Abstract
To perform a systematic review of the techniques for transient circulatory arrest during intracerebral aneurysm surgery according to the PRISMA guidelines. Search of PubMed and Google Scholar using the following: (“heart arrest” OR “cardiac standstill”[All Fields]) AND (“intracranial aneurysm” OR “intracranial”[All Fields] AND “aneurysm”[All Fields]). A total of 41 original articles were retrieved, of which 17 were excluded (review articles, editorials and single-case reports). A total of 24 separate articles published between 1984 and 2018 were included in the final analysis, where the majority of patients harbored anterior circulation giant or large aneurysms. Adenosine-induced cardiac arrest gave a short, temporary asystole. The method had benefits in aneurysm with a broad neck, a thin wall, in specific localizations with narrow surgical corridors or in case of intraoperative rupture. Rapid ventricular pacing (RVP) allows a longer and more easily controlled hypotension. Its use is largely limited to elective cases. Deep hypothermic circulatory arrest required a complex infrastructure, and fatal procedure complications lead to a 11.5–30% 30-day mortality rate, limiting its application to giant or complex aneurysm of the basilar artery or to residual posterior circulation aneurysm after endovascular treatment. Adenosine and RVP are both effective options to facilitate clipping of complex aneurysms. However, their use in patient with ischemic heart disease and cardiac arrhythmias should be avoided, and their safety in the context of subarachnoid hemorrhage is yet to be determined. Today, deep hypothermic circulatory arrest is almost obsolete due to endovascular alternatives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aneurysm surgery can be challenging and requires several steps prior to permanent clip application (Fig. 1) [1]. It classically involves establishment of proximal control and temporary clipping to soften the aneurysm sack, in order to safely dissect the aneurysm neck and to achieve optimal permanent clip positioning (Fig. 2) [1]. However, the use of temporary clips during aneurysm surgery may expose the patient to vessel injury, thromboembolic stroke, and aneurysm rupture [1]. In complex aneurysm surgery, temporary clipping is more challenging as it can require several clips to control both the afferent and efferent arteries (trapping) (Fig. 2) [1, 2]. Moreover, these temporary clips take up space and may obstruct the surgeon’s view during the placement of a permanent clip [2]. Lastly, temporary arterial occlusion is difficult in some situations, including the aneurysm of the paraclinoid internal carotid artery (ICA), and in deeply located aneurysm, such as in the basilar artery [2].
In contrast, aneurysm softening can also be done by lowering the mean systemic arterial blood pressure (Fig. 3) [1]. Transient cardiac standstill allows a controlled hypotension, in order to soften the aneurysm sack, to decrease the risk of rupture and bleeding and finally facilitate the permanent clipping. This is particularly important during surgery of giant or large aneurysm, when the use of temporary clipping is technically impossible [2].
However, the ideal method of transient cardiac standstill should have predictable effects, few side effects, be simple to use, titrable, and carry a low risk of procedure-related complications. In the literature, three options are described, namely, adenosine-induced hypotension, rapid ventricular pacing (RVP), and cardiac standstill during hypothermia (Fig. 3) [1]. The objective of this article was to perform a systematic review of the techniques for transient circulatory arrest during intracerebral aneurysm surgery according to the PRISMA guidelines.
Material and methods
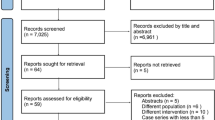
PubMed and Google Scholar searched 31.08.2018 using the following search terms: “heart arrest” OR (“heart”[All Fields] AND “arrest” ”[All Fields]) OR “cardiac standstill”[All Fields]) OR (“cardiac”[All Fields] AND “standstill” ”[All Fields]) AND (“intracranial aneurysm” OR (“intracranial”[All Fields] AND “aneurysm”[All Fields]) OR “cerebral aneurysm”[All Fields] OR (“cerebral”[All Fields] AND “aneurysm”[All Fields]). A total of 41 original articles were retrieved, of which 17 review articles, editorials, and case reports were excluded. A PRISMA Flow Diagram was created to illustrate the review process (Fig. 4). A total of 24 separate articles published between 1984 and 2018 were included in the final analysis (Table 1).
