Abstract
Patients with moyamoya angiopathy (MMA) are known to have an increased risk of impaired executive function (dysexecutive cognitive syndrome (DCS)). Numbers of moyamoya patients with DCS vary strongly in the literature; evidence of a correlation to affected vascular territories is low. This study aims to identify cognitive impairment in adult moyamoya patients and to correlate findings with imaging results. In addition, the predictive value of individual tests for the identification of DCS was analyzed. Neuropsychological test data of 41 adult moyamoya patients was analyzed for a possible correlation with territorial hypoperfusion on H215O PET with acetazolamide (ACZ) challenge (cerebrovascular reserve—CVR) and infarction patterns observed in MRI. Each vascular territory was analyzed separately and correlated to neuropsychological test results and to the presence of DCS. In total, 41.5% of patients presented with DCS. Significant association of DCS and affection of the right middle cerebral artery (MCA) territory was seen for insufficient CVR in PET (p = 0.030) and for patients with infarctions seen in MRI (p = 0.014). Analysis of individual neuropsychological test results confirmed the main association with the right MCA territory, as well as some association with the right anterior cerebral artery (ACA) territory. Analysis of a subgroup of patients with chronic disease on MRI (presence of large post-infarction gliosis and brain atrophy in affected territories) revealed a significantly higher risk for DCS (85% affected) than non-chronic patients (21% affected) (p < 0.001). Analysis of neuropsychological test data in this moyamoya cohort reveals DCS in 41.5% of all patients. Correlation between DCS and an impairment of CVR seen in PET and/or infarctions seen in MRI was significant for the right MCA territory. Patients with chronic disease had a significantly higher risk for DCS than non-chronic patients (p < 0.001).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with moyamoya angiopathy (MMA) radiographically show bilateral stenosis of the Circle of Willis with fine collateral networks resembling a puff of smoke, giving the disease its name [1]. Initial symptoms are transient ischemic attacks (TIAs), ischemic strokes, headache, epilepsy, or hemorrhage [2]. Additionally, patients may suffer from subtle neuropsychological deterioration caused by chronic cerebral hypoperfusion, even without having strokes [3, 4]. Recognition and understanding of these changes are of great importance as a progressive cognitive decline might be preventable and even partly reversible with timely extracranial–intracranial revascularization procedures [5, 6]. Identification of impaired cerebrovascular reserves (CVR) may be performed with H215O positron emission tomography (PET) or 99mTc-labeled hexamethylpropyleneamine oxime (99mTc-HMPAO) single photon emission tomography (SPECT) with acetazolamide (ACZ) challenge. However, PET seems to be more sensitive in detecting restricted perfusion reserves [7, 8]. Several studies on neuropsychological testing of adult moyamoya patients before and after revascularization surgery or with only conservative treatment have been published [3, 9]. However, none of these studies specifically focused on the relationship between territorial perfusion reserve deficits measured with H215O PET (impaired cerebrovascular reserve—CVR) and MRI findings in correlation to cognitive deficits. Also, evidence of neuropsychological deficits in European patients is very limited [10,11,12]. This study aims to identify a possible correlation of impaired executive function (dysexecutive cognitive syndrome (DCS)) in adult moyamoya patients with territorial cerebrovascular reserve impairment measured with H215O PET and infarction patterns seen in conventional MRI. Furthermore, neuropsychological tests were evaluated to identify highest predictive values for DCS.
Methods
A study of baseline neuropsychological assessment of consecutive adult patients treated at our Moyamoya Center was performed.
Main inclusion criteria were angiographically proven moyamoya, availability of cerebral MRI, H215O PET with ACZ challenge, and neuropsychological testing. These examinations are part of the routine workup of all newly admitted moyamoya patients at our center. All examinations had to be in chronological proximity of less than 6 months with no surgical intervention and no new symptoms or stroke events in between. Ethical approval was obtained from the University of Tübingen Ethics Committee. General patient data were obtained from the patients’ clinical files, any imaging data was stored and analyzed in the hospital’s PACS system.
