Abstract
The basilar artery (BA), as a reference vessel for laboratory investigations of cerebral vasospasm (CVS) in many experimental models, warrants a sufficient blood supply despite hemodynamic changes during CVS. In a prospective evaluation study, we analyzed patients who were admitted to our department with subarachnoid hemorrhage (SAH) for the occurrence and sequelae of CVS. Specifically, we sought to identify patients with CVS of the BA. As per institutional protocol, all patients with CVS detected in the posterior circulation had magnetic resonance imaging (MRI) examinations instead of CTA. Between January and December 2016, 74 patients were treated for spontaneous SAH. CVS occurred in 45 (61%) patients, and 31 (42%) patients developed associated cerebral infarctions (CI). CVS was significantly associated with CI (p < 0.0001; OR 44). In 18 (24.3%) patients, CVS significantly affected the basilar artery. Poor admission clinical state, younger age, and treatment modalities were significantly associated with BACVS. BACVS was more often detected in patients with severe CVS (p < 0.046; OR 4.4). Patients with BACVS developed cerebral infarction in a frequency comparable to other patients with CVS (61% vs. 70%, p = 0.7), but none of these infarctions occurred in the brain stem or pons even though vessel diameter was dramatically reduced according to CT- and/or MR-angiography. BACVS does not appear to be followed by cerebral infarction in the BA territory, presumably due to a vascular privilege of this vessel and its perforating branches. In contrast, brain ischemia can frequently be observed in the territories of other major arteries affected by CVS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aneurysmal subarachnoid hemorrhage (SAH) still leads to high rates of morbidity and mortality [30]. Natural history estimates an overall mortality rate of about 50% [1, 14]. The most important prognostic factors are initial neurological grade at admission, patient characteristics (smoking, hypertension), SAH grade (H/H, Fisher score/volume of blood on initial CT), and in particular cerebral infarction [2, 7, 15, 24, 31]. Studies on optimization SAH treatment continue to be subject of clinical and experimental research. Changes in microcirculation and increased expression of various biomarkers in association of inflammatory processes are possible reasons for the etiology of CVS and cerebral infarction (CI). The basilar artery (BA) serves as a well accessible vessel for experimental (animal) research. Our data shows differences in the distribution of CI and hemodynamic characteristics between the anterior and vertebrobasilar circulation. To our knowledge, the reasons for this are not clearly described.
Methods
Patient data
Patients were selected from our prospective collected clinical database with SAH admitted to our department of neurosurgery between January 2016 and December 2016. Evaluation of data was performed retrospectively. Since the study question is the amount and location of cerebral infarction after CVS of the basilar artery, inclusion criteria were aneurysmal or non-aneurysmal SAH without a traumatic event. Patient characteristics, such as neurological score at admission (based on the Glasgow coma scale (GCS) and the World Federation of Neurological Surgeons (WFNS)), SAH grade (Hunt and Hess (H/H), Fisher score), aneurysm size, age, gender, course of SAH (vasospasm and cerebral infarctions, volume of cerebral infarction), the use of antiplatelet drugs, and aneurysm treatment were evaluated and investigated.
Clinical management
All patients underwent digital subtraction angiography (DSA) for diagnostic purpose. Based on interdisciplinary consensus, aneurysms were treated by endovascular or microsurgical occlusion. If microsurgical clipping was performed, repeated DSA was done a few days later to demonstrate occlusion of the aneurysm. All patients were equally exposed to our institutional treatment protocol following SAH for the treatment of CVS, blood pressure management, and hydrocephalus at our neurosurgical ICU. Considering our treatment protocol, oral nimodipine was administered to all patients with SAH started from the day of admission for 21 days. All patients received low molecular-weight heparin. Depending on the method of treatment (stenting) or their cardiovascular risks, patients received acetylsalicylic acid or other anticoagulants (defined as “additional anticoagulants”). Patients were monitored daily with transcranial Doppler (TCD). In case of increase in velocity (TCD mean > 150 cm/s or more than 50 cm/s in 24 h) or delayed neurological deterioration, a CT or MRI was initiated to confirm CVS. On day 7 ± 2 after ictus, patients who could not be sufficiently evaluated clinically received a regular scheduled CTA or MRA to detect CVS. The grading of CVS was divided into mild (< 33% luminal narrowing), moderate (33–66% luminal narrowing), and severe (> 66% luminal narrowing). Patients with moderate and severe CVS were included in further analysis. In patients with CVS, hypertension was induced, with the goal of a mean arterial pressure of > 110 mmHg. Neither transluminal balloon angioplasty nor intraarterial nimodipine treatment was the subject of our treatment protocol since data on their efficacy in the prevention of cerebral infarction are inconsistent.