Results
Adenosine-induced hypotension
Aneurysm sac softening by systemic administration of adenosine is a well-described method and was reported in 14 articles. Because of the very short half-life of adenosine (< 10 s), the drug allows a rapid induction and rapid restoration of the mean arterial blood pressure (MAP) after its termination. Adenosine acts both on cardiac tissue and on vascular smooth muscle cells. By binding to A1 receptors on cardiac pacemaker cells located in the sinoatrial node, adenosine decreases the heart rate (negative chronotropic effect), and by binding to cells in the AV nodes, adenosine also has a negative dromotropic effect to decrease the conduction velocity [3]. By binding to A2 receptors on vascular smooth muscle cells, adenosine also induces a profound reduction of the systemic vascular resistance [3]. The adenosine-induced negative chronotropic effect on cardiac tissue is particularly important as it induces only a limited reflex tachycardia. Therefore, adenosine does not cause a rebound hypertension or tachyphylaxis, allowing for multiple administrations during a single procedure. Usually, adenosine slows the heart rate gradually for about 20 to 30 s before asystole is reached for a mean time of 15 s [2].
The optimal dose and the best way of administration of adenosine have not yet been established. Some author reported a dose titration exercise to obtain 30 s of asystole [2, 4, 5]. The most common way to obtain asystole is the incremental way. The duration of adenosine-induced asystole is dose-dependent, but the duration is variable across patients. The actual starting dose is variable across the studies [2, 4,5,6]. In some studies, it starts with a 6-mg dose [5, 6]. Lee et al. [6] started with a 6-mg dose for the dose test, resulting in an immediate and short asystole for some patients (less than 5 s). For some of the patients, they increased the dosage up to 60 mg, obtaining a 27-s asystole. The duration of asystole increases with the dose, but some patients are more sensitive to adenosine, as some patients in this study experienced a 30-s asystole with 30 mg of adenosine only. In a study by Guinn et al. [5], in which the starting dose was the same, the median dose of adenosine that resulted in < 30 s of hypotension was 12 mg. Luostarinen et al. [7] started with the same starting dosage of 6 mg, with the same median dose of 12 mg. Powers et al. [8] grossly established that 1 mg of adenosine result in 1-s asystole. However, in case of an unexpected event, like an intraoperative aneurysm rupture, the incremental way for the administration of adenosine cannot be used as the dose-response specific to the patient has not been established.
On the other hand, some authors propose to use an estimated dose of adenosine to obtain asystole. Meling et al. [2] used 0.4 mg/kg IBW (ideal body weight) with a median dose of 30 mg to obtain a median of 20 s of asystole (5–30 s) per dose. Bebawy et al. [9] used a dose estimation to predict a 45-s controlled hypotension, using the IBW. There, a dose of 0.3 to 0.4 mg/kg IBW was used. This method of administration was also used by Lee et al. [6] for 10 other patients, resulting in 13–41 s of asystole. The duration of asystole in this study was predicted following the function: 7.774 = 24,637 × Dose/IBW. Several doses of adenosine can be administered to the patient, if needed [2].
The placement of external defibrillator pads on all patients who might receive adenosine should be considered to provide external pacing capability if prolonged asystole/bradycardia were to develop or cardioversion in the face of hemodynamically unstable atrial fibrillation.
Scenarios, where adenosine is indicated and has proven useful, were described by Meling et al. [2] who used adenosine in cases of intraoperative rupture to reduce dangerous drilling of the anterior or posterior clinoid process, to reduce the potential risk of ischemia due to temporary clipping, to reduce the risk of cranial nerve injury by temporary clipping, and to control an atherosclerotic or particularly fragile parent vessel like with blood-blister aneurysms [10] and, finally, in cases of aneurysms previously treated endovascularly. In the same way, Bendok et al. [11] considered that adenosine can help to obtain aneurysm softening in case of a broad atheromatous neck with thin wall, in cases associated with connective tissue disease or for clip adjustment. Similar indications for adenosine are described in other studies like the one of Lee et al. [6] and Guinn et al. [5] in which adenosine was used mostly because of large or deep aneurysm and in Andrade-Barazate et al. [12] in which temporary clipping was impossible to on the proximal portion of the contralateral ophthalmic ICA segment.
