Abstract
We retrospectively compared the outcome of aneurysmal subarachnoid hemorrhage (aSAH) patients treated in a neurosurgical ICU (nICU) between 1990 and 2005 with that of patients treated in a general ICU (gICU) between 2005 and 2013 with almost identical treatment strategies. Among other parameters, we registered the initial Hunt and Hess grade, Fisher score, the incidence of vasospasm, and outcome. A multivariate analysis (logistic regression model) was performed to adjust for different variables. In total, 755 patients were included in this study with 456 patients assigned to the nICU and 299 patients to the gICU. Multivariate logistic regression analysis revealed no significant difference between the patient outcome treated in a nICU versus gICU after adjusting for different variables. The outcome of patients after aSAH is not influenced by the type of ICU (gICU versus nICU). The data do not allow claiming that aSAH patients need to be treated in a specialized ICU for obtaining better results. Parameters which might differ from hospital to hospital, especially warranty of neurosurgical expertise on gICU, have the potential to influence the results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aneurysmal subarachnoid hemorrhage (aSAH) is a common and often devastating intracranial bleeding with population-based mortality rates as high as 45% and significant morbidity among survivors [2]. After successful surgical or endovascular treatment of the ruptured aneurysm, the patients’ outcome is threatened by arterial vasospasm, delayed ischemic neurological deficits (DIND), and cerebral infarction, requiring early detection and instant medical and sometimes surgical or endovascular treatment. In patients with high-grade aSAH, monitoring and treatment of brain edema and metabolic disbalances might be in the focus as well. Thus, aSAH patients have to be treated within an intensive care unit (ICU) during this risky period. Guidelines for the management of aSAH recommend an early referral to high-volume centers, but even these guidelines do not specify the type of ICU (neurosurgical ICU [nICU] versus general ICU [gICU]) where patients suffering from SAH should be transferred to.
As not yet performed, it was the aim of this study to evaluate the impact of a gICU versus a nICU on the patients’ outcome after aSAH at two different university hospitals with almost identical treatment strategies.
Methods
Patient population
We retrospectively compared two databases including patients suffering from aSAH admitted to two primary care university hospitals. The first database includes patients identified retrospectively between 1990 and 2004 admitted to the nICU at a high-volume university center. The second database includes aSAH patients admitted to the gICU at a high-volume university center between 2005 and 2013. The general treatment strategies in both institutions did not significantly differ, since the strategy has been transferred by the senior author from the first to the second center. The same strategy was followed in both institutions concerning the treatment in the acute phase, the aneurysm treatment, and the treatment of early and delayed complications in patients with aSAH.
Exclusion criteria were insufficient data for analysis, patients with traumatic subarachnoid hemorrhage, patients with arterio-venous malformation-related aneurysms, and an age < 18 years.
Baseline characteristics of the patients such as age, sex, aneurysm location, number of aneurysms, and aneurysm treatment modality were documented. Initial clinical presentation was documented according to the Hunt and Hess grading system (Hunt and Hess) [5]. Additionally, the initial Glasgow Coma Scale (GCS) [8] was recorded. Initial GCS score was divided into the following groups for statistical analysis: 3–6, 7–12, and 13–15.
Type of ICU
The nICU is part of the department of neurosurgery. Primary care is being provided by neurosurgeons with anesthesiologists serving as consultants. An experienced neurosurgeon is on duty during daytime, supported by rotating residents. Night-time coverage is provided by the neurosurgeon on call. A neurosurgical staff member makes rounds twice a day, accompanied by the attending neurosurgeon in the afternoon. The physician:patient ratio is 1:7, the nurse:patient ratio varied in the study period between 1:3 and 1:2.5. Due to a shift system, the night-time coverage was the same as during daytime.
The gICU is part of the department of anesthesiology. Primary care is being provided by general intensivists. An experienced neurosurgeon serves as a consultant and performs rounds at the gICU twice a day. Neurosurgical residents in the first to second year are part of the interdisciplinary ICU team. The neurosurgical resident on duty and the attending neurosurgeon on duty participate in the rounds at least once a day. The physician:patient ratio is 1:8 and the nurse:patient ratio is 1:2. Due to a shift system, the night-time coverage was the same as during daytime.
