiplatelet use. This is explained by several factors that may influence platelet function at the time of injury and that cause a transient coagulopathy. The study of Parry et al. [25] showed an effective utility of the test in the TBI population but suggested that cutoff thresholds for therapeutic platelets based on the cardiac literature may not be valid in the traumatic brain injured population. Further work to validate the utility of the VerifyNow assays in the TBI population as a prognostic and management tool is required.
The Multiplate analyzer (Roche Diagnostics International, Rotkreuz,Switzerland) allows platelet aggregation to be measured after addition of commonly used agonists. This method is used for diagnosing platelet defects and monitoring aspirin and clopidogrel.
Beynon showed that the Multiplate® analyzer may represent a valuable tool in the management of patients undergoing urgent neurosurgical therapy on antiplatelet therapy. It allows an immediate assessment of the effects of hemostatic measures to restore platelet activity if necessary. According to Beynon experience, Multiplate® analysis is sensitive of hemostatic drug administration or platelet transfusion, reporting an improvement of platelet activity at repeated test. Multiplate® analyzer may also represent a valuable device in non-urgent, elective neurosurgery because assessment of antiplatelet activity may reduce the time until surgery can be initiated and it also validates normal platelet activity prior to starting neurosurgical care, reducing the risk of thromboembolic complications during antiplatelet medication withdrawal on one side and on the other side the risk of bleeding complications during surgery.
Testing for clopidogrel can also be done using the flow cytometric vasodilator-stimulated phosphoprotein phosphorylation.
VASP assay: The VASP assay aims at the specific intraplatelet pathway blocked by clopidogrel. Clopidogrel is an antiplatelet prodrug, whose active metabolite inhibits platelet function by irreversible binding to the (adenosine diphosphate) platelet receptor P2Y12. VASP is an intracellular platelet protein which is non-phosphorylated at basal state. Since its relation in cascade with P2Y12 receptor, VASP phosphorylation correlates with inhibition of P2Y12 which is the receptor of prime importance in adenosine diphosphate receptor (ADP)-mediated activation of platelets and is primary target of ADP inhibitors’ action. Outcome of the assay is represented as the value of platelet reactivity index. VASP flow cytometric assay is emerging as the most promising and most sensitive method to detect patients with suboptimal response. This method is strongly correlated with the inhibition of ADP-induced platelet aggregation due to in vitro specific P2Y12 blockade.
In conclusion, platelet function tests seem promising in detecting the presence of platelet inhibitor in both emergency and planned neurosurgery; more studies are needed to demonstrate the usefulness of these tests in neurosurgical patients.
Perioperative management
Question 8: In antiplatelet patients (aspirin) in whom surgery can be postponed, how long do you wait before planning surgery?
Question 9: In patients on antiplatelet therapy other that aspirin (P2Y12 receptor inhibitors) in whom surgery can be postponed, how long do you wait before planning surgery?
For question 7, the answer “5–7 days” was chosen by the majority of responders (77%), “less than 5 days” was answered by 15%, and the others (5%) answered “7–10 days.” On the other hand, for question 8, the answer “7–10 days” was chosen by the majority of responders (53%), while “5–7 days” was answered by 34% (Fig. 3).
Guidelines for antiplatelet use in elective neurosurgery are also scarce. Clearly, a risk is associated with both the choice to continue antiplatelet agents during surgery due to increased bleeding in neurosurgery and the discontinuation of antiplatelets prior to surgery because of the risk of thrombosis.
Generally, it should be stated that in the setting of neurosurgery, discontinuation is likely the right decision nearly every time. In fact, it is improbable that in patients without stents, the risk of cardiac events will balance the risk of bleeding.
Since aspirin irreversibly inhibits circulating platelets, it should be suspended about 5–7 days (some suggest a period of 7–10 days) before the scheduled surgery [20]. Regarding P2Y12 receptor inhibitors, prasugrel should be discontinued at least 7 days before surgery, clopidogrel at least 5 days before surgery, and ticagrelor at least 3 days before surgery [22].