The predominant location for aneurysms clipped under adenosine is the paraclinoidal ICA, representing 35–54% of the aneurysms in clinical series [2, 6, 11,12,13]. Other common locations are anterior communicating artery (37%) and posterior communicating artery (22%) [5, 11].
Most of the studies were conducted on unruptured, elective cases of cerebral aneurysm [2, 5, 6, 11, 12]. The largest number of patient with adenosine-assisted clipping in an emergency is the one conducted by Meling et al. [2], with a total of 44% of subarachnoid hemorrhage.
Adenosine has been associated with some cases of prolonged hypotension. In the retrospective study by Owall et al. [14], 47 patients had mean hypotensive period lasting 29 ± 5 min. Within 3 min after the termination of adenosine infusions, the MAP levels were restored, except in two patients in whom the prehypotension MAP level was not restored until 10 and 14 min after termination due to arrhythmias. The mean dose of adenosine was similar to other studies, at 0.21 mg/kg/min. Guinn et al. [5] also described one case of prolonged hypotension, due to a rapid re-dosing of adenosine, in which closed chest compressions had to be initiated. In the Owall study, adenosine was given as a continuous infusion to obtain a 29-min controlled hypotension period [14]. Continuous infusion was also the way of administration of adenosine in a study by Sollevi et al. from 1984 [15], where 10 patients underwent aneurysm surgery. MAP was decreased to 46 mmHg within 1 to 2 min, and previous MAP levels were restored after 1 to 5 min. All the recent studies reporting adenosine use in aneurysm surgery used boluses of adenosine rather than infusion way of administration to allow a rapid and very transient effect on AV node and to protect patients from prolonged hypotension. But adenosine in infusion had also rapid reversible effects, and no evidence shows that bolus rather than infusion is a safer way to administer adenosine. It is important to notice, also, that adenosine-induced hypotension in the MAP described here (40–50 mmHg) does not compromise cerebral oxygenation and may rather be neuroprotective [16].
With respect to contraindications, in patients with coronary disease, such as proximal coronary stenosis, adenosine may cause regional myocardial ischemia due to redistribution of blood flow away from areas supplied by the stenotic vessel. Therefore, these patients should not receive adenosine. In the study by Owall et al. [14], 2 patients with an history of previous myocardial infarction developed ST-T segment depression followed by ventricular tachycardia for 2 min, 5 min after the induction of hypotension. In addition, Owall et al. described a AV block 1 in 3 patients [14]. The influence on AV conduction was dose-dependent; however, AV block type 1 occurred at variable infusion rates from 140 to 350 pg/kg/min. In patients without any documented cardiopathy, Bebawy et al. [17] described a mild post-operative increase in troponin levels (> 0.03 ng/mL), without any clinical or transthoracic echocardiographic evidence of cardiac dysfunction, in 2 of the 24 patients. These findings are consistent with the Bendok study, in which 2 of the 40 patients had elevated troponin levels postoperatively, but no echographic abnormalities [11].
Other relative contraindications to adenosine include patients with gout or other conditions of disturbed purine metabolism, because of the slight increase in uric acid level [14]. Patients with significant reactive airway disease (asthma or chronic obstructive pulmonary disease) should not receive adenosine, because of the potential effect of adenosine of bronchoconstriction [14].
Rapid ventricular pacing
An alternative option to obtain aneurysm softening is by reducing the systemic blood pressure via rapid ventricular pacing (RVP). In RVP, a pacemaker-induced tachycardia is created via an electrode placed in the right ventricle through the internal jugular vein. The tachycardia impedes ventricular filling and reduces ventricular contraction, leading to a reduced stroke volume and and blood pressure without causing cardiac standstill.