In addition, magnetic resonance imaging of the brain was performed on patients who suffered cerebral vasospasm in the vertebrobasilar circulation for detecting suspected CI of the brain stem. Also, patients with long-lasting CVS (> 21 days) received an MRI and served as control group for the CI rate. The rate of cerebral infarction, volume and location (anterior/posterior circulation, global), duration of vasospasm, and predictive factors for the incidence of BACVS were evaluated. Outcome was measured based on the modified Rankin Scale (mRS) at discharge (mRS 0–2 favorable vs. 3–6 unfavorable).
Cerebral ischemia
Imaging was performed and interpreted by an experienced neuroradiologist. For detecting late hyperacute (6–24 h) and acute (24 h-1 week) infarction, DWI-, FLAIR-, T1- and T2-weighted sequences were performed. MR-perfusion was not a subject of imaging protocol and was therefore not performed routinely. Infarction was defined as low attenuating regions on CT or on MR image displaying low T1, bright FLAIR signal intensity, and mixed signal on diffusion-weighted image (DWI/ADC), corresponding to a vascular distribution. Hypoattenuating regions on postoperative day 1 were considered to be related to the procedure. The volume of infarction was evaluated to establish a cut-off value of volume related to poor outcome.
The CT and MRI scans at admission were used for computer-assisted volumetric measurements. The method was used by an independent clinician. For computer-assisted measurement, the Brainlab® elements software (Brainlab Germany Headquarters, Munich, Germany) was used. The infarction margins were traced and the volume (cm3) was automatically calculated. Infarction areas were added up slice by slice automatically by the computer. For calculating infarction volume, the region of interest method (ROC) was used.
Statistical analysis
Statistical analysis was performed using the statistical software package SPSS (IBM SPSS Statistics for Windows, Version 22; Armonk, NY, USA. IBM Corp.) Categorical variables were analyzed in contingency tables using the Fisher exact test, an unpaired t test was used for parametric statistics. For univariate analysis, statistical significance was set at p < 0.05. Variables with a possible association with BACVS (p < 0.1) were entered into a forward stepwise multiple logistic regression analysis. The Kruskal–Wallis test was used for detecting predictors for outcome. Data and statistical analysis were approved by the Institute of Biostatistics and Mathematical Modeling.
Results
Patient characteristics
Seventy-four patients were included in our study. In 18 (24.3%) patients, BACVS could be detected. The majority of patients were female (n = 41; 55.4%). The mean age was 55 years. Thirty-six (48.6%) patients showed a good clinical state at admission (WFNS I-III), 38 (51.4%) patients suffered from a severe SAH at admission (WFNS IV-V). 15 (20.3%) patients died during the course, 7 (9.5%) after admission, before detecting CVS. Further characteristics were described in Table 1.
The majority of patients showed a Fisher 3 bleeding pattern (36; 48.6%). In 43 (58.1%) cases, the ruptured aneurysm was localized in the anterior circulation, in 11 (14.9%) cases in the posterior circulation. In 20 (27.0%) patients, aneurysm could not be detected. Microsurgical clipping was performed in 28 (37.8%) patients, 24 (32.4%) patients underwent endovascular treatment.