Outcome parameters
Outcome parameters were incidence of vasospasm, DIND, ischemic lesions, and clinical outcome.
Transcranial Doppler sonography (TCD) was carried out routinely on a daily basis between day 1 and day 14 after the initial bleeding. The mean blood flow velocities (BFVs) of the middle cerebral artery were measured by a 2-MHz Doppler ultrasound probe using a transtemporal approach as described by Aaslid et al. [1]. The TCD measurements were performed by a neurosurgical resident at the nICU and controlled by a board-certified neurosurgeon in cases of inconclusive data and/or BFV possibly requiring a management change. At the gICU, TCD measurements were done by either a general intensivist or a resident and were reported to the neurosurgical consultant for quality control and management planning. An increase of mean BFV over 120 cm/s was defined as TCD vasospasm [1, 6, 22].
The BFV in the cervical carotid artery for calculation of the Lindegaard index was only measured sporadically, if hyperaemia was strongly suspected.
The diagnosis of symptomatic vasospasm was made when new focal or global neurological deficits occurred that were not explained by other complications such as hydrocephalus, rebleeding, infection, metabolic abnormalities, surgical, or endovascular-associated complications as described before [22]. Delayed infarction was defined as new diagnosed infarction on the CT scan after exclusion of treatment-associated infarction.
The Glasgow Outcome Scale (GOS) score was used to describe the clinical condition by the time of discharge as well as approximately 6 months after the initial bleeding [7]. GOS 1 was assigned for patients who died and GOS 5 for patients with good recovery, GOS 2 for vegetative state, GOS 3 for severe disability, and GOS 4 for moderate disability [7].
Measurements of functional neurological recovery, like the modified Rankin score, were not made.
Imaging
Diagnosis of SAH was made by cerebral computerized tomography (CT). The amount of blood seen on the initial CT scan was classified according to the Fisher grading system [4]. Patients received either CT angiography or four-vessel digital subtraction angiography (DSA) before surgery or neuroradiological intervention (coiling). Preoperative MRI was not routinely performed. All patients underwent early post-operative CT scan (4 hours after surgery) and repeated CT scan in an event of neurological decline. This strategy was followed in both institutions. CT perfusion imaging became available in 2012 and, therefore, was not used in all patients treated on the nICU and in a minority of patients treated on the gICU.
Treatment
Irrespective of the type, the patients stayed on the ICU at least for 14 days after the initial rupture of the aneurysm. The aneurysm treatment was performed within the first 72 h after the bleeding by clipping or coiling. The number of coiled patients increased during the time period of the study. All patients with aSAH, submitted within 72 h after the initial bleeding event, underwent treatment, irrespective of the Hunt and Hess grade. In poor-grade patients (Hunt and Hess grades IV and V), an external ventricular drainage (EVD) and, in good-grade patients (Hunt and Hess grades I to III), a lumbar drainage were placed for intraoperative brain relaxation and, if present, treatment of acute hydrocephalus. This policy was performed in both institutions. If necessary, patients with insufficient lumbar drainage received an additional EVD. Intracranial pressure (ICP) > 20 mmHg was treated first by CSF drainage, then by mannitol. Starting in 2000, hemicraniectomy in otherwise uncontrollable ICP was occassionally performed. During the whole study period, other monitoring techniques despite daily TCD and ICP (if required) were not performed. The operative procedure has been described in detail before [23]. Microdoppler was used after clipping to assure patency of the parent artery and its branches. An irrigation with nimodipine was performed during the intradural steps of the operation.