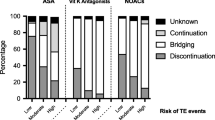
A challenging dilemma is represented by patient candidates for elective neurosurgery under antiplatelet therapy for recent stent implant. In these patients, the risk of stent thrombosis may outweigh the risk of bleeding, and it should be individually estimated in teamwork with a cardiologist. The type of stent used, the time from stent placement and from cardiac events, and the antiplatelet agents used have to be accurately evaluated [28]. Bare metal stents are fully endothelialized in 4–6 weeks; during this time, there is the highest risk of thrombosis. The risk is further increased when antiplatelet therapy is suddenly discontinued. Because of this reason, any type of not-deferrable surgery in patients with new stent placement should be performed at least 1 month after placement, nevertheless maintaining at least the therapy with acetylsalicylic acid in the postoperative period [22]. If particular clinical-angiographic risk factors are present (e.g., patients with stent placement for recent acute coronary syndrome), surgery should be postponed to at least 6 months [22].
Regarding the specific management of the neurosurgical patient, the hemorrhagic risk related to the planned treatment should be considered. A useful stratification of the bleeding risk and the indication for the management of antiplatelet therapy in patient with a recent stent implant is provided by Rossini et al. [28]). In the case of intermediate bleeding risk, it should be reasonable to postpone the intervention in cases of concomitant intermediate/high thrombotic risk, while if the intervention is not deferrable, the advice is to maintain the therapy with ASA in the perioperative period suspending instead the P2Y12 receptor inhibitor. In the case of low thrombotic risk, the double antiplatelet therapy can be suspended and resumed within 24–72 h from the intervention with a loading dose. In the case of surgery at high bleeding risk, it is suggested to postpone the surgery if the thrombotic risk is intermediate/high (if the thrombotic risk is low, antiplatelet therapy can be suspended). If surgery is not deferrable, the antiplatelet therapy must be suspended. Finally, in surgery at low bleeding risk (e.g., surgery for lumbar disk herniation, simple laminectomy, placement of an external ventricular drainage), surgery should be postponed if the thrombotic risk is intermediate/high. If surgery is not deferrable, the P2Y12 receptor inhibitor must be suspended.
Question 10: Do you usually ask for a cardiological evaluation for the perioperative management of antiplatelet patients?
Most of the participants usually request a cardiological evaluation (68%).
In antiplatelet patients in whom surgery is required, a consensus decision among treating clinicians as to the relative risks of surgery and discontinuation or continuation of antiplatelet therapy can be useful, especially in the eventuality of complex cases (e.g., patients with a recent percutaneous coronary intervention) [37].
Conclusion
There is a considerable lack of consensus regarding management of antiplatelet therapy in neurosurgery, with critical impact on patient’s treatment. What is clearly evident from the present survey is the considerable variability in neurosurgical care for antiplatelet patients; it is reasonable to assume that this scenario reflects the paucity of evidence regarding this issue.