Neuropsychological testing
Four different tests were performed to analyze parts of executive function: Trail making test A (psychomotor speed) and B (mental flexibility) (TMTA & TMTB), selective attention (D2) subdivided into speed and mistake, and Chapuis Labyrinth test (Ch-L) (problem solving) were performed in each patient. TMTA and TMTB were rated as pathological if patients’ z-score was worse than 1 SD from the normative mean data or if patients were not capable to finish the test. D2 was rated pathological if the score was below 90 (Z-Value worse than 1 SD). Results of D2 were separated in speed and general mistakes; different types of mistakes were not rated separately. Ch-L was rated pathological if the score was below 1 SD from the normative mean value. Existence of a dysexecutive cognitive syndrome (DCS) was defined if 2 or more of the abovementioned tests showed pathological results. This benchmark was adapted based on previous reports and fitted to our test battery [11]. All tests were performed under supervision of a board-certified neuropsychologist.
H215O PET with ACZ challenge and MRI
Cerebral perfusion reserve (CVR) was evaluated with PET for each vascular territory (ACA, MCA, and posterior cerebral artery (PCA)) for both hemispheres after ACZ challenge (dichotomized as sufficient CVR (0) or impaired CVR (1)) Further, PET was evaluated for perfusion deficits at baseline (normal (0) or decreased (1)). Acquisition of imaging was performed as described before [13] [14].
Quantification of ischemic lesions in MRI was done by analyzing fluid attenuated inversion recovery (FLAIR) and/or T2 sequences for every cerebrovascular territory. The rating was done as published before, but with some adaptations [7]. Ischemic lesions (cortical and/or subcortical) were rated as no lesions (0), < 3 lesions (1), ≥ 3 lesions (2), and confluence of lesions/territorial infarctions (3). For further detailed analysis, we divided patients into a chronic disease group if the MRI showed signs of old ischemic lesions with the presence of larger gliotic changes and resulting brain atrophy.
Analysis of MRI and CVR (C.R.) was performed blinded to the results of neuropsychological testing (P.H., M.F., and M.M.).
Data and statistical analysis
All data were stored and analyzed in JMP (SAS Institute Inc. 2018, Version 14.2.0, Cary, USA). Descriptive statistics were calculated with absolute numbers and percentages. Chi-squared and 2-tailed Fisher-Yates exact tests (in case of small sample size) were used for analysis of cognitive impairment in relation to cerebrovascular reserves and infarctions in MRI. Correlation was calculated with phi (ϕ) and rated small for ϕ ≤ 0.29, moderate for ϕ = 0.3–0.49, and strong for ϕ ≥ 0.5. The chi-squared test was used to analyze a possible impact of the size of infarction in MRI on neuropsychological deficits. Shapiro–Wilk test was performed as test of normality. Wilcoxon test was used to analyze a possible impact of quantity of impaired vascular territories related to neuropsychological deficits. Positive and negative predictive values were calculated to describe the likeliness of predicting neuropsychological deficits by analyzing perfusion reserves and infarction patterns in MRI. Level of statistical significance was p < 0.05. Given the exploratory nature of the present work, we performed no correction for multiple comparisons.
Results
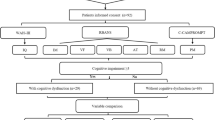
Forty-one patients (30 female, 11 male) fulfilled the inclusion criteria. The median age was 45 years with a range of 16–67 years. Majority (38 patients) of the patients were European Caucasians with only 3 patients of Asian descent. Bilateral angiopathy on conventional angiography was present in 32 patients, 7 patients had unilateral angiopathy. Two patients had moyamoya syndrome (1 patient with neurofibromatosis type 1 and 1 patient with multiple sclerosis) (Fig. 1).