Antivasospastic therapy
In all patients, hypovolemia was avoided and the patients were kept at least normovolemic during the first 14 days of therapy; colloidal substances were only occasionally used. All patients routinely received standard prophylactic therapy with nimodipine 10 ml/h (2 mg/h over 14 days intravenous) for 14 days. In patients with BFV exceeding 120 cm/s, a prophylactic “triple-H” therapy focusing on hypertension (systolic blood pressure between 160 and 180 mmHg) induced by catecholamines (predominantly noradrenaline) and normo- to mild hypervolemia (central venous pressure between 8 and 12 mmHg) was initiated. Hemodilution was not performed. Those criteria for the initiation of prophylactic therapy were standardized throughout the whole study period between 1990 and 2013 and did not differ between the two institutions.
Patients with TCD vasospasm kept bed rest. In patients with symptomatic vasospasm, reversal of the neurological deficit by further increase of blood pressure was attempted. Treatment with nimodipine or balloon angioplasty was not performed during the first study period (nICU) because of the not proven effect on outcome in matched-pair studies available during this time period [19]. On the other hand, interventional balloon angioplasty or local application of nimodipine was performed in selected cases after repeated DSA during the second study period (gICU) after interdisciplinary discussion of each specific case.
Statistical analysis
Statistica 8 (Statsoft, Tulsa, OK, USA) and SAS version 9.2 statistical software (SAS Institute) were used for analysis. Patient characteristics were summarized as percentiles, range, and median as reasonable. A p value < 0.05 was considered significant. Statistical significance was calculated using χ2 test for categorical variables, t test for continuous variables, and Mann-Whitney U test for non-parametric analyses. To assess the risk of the type of ICU-related outcome, we performed multivariate analyis using the logistic regression model to adjust for differences between the two different groups (p < 0.05).
Results
Description of the sample
A total of 755 patients with an average age of 50.5 years were included. Four hundred and fifty-six patients were registered in the nICU group and 299 patients in the gICU group. The median age of the nICU patients was 47.4 ± 11.8 years and of the gICU patients 55.2 ± 13.8 years. The age distribution was significantly different between the two groups with more than 90% of the patients younger than 60 years in the nICU group compared to 66% in the gICU group (p < 0.001). There was a significant difference between both groups concerning the initial Hunt and Hess grade with a higher grade in the gICU group (p = 0.001). GCS score was 3–6 in 114 patients (26.6%), 7–12 in 49 patients (11.4%), and 13–15 in 266 (62.0%) patients in the nICU group, respectively. In the gICU group, GCS score was 3–6 in 127 patients (42.9%), 8–12 in 22 patients (7.4%), and 13–15 in 147 patients (50.0%), respectively. A detailed analysis is summarized in Table 1. Mean arterial blood pressure by the time of admission to hospital was 142.8 mmHg systolic (SD 30.3 mmHg) and 90.3 mmHg diastolic (SD 18.3 mmHg) in the nICU group and 147.1 mmHg systolic and 80.7 mmHg diastolic (SD 16.5 mmHg) in the gICU group, respectively. Mean body temperature was 36.7 °C in the nICU group and 35.9 °C in the gICU group, respectively.
Univariate analysis
Incidence of vasospasm, DIND, and ischemic lesions
In the univariate analysis, lower age (OR 0.95, 95% CI 0.92–0.96, p < 0.001), higher GCS score (OR 1.38, 95% CI 1.01–1.87, p = 0.03), higher Fisher score (OR 0.51, 95% CI 0.32–0.81, p = 0.004), presence of EVD (OR 0.70, 95% CI 0.51–0.96, p = 0.02) or LD (OR 0.71, 95% CI 0.52–0.98, p = 0.03), and aneurysm clipping (OR 1.88, 95% CI 1.33–2.65, p < 0.001) were associated with higher incidence of vasospasm. Predictors of DIND were female sex (OR 0.62, 95% CI 0.42–0.93, p = 0.02), lower initial GCS score (OR 1.84, 95% CI 1.27–2.67, p = 0.001), higher Hunt and Hess grade (OR 0.67, 95% CI 0.46–0.97, p = 0.03), higher Fisher grade (OR 0.28, 95% CI 0.12–0.67, p = 0.004), presence of EVD (OR 0.39, 95% CI 0.27–0.58, p < 0.001) or LD (OR 0.55, 95% CI 0.37–0.81, p = 0.003), and treatment on the gICU (OR 0.35, 95% CI 0.24–0.51, p < 0.001). Lower initial GCS (OR 2.02, 95% CI 1.29–3.17, p = 0.002), higher Hunt and Hess grade (OR 0.54, 95% CI 0.35–0.85, p = 0.008), presence of an EVD (OR 0.52, 95% CI 0.33–0.82, p = 0.005) or LD (OR 1.57, 95% CI 1.01–2.46, p = 0.04), and treatment on the gICU (OR 2.04, 95% CI 1.28–3.24, p = 0.003) were predictors of delayed infarction.