During RVP, one typically starts with a heart rate of 150 beats per minute, then the heart rate is gradually increased in order to obtain satisfying value of MAP (under 50 mmHg). RVP at a frequency between 130 and 160 beats per min is considered effective in inducing a controlled hypotension in cerebral neurosurgery in conjunction with an already existing moderate hypotension. After cessation of RVP, the normalization of the systolic arterial blood pressure is almost immediate, and in contrary to the adenosine way of inducing hypotension, RVP has not been associated with prolonged hypotension in the studies [18, 19]. It is important to notice that prolonged intraoperative periods of hypotension are potentially harmful to the brain. Intraoperative systolic blood pressure < 60 mmHg has been associated with adverse neurological outcome. None of the patient in a retrospective study by Saldien et al. [20] had new microinfarcts lesion on the postoperative MRI compared to the preoperative MRI.
RVP can be more easily tailored (the faster the tachycardia, the lower the BP), and the hypotension is more easily controlled compared to adenosine. The hypotension can be sustained for much longer periods of time than with adenosine, as it can be made to be above an ischemic threshold (prolonged for many minutes since the MAP can be kept around 40 mmHg), whereas adenosine drops the MAP almost to 0 mmHg. Moreover, the large drop in MAP associated with adenosine might be potentially harmful for SAH patients with vasospasm.
In a prospective study by Konczalla et al. [18], RVP was applied during a mean duration of 60 ± 25 s, in order to obtain satisfying value of MAP. These values are consistent with other reviews [19, 20]. Several episodes of RVP could be used. In a retrospective study of Saldien et al. [19], an average of 3 pacing episodes was used during surgery with a maximum of 9 pacing episodes. In the Konczalla study, one patient received RVP up to four times [18].
Indications for the use of RVP in aneurysm surgery are comparable to those for adenosine to induce a controlled hypotension. In the study by Saldien et al. [20], softening of the aneurysm sac was the most common indication for RVP. Other indications included intraoperative bleeding, thin wall, and previous coiled aneurysms. RVP can be applicable for all the indications where adenosine can be used. Konczalla et al. [18] included in their prospective study seven patients with aneurysms being large (≥ 10 mm) or giant (30 mm), with a mean aneurysm size of 11.6 mm (± 6.2 mm). The majority of aneurysms in the study had a wide neck or were dysplastic.
The reported incidence of complications related to the RVP technique is low. Most complications are tachyarrhythmias, which can be treated most of the time by cardioversion. Konczalla et al. [18] reported a patient who developed a ventricular fibrillation while another patient developed atrial fibrillation, both resolved uneventfully. Small, temporary increases in troponin I levels can appear. In the review of Saldien et al. [20], there were no perioperative arrhythmias, but an increase in troponin I level that normalization within 24 h was frequently found. Of importance is that in the SAH group, the troponin levels 6 h after RVP were much higher in those patients compared with the other groups but without any acute coronary syndrome. In the Konczalla et al. study [18], the troponin I level also increased, but these small increases of troponin levels followed by a rapid normalization are suggestive of a reversible effect on cardiomyocytes rather than irreversible damages.
RVP involves the placement of intracardiac electrodes. Therefore, surgeons and anesthetists should be aware of the potential risk of cardiac perforation, cardiac tamponade, arrhythmias such as ventricular tachycardia and ventricular fibrillation, and myocardial infarction, during the intervention and pneumothorax if the pacing catheter is inserted from a jugular/subclavian vein. These are very rare complications and none of these were observed by Saldien et al. [20] or Konczalla et al. [18]. Sometimes, RVP is not necessary during the clipping, but the placement of cardiac electrodes exposes the patient to the risks described previously, without the advantages of the pacing (Table 2).
Some contraindications to this technique are important to notice, first and foremost in patients with history of severe coronary ischemic heart disease, cardiac arrhythmias, severe left ventricular dysfunction, or severe valvar heart disease, due to the risk of tachyarrhythmia.
Deep hypothermia
Deep hypothermia with extracorporeal circulation is also a possibility for the treatment of complex aneurysms. It is a way to obtain a cardiac standstill for quite a period of time because the hypothermia confers cerebro-protective effects, i.e., decreases the metabolic demands of neurons and increases the brain’s tolerance for the absence of cerebral blood flow.