Cerebral vasospasm, cerebral infarction, and basilar artery vasospasm
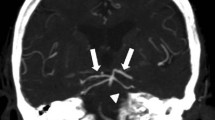
Forty-five (60.8%) patients suffered from CVS. The majority of patients showed severe CVS (n=24; 53.3%). In patients with BACVS, severe CVS was more frequent (64.3% vs. 35.7%; p < 0.05). Figure 1 shows a patient with non-aneurysmal SAH and severe BACVS. The duration of CVS lasted between 4 and 47 days (mean 14.1 days). Vessels in the anterior circulation were mainly affected (n = 25; 55.6%), followed by global CVS (n = 15; 33.3%) and vessels of the posterior circulation (n = 5; 11.1%).
a Digital subtraction angiography after SAH shows an inconspicuous width of the basilar artery. An aneurysm could not be found. The MRI follow-up after 7 days shows cerebral vasospasm and a high degree of narrowness of the basilar artery (b) what is regressed after 18 days (c). Cerebral infarctions did not occur (d)
Cerebral infarction (CI) occurred in 31 out of 74 patients (41.9%) patients and took place predominantly in the anterior circulation (n = 24; 77.4%).
Patients with long-lasting CVS (> 21 days) had more frequent CI in the MR imaging than patients with a shorter duration of CVS. The mean volume of infarction was 82.6 cm3. A cut-off point with a value of 7.3 cm3 was calculated for infarction volume. Infarction volume was statistically significant related to poor outcome (AUC = 0.85; p = 0.013; OR 16.8). In univariate analysis, admission state was significantly associated with CI (p < 0.0001; OR 0.11). CI was statistically significant based on the incidence of CVS (p < 0.0001; OR 0.02). Seventeen (23%) patients received additional antiplatelet drugs or additional anticoagulants. Treatment with additional antiplatelet drugs/anticoagulants was not statistically significant for the prevention of CVS-related CI (p = 0.26; OR 2.04). In 43 (58.1%) patients, the insertion of an external ventricular drainage was necessary. The need for external ventricular drainage due to hydrocephalus was significantly associated with the incidence of CI (p < 0.001; OR 0.13). Treatment modality (clipping vs. coiling) had no effect on the development of CI (p > 0.3). A detailed list of distribution and association between CVS location and CI is shown in Table 1.
Patients without BACVS were older than patients with detected BACVS (p < 0.01; OR 5.75). The mean CVS duration in patients with BACVS was 15.5 days and in patients without BACVS 13.2 days (p > 0.05). BACVS was not a significant predictor of CI. Brain stem infarction did not occur in any cases (Figs. 1 and 2).
In multiple logistic regression analysis, younger age (OR 3.9), worse admission status (OR 5.5), endovascular treatment (OR 4.9), and severe CVS (OR 4.4) were significant independent predictors to receive BACVS (Table 2).
Outcome at discharge
The outcome of patients with BACVS was significantly worse (p < 0.01). However, if only patients with CVS are examined, there is no statistical significant difference (Kruskal–Wallis test p = 0.07). Thirty-one (41.9%) patients showed a favorable outcome (mRS 0–2) at discharge and 28 (37.8%) patients suffered severe neurological deficits (mRS 3–5). Fifteen (20.3%) patients died during the course. Table 3 shows the predictive variables for unfavorable outcome.
Discussion
Many investigations have been proposed to explain the etiology of cerebral infarction according to SAH [1, 3,4,5,6, 12, 19, 22,23,24, 29]. CVS is one of the linked causes for the development of CI. However, there are many theories that refute these considerations. Foreman et al. described in their recent study the pathophysiology of early arteriolar vasospasm with microthrombosis, perfusion mismatch, and neurovascular uncoupling as one reason for cortical infarction. Furthermore, large vessel vasospasm serves as a late contributor. He depicts a process of inflammatory response after ictus that leads to neuronal injury and probably to cortical ischemia [9,19]. Ultra-early angiographic vasospasm appears to be associated with delayed cerebral ischemia and infarction. In their study, Al-Mufti et al. described a twofold increased risk of cerebral infarction in patients with ultra-early CVS (within the first 48 h) [1]. In addition to the clinical state of admission, the amounts of intracerebral blood on CT are strong predictors for CVS and DCI [10]. Patient characteristics such as nicotine abuse, hyperglycemia, hydrocephalus, a history of diabetes mellitus, and early systemic inflammatory response syndrome showed evidence for a higher risk of ischemia after SAH [6]. Other studies postulate the disturbance of the microcirculation as a presumptive predictor for infarction [25, 26, 32].