Clinical outcome
Predictors of good clinical outcome were lower age (OR 0.95, 95% CI 0.94–0.96, p < 0.001), higher initial GCS score (OR 0.06, 95% CI 0.04–0.09, p < 0.001), lower Hunt and Hess grade (OR 16.37, 95% CI 11.09–24.16, p < 0.001), lower Fisher grade (OR 7.71, 95% CI 3.92–15.14, p < 0.001), absence of an EVD (OR 13.73, 95% CI 9.53–19.79, p < 0.001) or LD (OR 0.37, 95% CI 0.27–0.51, p < 0.001), endovascular aneurysm treatment (OR 1.76, 95% CI 1.26–2.46, p < 0.001), absence of DIND (OR 4.30, 95% CI 2.88–6.41, p < 0.001) or delayed infarction (OR 4.54, 95% CI 2.73–7.53, p < 0.001), and treatment on the nICU (OR 2.07, 95% CI 1.53–2.78, p < 0.001). Lower age (OR 0.94, 95% CI 0.92–0.95, p < 0.001), higher initial GCS score (OR 0.09, 95% CI 0.06–0.14, p < 0.001), lower Hunt and Hess grade (OR 10.59, 95% CI 6.96–16.09, p < 0.001), lower Fisher grade (OR 10.40, 95% CI 3.71–29.17, p < 0.001), absence of EVD (8.65, 95% CI 5.70–13.14, p < 0.001) or LD (OR 0.33, 95% CI 0.22–0.48, p < 0.001), endovascular aneurysm treatment (OR 1.77, 95% CI 1.20–2.63, p = 0.004), absence of DIND (OR 3.04, 95% CI 1.99–4.64, p < 0.001), or delayed infarction (OR 4.36, 95% CI 2.53–7.52, p < 0.001) was associated with better long-term outcome.
Multivariate analysis
Incidence of vasospasm, DIND, and ischemic lesions
Two hundred and fifty-four patients (55.7%) developed TCD vasospasm in the nICU group, of whom 55 patients (14.8%) developed a DIND. Regarding the gICU group, TCD vasospasm was detected in 170 patients (56.9%), of whom 78 (26.1%) patients developed a DIND. Aneurysm surgery was an idependent predictor of vasospasm (OR 2.4, 95% CI 1.57–3.53, p < 0.001), while the absence of EVD (OR 0.38, 95% CI 0.25–0.56, p < 0.001), lower Fisher grade (OR 0.45, 95% CI 0.26–0.78, p = 0.005), and higher age (OR 0.94, 95% CI 0.93–0.96, p < 0.001) were associated with lower incidence of vasospasm. An independent predictor of DIND was aneurysm surgery (OR 1.65, 95% CI 1.02–2.67, p = 0.04), while the absence of an EVD (OR 0.33, 95% CI 0.21–0.52, p < 0.001) or LD (OR 0.39, 95% CI 0.24–0.61, p < 0.001) and the treatment on the nICU were associated with lower incidence of DIND. Lower initial GCS score (OR 1.9, 95% CI 1.1–3.3, p = 0.02) and the treatment on the nICU were associated with higher incidence of delayed infarction, while the absence of an EVD (OR 0.45, 95% CI 0.26–0.81, p = 0.008) was associated with less delayed infarction (Table 2).