Exemplary case of a 50-year old female patient with unilateral moyamoya angiopathy. The patient suffered a first episode of hemiparesis more than 10 years ago and was treated conservatively at an external hospital. After an extensive stroke with severe hemiparesis, the patient was admitted to our hospital for further treatment after she had recovered partly from her initial symptoms. MRI was categorized as chronic according to MRI. a FLAIR images, note the significant brain atrophy due to chronic hypoperfusion and recurrent infarctions in the ACA and MCA territories (grade 3). b Results of H215O PET at baseline and with ACZ challenge (upper row baseline, lower row after ACZ challenge). Limited baseline perfusion in the right ACA and MCA territories can be seen in the upper row, a lack of signal increase after ACZ application in the lower row indicating reduced CVR. This patient was diagnosed with a DCS with 3 of 4 tests showing pathological results. Surgical revascularization was scheduled
One hundred thirty-five cerebral arteries, i.e., vascular territories were seen to be involved on conventional angiography. Thirty-one patients were right-handed, 1 was left-handed, and 9 were ambidexters. All patients had their testing after the initial presentation at our Moyamoya Center.
Imaging data
All imaging data were of good quality and no data had to be excluded from the analysis. A total of 246 cerebral vascular territories were analyzed for PET and MRI, respectively. According to PET, 84 territories showed noticeable changes at baseline, 99 territories showed a reduced CVR after ACZ challenge. Infarctions were seen in 94 territories in MRI, of which 34 were grade 1, 49 grade 2, and 11 grade 3 (Fig. 2). General distribution of perfusion impairment and infarctions can be seen in Table 1. Chronic disease on MRI was seen in 13 patients. Exemplary cases can be found in Figs. 1 and 2.
Exemplary case of a 64-year old female patient with unilateral moyamoya angiopathy. The patient suffered from temporary brachio-facial paresis caused by small infarctions which recovered well. A disease-history of less than 1 year was known when she was admitted to our hospital for the first time. MRI findings were categorized as non-chronic according to MRI. a FLAIR images, note the age-related symmetric bilateral brain atrophy with only small infarctions in the MCA territory (grade 2) on the right side, in contrast to patient of Fig. 1. b Results of H215O PET at baseline and with ACZ challenge (upper row baseline, lower row after ACZ challenge). Limited baseline perfusion in both ACA and the right MCA territory can be seen in the upper row; a lack of signal increase after ACZ application in the lower row indicates a reduced CVR. This patient was diagnosed with a DCS with 3 of 4 tests showing pathological results. Surgical revascularization was scheduled
Neuropsychological testing
All patients completed the test battery, the respective neuropsychological data sets were complete and used without any exclusions. An overview of the test results for the entire cohort can be found in Table 2. No pathological test result was seen in 12 (29%), 1 pathological result in 12 (29%), 2 pathological results in 5 (12%), 3 pathological results in 3 (7%), and 4 pathological results in 9 (22%) patients. In total, 17 (41.5%) of our patients had a DCS (≥ 2 tests with pathological results).
Neuropsychological deficits in relation to perfusion seen in PET
Results of baseline PET (data not shown) did not reveal any statically significant results (besides p = 0.043 for the left PCA territory, which only affect 2 patients). CVR as determined by PET at baseline and after ACZ challenge showed significant association (p = 0.035, φ = 0.345) for the D2 test and the right MCA territory, as well as for DCS and the right MCA territory (p = 0.030, φ = 0.382). Further, a trend towards association of the right ACA territory was found for the TMTB (p = 0.060, φ = 0.310), D2 total (p = 0.060, φ = 0.310), and DCS (p = 0.062, φ = 0.310). ϕ values for all significant results were of moderate strength of effect. All results can be seen in Table 3. A tendency towards association (p = 0.057) with DCS was also seen for the correlation of total number of territories with impaired CVR.
Neuropsychological deficits in relation to infarctions seen in MRI
Neuropsychological testing and MRI data were analyzed to identify a possible relation of infarctions in each vascular territory. Significant association with DCS was seen in patients with infarctions in the right MCA territory (p = 0.014, φ = 0.400). The analysis of the extent of infarctions also showed a significant association to have a DCS (p = 0.004, φ = 0.542 (chi-squared test)) for the right MCA territory.