Concerning the technical steps, 2 cannulas are inserted in the femoral artery and vein in order to obtain an extracorporeal circulation. The intracranial aneurysm is exposed and dissected. Then, the body temperature is gradually lowered and, at a temperature of 18–22 °C, the heart becomes asystolic. In a study by Lawton et al. [21], after attaining a nasopharyngeal temperature of 16 °C, the patients were exsanguinated by draining 30% of the blood volume into a venous reservoir to facilitate aneurysm exposure. This allowed the aneurysm to collapse and facilitated the final dissection and neck clipping. The safe limit of cardiac arrest in this study was 45 min, and the mean time of cardiopulmonary bypass was 133 min (100–172), with a mean 32 min of cardiac arrest. These values are consistent with other studies, like the Schebesch et al. [22] for example, in which the mean cardiac arrest time was 23.4 min (3 to 102 min) with a mean brain temperature of 18.4 °C. The cardiopulmonary bypass was performed in a mean duration of 136 min (45 to 240 min).
However, a very complex infrastructure is necessary, because the cardiac standstill requires an extra-corporeal circulation system [23]. Several probes are used to register temperature, and the pulmonary and arterial pressures are registered invasively. Moreover, intraoperative monitoring when using deep hypothermia requires electroencephalographic (EEG) activity, somatosensory evoked potentials (SSEP’s), and brain stem auditory evoked potentials (BAEP’s).
Nowadays, cardiac arrest with extracorporeal circulation under deep hypothermia is reserved to giant or large posterior circulation aneurysm, but virtually, all the indications for adenosine or RVP are also valuable for deep hypothermia. In a study by Lawton et al. [21], only 39% of the aneurysm would have been clippable without deep hypothermia, in contrast of the 97% which were clipped successfully. Thus, hypothermic circulatory arrest may facilitate direct clipping of giant and complex posterior circulation aneurysms.
Unfortunately, the method is associated with very high morbidity and mortality rates [24]. Some of the surgical complications include post-operative hematomas, perforator infarctions, MCA infarctions, and pontine infarctions. Deep hypothermia is also associated with major medical complications, such as sepsis, myocardial infarction, transfusion related hepatitis, pulmonary embolism, and pneumonia. These complications are described frequently across the literature [16, 21, 22, 24,25,26,27]. Lawton et al. [21] found a 37% rate of major complications, including surgical major complications, with 9 post-operative hematomas, and 8 perforators infarction across the 60 patients of the study. Major medical complications included four cases of sepsis, one case of myocardial infarction, one case of transfusion related hepatitis, one herpetic encephalitis, and one pulmonary embolism. Similar medical complications were found by Ponce et al. [24] and Mack et al. [25], in which surgical complications included tamponade and aortic root rupture: one patient developed seizures, 2 patients developed arrhythmias, 2 other developed SIADH, and 3 cases of sepsis were also diagnosed after the operation. Lawton et al. [21], reported a quite high rate of permanent treatment-associated neurological morbidity of 13%, with a similar rate of morbidity across the studies [21, 22, 24,25,26, 28]. Lastly, the mortality associated to this technique is variable across the studies, from 8.3% in the Lawton et al. [21] to 14% in the Ponce et al. [24]. This high mortality rate can be related to the high proportion of patients with SAH (45%).
Discussion
In this study, we systematically reviewed 24 articles, published between 1984 and 2018, in order to compare the techniques used for transient circulatory arrest during intracerebral aneurysm surgery.
The classical way to obtain aneurysm softening using temporary clips to temporary obstruct mechanically the blood flow through the parent vessel has the advantage to be easy to use, can be repeated, and to be noninvasive. This technique allows the surgeon to use one or several clips, with an easy on-off demand. However, temporary clipping can be complex and unrealizable for giant aneurysms or for aneurysms with wide necks, blood blisterlike appearances, previous endovascular treatment, involvement of perforators, or incorporation of branching vessels.