Clinical outcome
In the nICU group, GOS 1 was seen in 62 patients (13.6%), GOS 2 in 10 patients (2.2%), GOS 3 in 101 patients (22.2%), GOS 4 in 100 patients (21.9%), and GOS 5 in 183 patients (40.1%), respectively, by the time of discharge from the hospital. In the gICU group, GOS 1 was observed in 69 patients (23.5%), GOS 2 in 29 patients (9.9%), GOS 3 in 64 patients (21.8%), GOS 4 in 19 patients (6.5%), and GOS 5 in 113 patients (38.4%) by the time of discharge from hospital. Predictors of better outcome at discharge were the absence of EVD (OR 5.6, 95% CI 3.3–9.4, p < 0.001), absence of delayed infarctions (OR 5.03, 95% CI 2.51–10.05, p < 0.001), or absence DIND (OR 4.2, 95% CI 2.32–7.53), while lower initial GCS score (OR 0.15, 95% CI 0.09–0.26, p < 0.001) and lower age (OR 0.95, 95% CI 0.94–9.74, p < 0.001) were associated with poorer outcome at discharge.
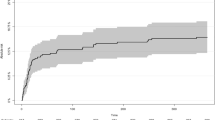
Long-term follow-up data (6 ± 17.9 months) were available in 60.7% (n = 277) of the nICU patients and in 87.0% (n = 260) of the gICU patients. GOS score after 6 months was as followed for the nICU group: 1 (n = 64, 23.1%), 2 (n = 1, 0.4%), 3 (n = 29, 10.5%), 4 (n = 36, 13.0%), and 5 (n = 147; 53.1%). GOS was as followed for the gICU group: 1 (n = 74, 28.5%), 2 (n = 8, 3.1%), 3 (n = 32, 12.3%), 4 (n = 24, 9.2%), 5 (n = 122, 46.9%). Predictors of good long-term outcome were the absence of an EVD (OR 3.2, 95% CI 1.8–5.69, p < 0.001), absence of delayed infarctions (OR 5.2, 95% CI 2.51–10.84, p < 0.001), or absence of DIND (OR 2.5, 95% CI 1.42–4.53, p = 0.002), while lower age (OR 0.95, 95% CI 0.93–0.97, p < 0.001) and lower initial GCS score (OR 0.30, 95% CI 0.17–0.54, p < 0.001) were predictors of poor long-term outcome. In the multivariate analysis, the type of ICU was not a significant predictor of short- and long-term outcomes.
In the nICU group, 72 patients (15.8%) required a ventriculo-peritoneal or ventriculo-atrial shunt, whereas 67 patients (22.4%) underwent shunt operation in the gICU group (p = 0.02).
Discussion
A meta-analysis of Kramer and Zygun identified 12 studies, including 24.520 patients, enrolled in different models of care for critically ill neurologic patients. Combining all of these data, mortality was clearly lower in specialized ICUs [11]. Wärme et al. demonstrated an improved clinical outcome after severe head injury in an organized nICU compared to a historical cohort treated in a gICU prior to the establishment of a nICU [24]. A review in 2014 supported these findings, but failed to exactly identify reasons for the better outcome [12]. Higher patient volumes, a more strict adherence to protocols, more intracranial and hemodynamic monitoring, more nutritional support, and less sedation were considered as possible reasons [13]. In addition, patient satisfaction is higher in a nICU [21]. Lott and coworkers performed a retrospective cohort study examining critical illness outcome in a variety of diagnoses in specialty ICUs versus gICU. This study included 11,984 patients admitted to 124 ICUs [15]. In contrast to the aforementioned studies, they found no significant differences in risk-adjusted mortality between general ICUs and specialty ICUs, among which were neurological ICUs. Neurosurgical ICUs were not specifically mentioned.
Recent studies have demonstrated that the presence of a neurointensivist on a gICU is associated with improved clinical outcomes in aSAH patients [9, 10, 14, 20, 21]. These studies and the data from the meta-analysis paved the way to our assumption that aSAH patients fare better if treated in a nICU.