The aim of our study is to carry out the rate and effect of BACVS, the rate and probably the association to brain stem infarction, and the peculiarities of this current area. We identified 18 patients with BACVS. While most of the patients showed vasospasm of the anterior circulation and infarction of the anterior territory during the course of CVS, none of the patients showed any brain stem infarction according to BACVS. Furthermore, the site of cerebral ischemia was not related to the location of SAH or aneurysm. As well, we detected a high rate of cerebral infarction (41.9%) in the entire study group. The group of patients with cerebral infarction presented a high rate of worse admission state (WFNS IV-V; 58.1%), a high rate of H&H grade 4 to 5 (45.2%), and a high rate of Fisher 3–4 bleeding (71%), as a presumptive reason for the increased number of infarction.
We detected a high grade of vessel diameter reduction in BAs without any affection, except for a difference in the duration of CVS. The first question is why only a small proportion of patients develop BACVS and, secondly, why patients do not develop brain stem infarcts in the course of BACVS despite an enormous narrowing of the vascular lumen?
Regarding the etiology of BACVS, younger age, worse admission status, and endovascular treatment were statistically significant predictors. BACVS occurred in patients with severe CVS, who were affected by the initial clinical state and SAH grade. Manipulation of the basilar artery during endovascular treatment could be one explanation of the prediction effect. Differences in outcome between the treatment modalities were not obvious. With regard to the blood supply of the brain stem, the anatomy of the BA itself could help to elucidate our observation: the BA, a large vessel, is formed by fusion of the longitudinal neural system, includes many perforating arteries, with a mean number of 6.1 and an average diameter of 393 μm. The BA perforators supply the pons and originate from the parent vessel, but often from its pial branches as well (91.7%) [8]. A natural extension of the segmental arrangement of the spinal cord concerning the arterial system of the cerebrum and cerebellum is postulated [20]. One explanation for the absence of brain stem ischemia during CVS could be the hemodynamic and quantitative peculiarities of the perforators. Image modalities with the possibility of optimized representation of the perforators is therefore of great importance for the detection of the etiology of cerebral ischemia. DSA in high-resolution and 3D-technique and high-resolution postcontrast time-of-flight MR-angiography at 7.0 T seems to be suitable in visualization of intracranial perforating arteries [13, 18].
Differences in posterior circulation and basilar artery anatomy are obvious in stroke management, too. Beside the low incidence of basilar artery occlusions, the time window may have a longer time for recanalization therapy [27]. Unlike the arteries of the anterior circulation, the basilar artery is not a terminal vessel, so the blood supply of the posterior territory, even with the help of the vertebral arteries is better suited in cases of vasospasm or narrowing and continues to ensure the supply of nutrients and oxygen. Many investigations are concerned with the pathophysiology of the BA during the course of CVS according to SAH. Besides studies of autoregulation [21, 26] and detection of biomarkers [16, 17, 19, 33], vascular microanatomy [11, 20] and image modalities [28] are subjects of many investigations by means of the BA. Considering these observations, a comparison of terminal vessel territories that seem to be more affected should be chosen in SAH research. Furthermore, the special features of the brain stem such as hemodynamic changes and compensatory mechanisms should be considered more closely in further vascular research. The small number, short follow-up duration, and the retrospective study design are limitations of our study; nevertheless, we evaluated 74 patients with SAH in a short time (12 months). Performing MRI in ventilated patients is time-consuming and carries risks. Therefore MRI was justifiable in cases of high suspicion of BACVS and long-lasting CVS. All study patients with detected BACVS received MRI diagnostic. Patients with BACVS, who could not receive MRI, were excluded. We described all ischemic changes, visualized in the MR modality as a cerebral infarction, even without clinical symptoms. This is one explanation for the high rate of cerebral infarction in our study. In addition, we include a high number of patients with severe SAH and long-lasting CVS. The study should serve as an incentive for further larger studies. Further studies require MRI comparison between patients with and without CVS, considering hemodynamic features in the vertebrobasilar territories. The main point of our study is to highlight hemodynamic properties in the brain stem despite high grade and long-lasting CVS.