References
Baharoglu MI, Cordonnier C, Salman RA-S, de Gans K, Koopman MM, Brand A, Majoie CB, Beenen LF, Marquering HA, Vermeulen M, Nederkoorn PJ, de Haan RJ, Roos YB, Investigators PATCH (2016) Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): a randomised, open-label, phase 3 trial. Lancet 387:2605–2613. https://doi.org/10.1016/S0140-6736(16)30392-0
Baschin M, Selleng S, Zeden JP, Westphal A, Kohlmann T, Schroeder HW, Greinacher A, Thiele T (2017) Platelet transfusion to reverse antiplatelet therapy before decompressive surgery in patients with intracranial haemorrhage. Vox Sang. https://doi.org/10.1111/vox.12542
Blais N, Pharand C, Lordkipanidzé M, Sia YK, Merhi Y, Diodati JG (2009) Response to aspirin in healthy individuals. Cross-comparison of light transmission aggregometry, VerifyNow system, platelet count drop, thromboelastography (TEG) and urinary 11-dehydrothromboxane B(2). Thromb Haemost 102:404–411. https://doi.org/10.1160/TH09-02-0126
Bolliger D, Seeberger MD, Tanaka KA (2012) Principles and practice of thromboelastography in clinical coagulation management and transfusion practice. Transfus Med Rev 26:1–13. https://doi.org/10.1016/j.tmrv.2011.07.005
Brouwers HB, Greenberg SM (2013) Hematoma expansion following acute intracerebral hemorrhage. Cerebrovasc Dis
Chen C-J, Ding D, Buell TJ, Testai FD, Koch S, Woo D, Worrall BB (2018) Restarting antiplatelet therapy after spontaneous intracerebral hemorrhage. Neurology. https://doi.org/10.1212/WNL.0000000000005742
Collins R, Peto R, Hennekens C, Doll R, Bubes V, Buring J, Dushkesas R, Gaziano M, Brennan P, Meade T, Rudnicka A, Hansson L, Warnold I, Zanchetti A, Avanzini F, Roncaglioni MC, Tognoni G, Chown M, Baigent C, Barton I, Baxter A, Bhala N, Blackwell L, Boreham J, Bowman L, Buck G, Emberson J, Godwin J, Halls H, Holland L, Kearney P, Reith C, Wilson K, Patrono C (2009) Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. https://doi.org/10.1016/S0140-6736(09)60503-1
Creutzfeldt CJ, Weinstein JR, Longstreth WT, Becker KJ, McPharlin TO, Tirschwell DL (2009) Prior antiplatelet therapy, platelet infusion therapy, and outcome after intracerebral hemorrhage. J Stroke Cerebrovasc Dis. https://doi.org/10.1016/j.jstrokecerebrovasdis.2008.10.007
Cucchiara B, Messe S, Sansing L, Kasner S, Lyden P (2008) Hematoma growth in oral anticoagulant related intracerebral hemorrhage. Stroke. https://doi.org/10.1161/STROKEAHA.108.520668
Davis SM, Broderick J, Hennerici M, Brun NC, Diringer MN, Mayer SA, Begtrup K, Steiner T (2006) Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. https://doi.org/10.1212/01.wnl.0000208408.98482.99
Douros A, Renoux C, Yin H, Filion KB, Suissa S, Azoulay L (2019) Concomitant use of direct oral anticoagulants with antiplatelet agents and the risk of major bleeding in patients with nonvalvular atrial fibrillation. Am J Med 132:191–199.e12. https://doi.org/10.1016/j.amjmed.2018.10.008
Ducruet AF, Hickman ZL, Zacharia BE, Grobelny BT, DeRosa PA, Landes E, Lei S, Khandji J, Gutbrod S, Connolly ES (2010) Impact of platelet transfusion on hematoma expansion in patients receiving antiplatelet agents before intracerebral hemorrhage. Neurol Res. https://doi.org/10.1179/174313209X459129
Flaherty ML, Kissela B, Woo D, Kleindorfer D, Alwell K, Sekar P, Moomaw CJ, Haverbusch M, Broderick JP (2007) The increasing incidence of anticoagulant-associated intracerebral hemorrhage. Neurolo. https://doi.org/10.1212/01.wnl.0000250340.05202.8b
Frontera JA, Lewin JJ, Rabinstein AA, Aisiku IP, Alexandrov AW, Cook AM, del Zoppo GJ, Kumar MA, Peerschke EIB, Stiefel MF, Teitelbaum JS, Wartenberg KE, Zerfoss CL (2016) Guideline for reversal of antithrombotics in intracranial hemorrhage: a statement for healthcare professionals from the Neurocritical Care Society and Society of Critical Care Medicine. Neurocrit Care. https://doi.org/10.1007/s12028-015-0222-x