Further, significant association was seen in the right MCA territory for the TMTB (p = 0.034, φ = 0.346), D2 total (p = 0.034, φ = 0.346), and the speed as a part of D2 (p = 0.038, φ = 0.352). Infarctions in the right ACA territory were significantly associated with pathologic results in the TMTB test (p = 0.041, φ = 0.370) as well. ϕ values for all significant results were of moderate strength of effect, besides the association of the intensity of infarctions and DCS, which showed a strong strength of effect. All results can be seen in Table 4. The analysis of a possible correlation of DCS and the number of territories with infarctions seen in MRI showed a significant association (p = 0.026).
Neuropsychological deficits in comparison of patients with non-chronic vs. chronic disease
We have defined two subgroups of patients according to MRI. Six of 28 patients (21%) with suggested non-chronic disease showed a DCS, whereas in the group of patients with suggested chronic disease 11 of 13 patients (85%) had a DCS (p < 0.001, ϕ = 0.597). Analysis of individual results also revealed highly significant differences in all tests, besides for D2 attention test, with moderate or strong strength of effect (Table 5).
Positive and negative predictive values of CVR assessed by PET and infarctions on MRI in the right MCA territory for DCS
Reduced CVR on PET of the right MCA territory revealed an negative predictive value (NPV) = 1.0, positive predictive value (PPV) = 0.50 with a sensitivity of 1.0 and specificity of 0.29 for DCS. All other territories showed no relevant prediction for DCS (data not shown). Analysis of infarctions in the right MCA territory revealed an NPV = 0.91, PPV = 0.53 with a sensitivity of 0.94 and a specificity of 0.42 for DCS. The combined analysis of CVR deficits and infarctions seen in MRI revealed an NPV = 0.88, PPV = 0.62 with a sensitivity of 0.94 and specificity of 0.41 for DCS.
Predictive values of individual neuropsychological tests for DCS
Predictive values were calculated for individual tests to identify possible indicator-tests for DCS. Highest predictive values were found for TMTB with PPV = 0.93, NPV = 0.89, Sensitivity = 0.82, and Specificity = 0.96. TMTA, D2, and Ch-L revealed positive predictive values < 0.85 (data not shown, see electronic supplemental material).
Discussion
This is the first study to investigate a possible association between regional CVR deficits observed on H215O PET (baseline and ACZ challenge) and infarction patterns seen on MRI with DCS finding in neuropsychological testing of adult moyamoya patients. Analysis of our cohort showed that 41.5% of all moyamoya patients have DCS, which seems to be well averaged in comparison to other studies with comparable patient cohorts [3, 4, 11, 15, 16].
An existing analysis of 30 adult patients with angiographically proven MMD but no or only very small subcortical infarctions in MRI revealed significant cognitive impairment in 23% despite not having large parenchymal lesions in MRI. However, functional imaging data (such as PET, among others) was not analyzed in this study [4].
In our study, we therefore aimed to analyze a possible correlation of cognitive impairment in moyamoya patients with MRI and H215O PET data for each cerebral vascular territory to understand the possible impact and relation of disease affection seen in imaging data and DCS.
Our results reveal that there seems to be a strong association of executive function deficits with the right hemisphere mainly in the MCA territory. The results of the independent analysis of PET (p = 0.030, φ = 0.382) and MRI (p = 0.014, φ = 0.400) show comparable results.
High negative predictive values of 0.91 for MRI and 1.0 for PET show a very high likelihood to not have a DCS if the right MCA territory is not affected functionally by this disease. This goes along with a high sensitivity of 0.94 for MRI and 1.0 for PET to reveal a cognitive impairment if the right MCA territory is affected on PET or MR imaging in this territory. A possible bias due to an asymmetry of affected territories in the respective patients appears unlikely, as the distribution of infarctions and perfusion impairment is well balanced, as seen in Table 1. Further, the analysis of the handedness of patients in this cohort reveals that only one patient was left-handed, all other patients were right-handed or ambidexters.