Conclusion
CVS still seems to be one of the main causes of CI after SAH. In 41.9%, CI was detected by MRI. However, BACVS does not appear to be followed by cerebral infarction in the BA territory, presumably due to a vascular privilege of this vessel and its perforating branches. In contrast, brain ischemia can frequently be observed in the territories of other major arteries (internal carotid, anterior/middle cerebral arteries) affected by CVS. Further investigations are required in the research of CVS.
References
Al-mufti F, Roh D, Lahiri S, et al. (2016) Ultra-early angiographic vasospasm associated with delayed cerebral ischemia and infarction following aneurysmal subarachnoid hemorrhage. J Neurosurg 1–7
Andreasen TH, Bartek J, Andresen M, Springborg JB, Romner B (2013) Modifiable risk factors for aneurysmal subarachnoid hemorrhage. Stroke 44(12):3607–3612
Badjatia N, Seres D, Carpenter A, Schmidt JM, Lee K, Mayer SA, Claassen J, Connolly ES, Elkind MS (2012) Free fatty acids and delayed cerebral ischemia after subarachnoid hemorrhage. Stroke 43(3):691–696
Crowley RW, Medel R, Dumont AS, Ilodigwe D, Kassell NF, Mayer SA, Ruefenacht D, Schmiedek P, Weidauer S, Pasqualin A, Macdonald RL (2011) Angiographic vasospasm is strongly correlated with cerebral infarction after subarachnoid hemorrhage. Stroke 42(4):919–923
Dankbaar JW, Rijsdijk M, van der Schaaf IC, Velthuis BK, Wermer MJH, Rinkel GJE (2009) Relationship between vasospasm, cerebral perfusion, and delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Neuroradiology 51(12):813–819
de Rooij NK, Rinkel GJE, Dankbaar JW, Frijns CJM (2012) Delayed cerebral ischemia after subarachnoid hemorrhage: a systematic review of clinical, laboratory, and radiological predictors. Stroke 43–54
Dinc N, Lescher S, Quick-Weller J, Berkefeld J, Platz J, Senft C, Seifert V, Konczalla J (2017) Outcome, prognostic factors, and follow-up results after subarachnoid hemorrhage from pericallosal artery aneurysms. World Neurosurg 99:566–571
Djulejić V, Marinković S, Milić V et al (2015) Common features of the cerebral perforating arteries and their clinical significance. Acta Neurochir (Wien) 157(5):743–754
Foreman B (2016) The pathophysiology of delayed cerebral ischemia. J Clin Neurophysiol 33(3)
Foreman PM, Chua MH, Harrigan MR, et al (2016) External validation of the Practical Risk Chart for the prediction of delayed cerebral ischemia following aneurysmal subarachnoid hemorrhage. 1–7
Ghantous CM, Azrak Z, Rahman FA, Itani HA, Zeidan A (2016) Assessment of basilar artery reactivity in stroke and subarachnoid hemorrhage using wire myograph. Methods Mol Biol 1462:625–643
Güresir E, Raabe A, Jaiimsin A et al (2010) Histological evidence of delayed ischemic brain tissue damage in the rat double-hemorrhage model. J Neurol Sci 293(1-2):18–22
Harteveld AA, De Cocker LJL, Dieleman N et al (2015) High-resolution postcontrast time-of-flight MR angiography of intracranial perforators at 7.0 tesla. PLoS One 10(3):1–11
Hop JW, Rinkel GJ, Algra A, van Gijn J (1997) Case-fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. Stroke 28(3):660–664
Jabbarli R, Reinhard M, Roelz R et al (2015) Early identification of individuals at high risk for cerebral infarction after aneurysmal subarachnoid hemorrhage: the BEHAVIOR score. J Cereb Blood Flow Metab 35(10):1587–1592
Jung CS, Lange B, Zimmermann M, Seifert V (2013) CSF and serum biomarkers focusing on cerebral vasospasm and ischemia after subarachnoid hemorrhage. Stroke Res Treat 2013:560305