Gaist D, Garcia Rodriguez LA, Hellfritzsch M, Poulsen FR, Halle B, Hallas J, Pottegard A (2017) Association of antithrombotic drug use with subdural hematoma risk. JAMA. https://doi.org/10.1001/jama.2017.0639
Green A, Pottegård A, Broe A, Diness TG, Emneus M, Hasvold P, Gislason GH (2016) Initiation and persistence with dual antiplatelet therapy after acute myocardial infarction: a Danish nationwide population-based cohort study. BMJ Open 6:e010880. https://doi.org/10.1136/bmjopen-2015-010880
Hovens MMC, Snoep JD, Eikenboom JCJ, van der Bom JG, Mertens BJA, Huisman MV (2007) Prevalence of persistent platelet reactivity despite use of aspirin: a systematic review. Am Heart J 153:175–181. https://doi.org/10.1016/j.ahj.2006.10.040
Karger R, Reuter K, Rohlfs J, Nimsky C, Sure U, Kretschmer V (2012) The platelet function analyzer (PFA-100) as a screening tool in neurosurgery. ISRN Hematol 2012:1–7. https://doi.org/10.5402/2012/839242
Li C, Hirsh J, Xie C, Johnston MA, Eikelboom JW (2012) Reversal of the anti-platelet effects of aspirin and clopidogrel. J Thromb Haemost. https://doi.org/10.1111/j.1538-7836.2012.04641.x
Loftus CM (2016) Anticoagulation and hemostasis in neurosurgery
Lovelock C, Molyneux A, Rothwell P (2007) Change in incidence and aetiology of intracerebral haemorrhage in Oxfordshire, UK, between 1981 and 2006: a population-based study. Lancet Neurol. https://doi.org/10.1016/S1474-4422(07)70107-2
Moťovská Z, Varvařovský I, Ošťádal P (2017) 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. Summary of the document prepared by the Czech Society of Cardiology. Cor Vasa
Moussouttas M, Malhotra R, Fernandez L, Maltenfort M, Holowecki M, Delgado J, Lawson N, Badjatia N (2010) Role of antiplatelet agents in hematoma expansion during the acute period of intracerebral hemorrhage. Neurocrit Care 12:24–29. https://doi.org/10.1007/s12028-009-9290-0
Naidech AM, Jovanovic B, Liebling S, Garg RK, Bassin SL, Bendok BR, Bernstein RA, Alberts MJ, Batjer HH (2009) Reduced platelet activity is associated with early clot growth and worse 3-month outcome after intracerebral hemorrhage. Stroke. https://doi.org/10.1161/STROKEAHA.109.550939
Parry PV, Choi PA, Bauer JS, Panczykowski DM, Puccio AM, Okonkwo DO (2016) Utility of the aspirin and P2Y12 response assays to determine the effect of antiplatelet agents on platelet reactivity in traumatic brain injury. Neurosurgery 1. https://doi.org/10.1227/NEU.0000000000001406
Rao A, Lin A, Hilliard C, Fu R, Lennox T, Barbosa R, Schreiber M, Rowell S (2017) The utility of thromboelastography for predicting the risk of progression of intracranial hemorrhage in traumatic brain injury patients. Neurosurgery 64:182–187. https://doi.org/10.1093/neuros/nyx210
Reiter RA, Mayr F, Blazicek H, Galehr E, Jilma-Stohlawetz P, Domanovits H, Jilma B (2003) Desmopressin antagonizes the in vitro platelet dysfunction induced by GPIIb/IIIa inhibitors and aspirin. Blood 102:4594–4599. https://doi.org/10.1182/blood-2002-11-3566
Rossini R, Tarantini G, Musumeci G, Masiero G, Barbato E, Calabrò P, Capodanno D, Leonardi S, Lettino M, Limbruno U, Menozzi A, Marchese UOA, Saia F, Valgimigli M, Ageno W, Falanga A, Corcione A, Locatelli A, Montorsi M, Piazza D, Stella A, Bozzani A, Parolari A, Carone R, Angiolillo DJ (2018) A multidisciplinary approach on the perioperative antithrombotic management of patients with coronary stents undergoing surgery: surgery after stenting 2. JACC Cardiovasc Interv
Sansing LH, Messe SR, Cucchiara BL, Cohen SN, Lyden PD, Kasner SE (2009) Prior antiplatelet use does not affect hemorrhage growth or outcome after ICH. Neurology. https://doi.org/10.1212/01.wnl.0000342709.31341.88