The identification of subgroups with MRI-based suggested chronic disease revealed that an assumed long-term hypoperfusion without treatment over several years might result in a chronic damage of the brain. The chronic status can be seen as strong territorial brain atrophy in MRI. A highly significant (p < 0.001, ϕ = 0.597) association with chronic disease and impairment in neuropsychological testing compared with the group with suggested non-chronic disease (despite having two groups of different size) was identified in this study. This finding underlines the need for timely revascularization of patients with moyamoya, once perfusion reserves appear insufficient in functional imaging.
Several tests are available for neuropsychological testing. We have focused on executive function and chose TMTA, TMTB, D2, and Ch-L for this analysis. When looking at predictive values for the respective tests to possibly identify one test as the best screening tool for patients, TMTB revealed the best results with a PPV of 0.93, NPV of 0.89, a sensitivity of 0.82, and a specificity of 0.96. In our cohort, 37% of all patients revealed pathologic results (z-scores > 1 SD below the mean) for the TMTB, which is comparable to previous studies with 42% (Karzmark et al. [15], z-scores > 1 SD below the mean), 33% (Karzmark et al. [4], patients with no large infarctions in MRI, z-scores > 1 SD below the mean), and 39% (Festa et al. [16], z-scores > 1.5 SD below the mean). As discussed before, TMTB seems to be the test which might be most useful for screening, as also shown in our analysis [11]. Nevertheless, we think that these remarkably good results for TMTB do not justify it to be the only test used to identify DCS. Missing the diagnosis in even only few patients might not be acceptable, especially when limited efforts are required to perform a more extensive neuropsychological testing battery. It remains open to discussion whether the test battery with four tests used for executive function in this analysis might be sufficient and precise in comparison to much more extensive testing performed in other publications, as the fraction of patients diagnosed with DCS vary in comparable cohorts from 31–66% [11, 15, 16]. However, our results of diagnosed DCS (41.5%) seem to be comparable to the results of these other studies.
Our results show that an impaired blood flow or infarctions of the ACA and MCA territories especially of the right hemisphere may have significant impact for the development of DCS. To our knowledge, this has not been shown before. Pathological test results, especially in TMTB, might be an expression of the patient’s impaired ability of oculomotor control or a disturbance of flexibility in decision making caused by hypoperfusion at the level of the basal ganglia in the non-dominant hemisphere [17].
Further, it has been described that reduced CVR in frontal regions might be related to DCS [11]. A significant association of the right ACA territory (infarctions in MRI) for TMTB (p = 0.041, φ = 0.370) and a tendency towards significant association for DCS (p = 0.062, φ = 0.310), TMTB (p = 0.060, φ = 0.310), and D2 (p = 0.060, φ = 0.310) in PET after ACZ challenge were also seen in this cohort. However, the “ACA territory” and the “frontal cortex” are anatomically not identical areas and a direct comparison is not possible. Therefore, we think that the global assumption of neuropsychological impairment caused by moyamoya, irrespective of the analysis of infarction patterns and perfusion imaging for each vascular territory might not be sufficient and should be addressed with specific imaging-based studies in correlation to neuropsychological testing in the future.
Limitations
The size of the patient cohort is limited. Nevertheless, to our knowledge, it is the largest cohort of mostly European moyamoya patients with neuropsychological assessment and one of the largest cohorts compared with analysis from the U.S. with mostly Caucasian patients. PET data have not been quantified in absolute terms (i.e., perfusion in ml/min/100 mg), requiring arterial blood sampling, error-prone blood analyses, and sophisticated kinetic analyses. Instead, we used a non-invasive, semi-quantitative method [13, 14]. Because these methods should be well suited to judge CVR on an ordinal scale, we refrained from further in-depth analyses (e.g., correlations analyses relying on semi-quantified perfusion and CVR indices). Also baseline scans on PET have limited predictive values if not quantified and normalized. Weak signal changes and a lack of asymmetry in visual analysis might cause to miss some impaired territories. Further, we did not differentiate between cortical and subcortical infarctions, but for the entire vascular territory. Specific differentiation between cortical and subcortical infarctions might only be possible in larger cohorts with then resulting sufficient statistical power.