Adenosine and RVP are very useful adjuncts to temporary clipping during intracranial aneurysm surgery in a number of scenarios. However, their use in patient with severe coronary ischemic heart disease, cardiac arrhythmias, severe left ventricular dysfunction, or severe valvar heart disease should be avoided, and their safety in the context of subarachnoid hemorrhage is yet to be determined. Furthermore, adenosine is contraindicated in patients with significant reactive airway disease. Thus, although adenosine-induced cardiac arrest is an easy method to utilise, it still should only be used by a well-trained team of neurosurgical and anesthesiologic staff.
Lastly, cardiac standstill under deep hypothermia is also a possibility, but its application is now limited to giant or complex aneurysm of the basilar artery or to residual posterior circulation aneurysm after endovascular treatment because of the important morbidity and mortality associated with this technique.
References
Meling TR (2018) Adenosine-assisted clipping of intracranial aneurysms. Acta Neurochir Suppl 129:11–18
Meling TR, Romundstad L, Niemi G, Narum J, Eide PK, Sorteberg AG et al (2018) Adenosine-assisted clipping of intracranial aneurysms. Neurosurg Rev 41(2):585–592
Shryock JC, Belardinelli L (1997) Adenosine and adenosine receptors in the cardiovascular system: biochemistry, physiology, and pharmacology. Am J Cardiol 79(12A):2–10
Hashimoto T, Young WL, Aagaard BD, Joshi S, Ostapkovich ND, Pile-Spellman J (2000) Adenosine-induced ventricular asystole to induce transient profound systemic hypotension in patients undergoing endovascular therapy. Dose-response characteristics. Anesthesiology 93(4):998–1001
Guinn NR, McDonagh DL, Borel CO, Wright DR, Zomorodi AR, Powers CJ et al (2011) Adenosine-induced transient asystole for intracranial aneurysm surgery: a retrospective review. J Neurosurg Anesthesiol 23(1):35–40
Lee SH, Kwun BD, Kim JU, Choi JH, Ahn JS, Park W et al (2015) Adenosine-induced transient asystole during intracranial aneurysm surgery: indications, dosing, efficacy, and risks. Acta Neurochir 157(11):1879–1886 discussion 86
Luostarinen T, Takala RS, Niemi TT, Katila AJ, Niemela M, Hernesniemi J et al (2010) Adenosine-induced cardiac arrest during intraoperative cerebral aneurysm rupture. World Neurosurg 73(2):79–83 discussion e9
Powers CJ, Wright DR, McDonagh DL, Borel CO, Zomorodi AR, Britz GW (2010) Transient adenosine-induced asystole during the surgical treatment of anterior circulation cerebral aneurysms: technical note. Neurosurgery 67(2 Suppl Operative):461–470
Bebawy JF, Gupta DK, Bendok BR, Hemmer LB, Zeeni C, Avram MJ et al (2010) Adenosine-induced flow arrest to facilitate intracranial aneurysm clip ligation: dose-response data and safety profile. Anesth Analg 110(5):1406–1411
Meling TR, Sorteberg A, Bakke SJ, Slettebo H, Hernesniemi J, Sorteberg W (2008) Blood blister-like aneurysms of the internal carotid artery trunk causing subarachnoid hemorrhage: treatment and outcome. J Neurosurg 108(4):662–671
Bendok BR, Gupta DK, Rahme RJ, Eddleman CS, Adel JG, Sherma AK et al (2011) Adenosine for temporary flow arrest during intracranial aneurysm surgery: a single-center retrospective review. Neurosurgery. 69(4):815–820 discussion 20-1
Andrade-Barazarte H, Luostarinen T, Goehre F, Kivelev J, Jahromi BR, Ludtka C et al (2015) Transient cardiac arrest induced by adenosine: a tool for contralateral clipping of internal carotid artery-ophthalmic segment aneurysms. World Neurosurg 84(6):1933–1940
Benech CA, Perez R, Faccani G, Trompeo AC, Cavallo S, Beninati S et al (2014) Adenosine-induced cardiac arrest in complex cerebral aneurysms surgery: an Italian single-center experience. J Neurosurg Sci 58(2):87–94