Snoep JD, Hovens MMC, Eikenboom JCJ, van der Bom JG, Jukema JW, Huisman MV (2007) Clopidogrel nonresponsiveness in patients undergoing percutaneous coronary intervention with stenting: a systematic review and meta-analysis. Am Heart J 154:221–231. https://doi.org/10.1016/j.ahj.2007.04.014
Taylor G, Osinski D, Thevenin A, Devys JM (2013) Is platelet transfusion efficient to restore platelet reactivity in patients who are responders to aspirin and/or clopidogrel before emergency surgery? J Trauma Acute Care Surg. https://doi.org/10.1097/TA.0b013e31828cca61
Tykocki T, Guzek K (2016) Anticoagulation therapy in traumatic brain injury. World Neurosurg
Uccella L, Zoia C, Bongetta D, Gaetani P, Martig F, Candrian C, Rosso R (2018) Are antiplatelet and anticoagulants drugs a risk factor for bleeding in mild traumatic brain injury? World Neurosurg. https://doi.org/10.1016/j.wneu.2017.10.173
Watson VL, Louis N, Seminara BV, Paul Muizelaar J, Alberico A (2017) Proposal for the rapid reversal of coagulopathy in patients with nonoperative head injuries on anticoagulants and/or antiplatelet agents: a case study and literature review. Neurosurgery
Wiegele M, Schöchl H, Haushofer A, Ortler M, Leitgeb J, Kwasny O, Beer R, Ay C, Schaden E (2019) Diagnostic and therapeutic approach in adult patients with traumatic brain injury receiving oral anticoagulant therapy: an Austrian interdisciplinary consensus statement. Crit Care 23:62. https://doi.org/10.1186/s13054-019-2352-6
Won S-Y, Dubinski D, Bruder M, Cattani A, Seifert V, Konczalla J (2017) Acute subdural hematoma in patients on oral anticoagulant therapy: management and outcome. Neurosurg Focus. https://doi.org/10.3171/2017.8.FOCUS17421
Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, Davila-Roman VG, Gerhard-Herman MD, Holly TA, Kane GC, Marine JE, Nelson MT, Spencer CC, Thompson A, Ting HH, Uretsky BF, Wijeysundera DN (2015) 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Developed in collaboration with the American College of Surgeons, American Society of Anesthesiologists, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Anesthesiologists, and Society of Vascular Medicine Endorsed by the Society of Hospital Medicine. J Nucl Cardiol 22(1):162–215. https://doi.org/10.1007/s12350-014-0025-z
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict(s) of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the IRCCS Ospedale Policlinico San Martino and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Statement of informed consent
Informed consent was obtained from all the patients included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Fig. 1
Question 1: In the last year, indicate the approximate percentage of patients admitted in your department with “acute” neurosurgical indication. Possible answers: less than 10%, between 10% and 25%, between 25% and 50%, between 50% and 75%, and more than 75% (JPG 41 kb)
Supplementary Fig. 2
Question 2: Indicate the approximate percentage of patients admitted at your department in the last year with “acute” neurosurgical indication (e.g., acute/chronic epidural-subdural hematomas, ICH, SAH, traumatic subarachnoid hemorrhage), in therapy with under association of antiplatelet and anticoagulant therapy (JPG 39 kb)
Rights and permissions
About this article
Cite this article
Fiaschi, P., Iaccarino, C., Stefini, R. et al. Clinical practice for antiplatelet and anticoagulant therapy in neurosurgery: data from an Italian survey and summary of current recommendations – part I, antiplatelet therapy. Neurosurg Rev 44, 485–493 (2021). https://doi.org/10.1007/s10143-019-01229-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-019-01229-7