We included 2 patients with MMS (multiple sclerosis and neurofibromatosis). Both diseases may per se have an effect on neurocognition. However, our direct focus on association with imaging data is very likely not influenced by this. Also, 4 patients who had previously undergone unilateral cerebral revascularization in outside hospitals in the left MCA territory were not excluded from our final analysis because they still need further revascularization in at least one more territory. Furthermore, it remains uncertain how EC-IC revascularization influences neurocognitive functions in patients, as a recent study showed no significant changes in patients after revascularization [18].
As we have analyzed preoperative patient data only, this study does not answer the question whether surgical revascularization might improve or worsen neurocognitive deficits over time. This question must be addressed once longitudinal data will be available in the future. Duration of illness (i.e., time between the origin of angiopathy and first presentation and/or time at revascularization surgery) varies among patients and is difficult to evaluate correctly. A state of chronic hypoperfusion might possibly result in accentuated neuropsychological deficits over time. The significant relationship between burden of infarction and brain atrophy seen on MRI and DCS does suggest that chronic disease may be a main DCS-causing factor.
Conclusion
Neuropsychological test results in correlation to perfusion imaging with H215O PET with ACZ challenge and infarction patterns on MRI of 41 moyamoya patients were analyzed. A total of 41.5% of all moyamoya patients were affected by DCS. Results revealed a strong association of imaging findings on PET and MRI and a DCS in the right MCA and ACA territory. Further, chronic disease defined by brain atrophy and gliosis secondary to major infarctions seen on MRI is significantly associated with DCS (p < 0.001, φ = 0.600) compared with patients with non-chronic moyamoya.
References
Suzuki J, Takaku A (1969) Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol 20(3):288–299
Scott RM, Smith ER (2009) Moyamoya disease and moyamoya syndrome. N Engl J Med 360(12):1226–1237. https://doi.org/10.1056/NEJMra0804622
Kronenburg A, van den Berg E, van Schooneveld MM, Braun KPJ, Calviere L, van der Zwan A, Klijn CJM (2018) Cognitive functions in children and adults with moyamoya vasculopathy: A systematic review and meta-analysis. Journal of stroke 20(3):332–341. https://doi.org/10.5853/jos.2018.01550
Karzmark P, Zeifert PD, Bell-Stephens TE, Steinberg GK, Dorfman LJ (2012) Neurocognitive impairment in adults with moyamoya disease without stroke. Neurosurgery 70(3):634–638. https://doi.org/10.1227/NEU.0b013e3182320d1a
Yanagihara W, Chida K, Kobayashi M, Kubo Y, Yoshida K, Terasaki K, Ogasawara K (2018) Impact of cerebral blood flow changes due to arterial bypass surgery on cognitive function in adult patients with symptomatic ischemic moyamoya disease. J Neurosurg:1–9. https://doi.org/10.3171/2018.7.jns18149
Baek HJ, Chung SY, Park MS, Kim SM, Park KS, Son HU (2014) Preliminary study of neurocognitive dysfunction in adult moyamoya disease and improvement after superficial temporal artery-middle cerebral artery bypass. Journal of Korean Neurosurgical Society 56(3):188–193. https://doi.org/10.3340/jkns.2014.56.3.188