Konczalla J, Wanderer S, Mrosek J et al (2016) Levosimendan, a new therapeutic approach to prevent delayed cerebral vasospasm after subarachnoid hemorrhage? Acta Neurochir (Wien) 158(11):2075–2083
Lescher S, Samaan T, Berkefeld J (2014) Evaluation of the pontine perforators of the basilar artery using digital subtraction angiography in high resolution and 3D rotation technique. Am J Neuroradiol 35(10):1942–1947
Li G, Wang Q, Lin T (2016) Alterations in the expression of protease-activated receptor�1 and tumor necrosis factor-α in the basilar artery of rats following a subarachnoid hemorrhage. Exp Ther Med 717–722
Mercier PH, Brassier G, Fournier HD, Picquet J, Papon X, Lasjaunias P (2008) Vascular microanatomy of the pontomedullary junction, posterior inferior cerebellar arteries, and the lateral spinal arteries. Interv Neuroradiol 14:49–58
Otite F, Mink S, Tan CO et al (2014) Impaired cerebral autoregulation is associated with vasospasm and delayed cerebral ischemia in subarachnoid hemorrhage. Stroke 45(3):677–682
Platz J, Berkefeld J, Singer OC et al (2014) Frequency, risk of hemorrhage and treatment considerations for cerebral arteriovenous malformations with associated aneurysms. Acta Neurochir (Wien) 156(11):2025–2034
Platz J, Güresir E, Wagner M, Seifert V, Konczalla J (2016) Increased risk of delayed cerebral ischemia in subarachnoid hemorrhage patients with additional intracerebral hematoma. J Neurosurg 1–7
Rabinstein AA, Friedman JA, Weigand SD et al (2004) Predictors of cerebral infarction in aneurysmal subarachnoid hemorrhage. Stroke 35(8):1862–1866
Rosengart AJ, Schultheiss KE, Tolentino J, Macdonald RL (2007) Prognostic factors for outcome in patients with aneurysmal subarachnoid hemorrhage. Stroke 38(8):2315–2321
Santos GA, Petersen N, Zamani AA et al (2016) Pathophysiologic differences in cerebral autoregulation after subarachnoid hemorrhage. Neurology 86(21):1950–1956
Schulz UG, Fischer U (2017) Posterior circulation cerebrovascular syndromes: diagnosis and management. J Neurol Neurosurg Psychiatry 88(1):45–53
Sviri GE, Britz GW, Lewis DH et al (2006) Brainstem hypoperfusion in severe symptomatic vasospasm following aneurysmal subarachnoid hemorrhage: role of basilar artery vasospasm. Acta Neurochir (Wien) 148(9):929–934
Ulrich CT, Fung C, Vatter H, et al (2013) Occurrence of vasospasm and infarction in relation to a focal monitoring sensor in patients after SAH: placing a bet when placing a probe? PLoS One 8(5)
van Gijn J, Kerr RS, Rinkel GJE (2007) Subarachnoid haemorrhage. Lancet 369(9558):306–318
Vergouwen MDI, Ilodigwe D, MacDonald RL (2011) Cerebral infarction after subarachnoid hemorrhage contributes to poor outcome by vasospasm-dependent and -independent effects. Stroke 42(4):924–929
Wagner M, Steinbeis P, Güresir E et al (2013) Beyond delayed cerebral vasospasm: infarct patterns in patients with subarachnoid hemorrhage. Clin Neuroradiol 23(2):87–95
Xiong Y, Wang X, Zhong M, et al (2016) Alterations of caveolin-1 expression in a mouse model of delayed cerebral vasospasm following subarachnoid hemorrhage. Exp Ther Med 1993–2002
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional committee (University Hospital Frankfurt) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Dinc, N., Quick-Weller, J., Tritt, S. et al. Vasospasm of the basilar artery following spontaneous SAH—clinical observations and implications for vascular research. Neurosurg Rev 42, 983–989 (2019). https://doi.org/10.1007/s10143-018-1015-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-018-1015-4