This is the first study to compare the outcome of patients suffering from aSAH, treated either in a nICU or in a gICU with a neurosurgeon who serves as a consultant. This comparison is only possible due the fact that the senior author worked first in an institution with a nICU and transferred the neurosurgical/neurointensive care treatment strategy in detail to the second center. Only two neurosurgical treatment aspects were not kept constant throughout the whole study period: (1) Interventional aneurysm treatment was principally established during the second observation period. Thus, only 7.3% of the patients in the nICU group, but 51.5% of the patients in the gICU group, were treated interventionally by coiling. The ISAT study and its follow-up analyses indicate that coiling offers early and late outcome benefits if compared with clipping [16,17,18]. However, the multivariate analysis of our data showed that endovascular aneurysm treatment was not an independent predictor of clinical outcome. Therefore, we believe that the imbalance of the numbers of coiled and clipped patients did not influence the results. (2) Magnesium was exclusively given on the gICU until the results of the MASH-2-study have demonstrated that the intravenous application of magnesium does not improve clinical outcome after aSAH [3]. Despite being negative in terms of outcome improvement, the MASH-2-study did not provide evidence that magnesium administration has a negative effect on outcome. Again, we believe that the use of magnesium exclusively on the gICU had no substantial influence on the results. In contrast to neurosurgical/neurointensive care aspects, the senior author did not keep aspects of general intensive care (ventilation, sedation, nutrition) constant during the whole study period. Probably, some aspects of general intensive care changed within the study period. However, the specific effects of single intensive care measures on outcome after aSAH are not well investigated, making control of that bias difficult.
The major finding of the study was that the outcome of aSAH patients shows no correlation with the type of the ICU. We detected significant differences in both groups concerning the patient’s age, severity of the bleeding, and amount of blood seen on the initial CCT, with all these factors strongly contributing to the patient’s outcome. However, in the multivariate analysis, the type of ICU was not an independent predictor of clinical outcome, while we were able to confirm again the well-known risk factors for poor outcome after aSAH such as the Hunt and Hess grade and the Fisher score, supporting the validity of our analysis.
The most likely explanation for this finding is that the earlier detection and initiation of treatment of aSAH sequelae such as hydrocephalus, vasospasm, or brain edema on a nICU by a neurosurgeon are outweighted by a less profound knowledge in general intensive care such as respirator therapy or sedation and vice versa on a gICU. Possibly, an increasing time of parallel presence of a neurosurgeon and an intensivist on an ICU allows improving the outcome of aSAH patients.
Limitations of the study
The present study has several limitations. (1) Its retrospective study design per se implies the comparison of two groups that has not been obtained by randomization. It has to be acknowledged that the current study shares this limitation with the vast majority of studies addressing the same topic [9,10,11,12, 14, 15]. Thus, the two groups in our and the other studies cannot be equally compared in detail, especially concerning aspects in treatment contents (e.g., specific medication, ventilation agents, and dosage) or the quality of treatment and staff resources. However, a monocenter prospective, randomized trial is extremely unlikely to set up because the infrastructure of a hospital barely allows to randomly allocate a patient to a nICU or gICU. The value of a multicenter prospective, randomized trial would be substantially hampered by the fact that the treatment strategies are not uniform and might differ even more (e.g., quality of staff resources). (2) It is a limitation that databases which had been obtained in two different university centers were compared. We consider this limitation to be minor because the constancy of the treatment strategy in the whole study period was guaranteed by the senior author, who transferred the strategy from the first to the second center. In addition, it has to be kept in mind that a study comparing nICU or gICU outcomes requires either a personnel position change introducing the flaw of two center data collection or a hospital infrastructure change, which is a rare event. (3) Another limitation might be the long study period. It is impossible to determine all factors that might influence the observed results in our study. Thus, we were not able to address different important aspects that have changed over this long time period especially aspects of general intensive care where no attempt was made by the senior author to keep these factors constant. However, previous studies shared the same limitation [10, 14]. In addition, the effect on outcome of single intensive care measures is not well investigated. (4) Endovascular therapy played a minor role in the management of aneurysms in the nICU group compared to patients treated in the gICU. However, in the multivariate analysis, endovascular treatment was not an independent outcome predictor. (5) It seems possible that parameters being outcome-relevant, such as age-related co-morbidities, but having been beyond the scope of interest during data collection, influenced the results. (6) The loss to follow-up in the nICU group was substantially higher than that in the gICU group, which might have had an influence on the results of the study. On the other hand, differences in the quality of intensive care, if present, should have a substantially higher effect on short- than on long-term outcome. (7) Furthermore, we are aware of the problem that the conclusions we have formulated should be specially audited by different countries, as the structure and timetable of services and units differ from those of other countries or even nationwide. Hence, the results should interpreted carefully based on the specificity of the national healthcare system organization and regional treatment protocols for patients with aSAH.