Roder C, Burkle E, Ebner FH, Tatagiba M, Ernemann U, Buck A, Meyer PT, Khan N (2018) Estimation of severity of moyamoya disease with [(15)O]water-positron emission tomography compared with magnetic resonance imaging and angiography. World Neurosurg 117:e75–e81. https://doi.org/10.1016/j.wneu.2018.05.163
Acker G, Lange C, Schatka I, Pfeifer A, Czabanka MA, Vajkoczy P, Buchert R (2018) Brain perfusion imaging under acetazolamide challenge for detection of impaired cerebrovascular reserve capacity: positive findings with 15O-water PET in patients with negative 99mTc-HMPAO SPECT findings. J Nucl Med 59(2):294–298. https://doi.org/10.2967/jnumed.117.195818
Weinberg DG, Rahme RJ, Aoun SG, Batjer HH, Bendok BR (2011) Moyamoya disease: functional and neurocognitive outcomes in the pediatric and adult populations. Neurosurg Focus 30(6):E21. https://doi.org/10.3171/2011.3.Focus1150
Calviere L, Ssi Yan Kai G, Catalaa I, Marlats F, Bonneville F, Larrue V (2012) Executive dysfunction in adults with moyamoya disease is associated with increased diffusion in frontal white matter. J Neurol Neurosurg Psychiatry 83(6):591–593. https://doi.org/10.1136/jnnp-2011-301388
Calviere L, Catalaa I, Marlats F, Viguier A, Bonneville F, Cognard C, Larrue V (2010) Correlation between cognitive impairment and cerebral hemodynamic disturbances on perfusion magnetic resonance imaging in European adults with moyamoya disease. Clinical article. J Neurosurg 113(4):753–759. https://doi.org/10.3171/2010.4.jns091808
Savolainen M, Mustanoja S, Pekkola J, Tyni T, Uusitalo AM, Ruotsalainen S, Poutiainen E, Hernesniemi J, Kivipelto L, Tatlisumak T (2018) Moyamoya angiopathy: long-term follow-up study in a Finnish population. J Neurol. 266:574–581. https://doi.org/10.1007/s00415-018-9154-7
Arigoni M, Kneifel S, Fandino J, Khan N, Burger C, Buck A (2000) Simplified quantitative determination of cerebral perfusion reserve with H215O PET and acetazolamide. Eur J Nucl Med Mol Imaging 27(10):1557–1563
Hauser TK, Seeger A, Bender B, Klose U, Thurow J, Ernemann U, Tatagiba M, Meyer PT, Khan N, Roder C (2019) Hypercapnic BOLD MRI compared to H215O PET/CT for the hemodynamic evaluation of patients with moyamoya disease. NeuroImage Clinical 22:101713. https://doi.org/10.1016/j.nicl.2019.101713
Karzmark P, Zeifert PD, Tan S, Dorfman LJ, Bell-Stephens TE, Steinberg GK (2008) Effect of moyamoya disease on neuropsychological functioning in adults. Neurosurgery 62(5):1048–1051; discussion 1051-1042. https://doi.org/10.1227/01.neu.0000325866.29634.4c
Festa JR, Schwarz LR, Pliskin N, Cullum CM, Lacritz L, Charbel FT, Mathews D, Starke RM, Connolly ES, Marshall RS, Lazar RM (2010) Neurocognitive dysfunction in adult moyamoya disease. J Neurol 257(5):806–815. https://doi.org/10.1007/s00415-009-5424-8
Karnath H-O, Thier P (2006) Neuropsychologie. Springer
Zeifert PD, Karzmark P, Bell-Stephens TE, Steinberg GK, Dorfman LJ (2017) Neurocognitive performance after cerebral revascularization in adult moyamoya disease. Stroke 48(6):1514–1517. https://doi.org/10.1161/strokeaha.116.016028
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethics standards. As to the retrospective character of this analysis, no specific formal consent was obtained.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 20 kb)
Rights and permissions
About this article
Cite this article
Roder, C., Haas, P., Fudali, M. et al. Neuropsychological impairment in adults with moyamoya angiopathy: preoperative assessment and correlation to MRI and H215O PET. Neurosurg Rev 43, 1615–1622 (2020). https://doi.org/10.1007/s10143-019-01192-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-019-01192-3