Owall A, Gordon E, Lagerkranser M, Lindquist C, Rudehill A, Sollevi A (1987) Clinical experience with adenosine for controlled hypotension during cerebral aneurysm surgery. Anesth Analg 66(3):229–234
Sollevi A, Lagerkranser M, Irestedt L, Gordon E, Lindquist C (1984) Controlled hypotension with adenosine in cerebral aneurysm surgery. Anesthesiology 61(4):400–405
Lagerkranser M, Bergstrand G, Gordon E, Irestedt L, Lindquist C, Stange K et al (1989) Cerebral blood flow and metabolism during adenosine-induced hypotension in patients undergoing cerebral aneurysm surgery. Acta Anaesthesiol Scand 33(1):15–20
Bebawy JF, Zeeni C, Sharma S, Kim ES, DeWood MS, Hemmer LB et al (2013) Adenosine-induced flow arrest to facilitate intracranial aneurysm clip ligation does not worsen neurologic outcome. Anesth Analg 117(5):1205–1210
Konczalla J, Platz J, Fichtlscherer S, Mutlak H, Strouhal U, Seifert V (2018) Rapid ventricular pacing for clip reconstruction of complex unruptured intracranial aneurysms: results of an interdisciplinary prospective trial. J Neurosurg 128(6):1741–1752
Saldien V, Menovsky T, Rommens M, Van der Steen G, Van Loock K, Vermeersch G et al (2012) Rapid ventricular pacing for flow arrest during cerebrovascular surgery: revival of an old concept. Neurosurgery 70(2 Suppl Operative):270–275
Saldien V, Schepens T, Van Loock K, Vermeersch G, Wildemeersch D, Van Hoof V et al (2018) Rapid ventricular pacing for neurovascular surgery: a study on cardiac and cerebral effects. World Neurosurg. 119:e71–ee7
Lawton MT, Raudzens PA, Zabramski JM, Spetzler RF (1998) Hypothermic circulatory arrest in neurovascular surgery: evolving indications and predictors of patient outcome. Neurosurgery 43(1):10–20 discussion -1
Schebesch KM, Proescholdt M, Ullrich OW, Camboni D, Moritz S, Wiesenack C et al (2010) Circulatory arrest and deep hypothermia for the treatment of complex intracranial aneurysms--results from a single European center. Acta Neurochir 152(5):783–792
Sullivan BJ, Sekhar LN, Duong DH, Mergner G, Alyano D (1999) Profound hypothermia and circulatory arrest with skull base approaches for treatment of complex posterior circulation aneurysms. Acta Neurochir (Wien) 141(1):1–11 discussion -2
Ponce FA, Spetzler RF, Han PP, Wait SD, Killory BD, Nakaji P et al (2011) Cardiac standstill for cerebral aneurysms in 103 patients: an update on the experience at the Barrow Neurological Institute. Clinical article. J Neurosurg 114(3):877–884
Mack WJ, Ducruet AF, Angevine PD, Komotar RJ, Shrebnick DB, Edwards NM et al (2008) Deep hypothermic circulatory arrest for complex cerebral aneurysms: lessons learned. Neurosurgery 62(6 Suppl 3):1311–1323
Massad MG, Charbel FT, Chaer R, Geha AS, Ausman JI (2001) Closed chest hypothermic circulatory arrest for complex intracranial aneurysms. Ann Thorac Surg 71(6):1900–1904
Koch F, Thompson J, Chung RS (1991) Giant cerebral aneurysm repair. AORN J 54(2):224–227
Aebert H, Brawanski A, Philipp A, Behr R, Ullrich O-W, Keyl C et al (1998) Deep hypothermia and circulatory arrest for surgery of complex intracranial aneurysms. Eur J Cardiothorac Surg 13(3):223–229
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable as no new patients were involved in this research.
Informed consent
Not applicable as no new patients were involved in this research.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Meling, T.R., Lavé, A. What are the options for cardiac standstill during aneurysm surgery? A systematic review. Neurosurg Rev 42, 843–852 (2019). https://doi.org/10.1007/s10143-019-01183-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-019-01183-4