Conclusion
The outcome is not better if patients with aSAH are treated on a neurosurgical ICU. The study does not allow to identify reasons for this result. It can be assumed that the higher competence of a neurosurgically trained team for early detection of secondary outcome-relevant neuroworsening is outweight by the higher knowledge of the intensivist in the more general aspects of intensive care.
References
Aaslid R, Huber P, Nornes H (1984) Evaluation of cerebrovascular spasm with transcranial Doppler ultrasound. J Neurosurg 60:37–41
Bederson JB, Sander Connolly E, Batjer H, Dacey RG, Dion JE, Diringer MN et al (2009) Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Assocation. Stroke 40:994–1025
Dorhout Mees SM, Algra A, Vandertop WP, van Kooten F, Kuijsten HA, Boiten J, MASH-2 Study Group et al (2012) Magnesium for aneurysmal subarachnoid haemorrhage (MASH2): a randomised placebo-controlled trial. Lancet 380:44–49
Fisher CM, Kistler JP, Davis JM (1980) Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery 6:1–9
Hunt WE, Hess RM (1968) Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg 28:14–20
Ionita CC, Graffagnino C, Alexander MJ, Zaidat OO (2008) The value of CT angiography and transcranial Doppler sonography in triaging suspected cerebral vasospasm in SAH prior to endovascular therapy. Neurocrit Care 9:8–12
Jennett B, Bond M (1975) Assessment of outcome after severe brain damage. Lancet 1:480–484
Jones C (1979) Glasgow coma scale. Am J Nurs 79:1551–1553
Josephson SA, Douglas VC, Lawton MT, English JD, Smith WS, Ko NU (2010) Improvement in intensive care unit outcomes in patients with subarachnoid hemorrhage after initiation of neurointensivist co-management. J Neurosurg 112:626–630
Knopf L, Staff I, Gomes J, McCullough L (2012) Impact of a neurointensivist on outcomes in critically ill stroke patients. Neurocrit Care 16:63–71
Kramer AH, Zygun DA (2011) Do neurocritical care units save lives? Measuring the impact of specialized ICUs. Neurocrit Care 14:329–333
Kramer AH, Zygun DA (2014) Neurocritical care: why does it make a difference? Curr Opin Crit Care 20:174–181
Kurtz P, Fitts V, Sumer Z, Jalon H, Cooke J, Kvetan V, Mayer SA (2011) How does care differ for neurological patients admitted to a neurocritical care unit versus a general ICU? Neurocrit Care 15:477–480
Lerch C, Yonekawa Y, Muroi C, Bjeljac M, Keller E (2006) Specialized neurocritical care, severity grade, and outcome of patients with aneurysmal subarachnoid hemorrhage. Neurocrit Care 5:85–92
Lott JP, Iwashyna TJ, Christie JD, Asch DA, Kramer AA, Kahn JM (2009) Critical illness outcomes in specialty versus general intensive care units. Am J Respir Crit Care Med 179:676–683
Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group et al (2002) International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 360:1267–1274
Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group et al (2005) International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 366:809–817
Molyneux AJ, Birks J, Clarke A, Sneade M, Kerr RS (2015) The durability of endovascular coiling versus neurosurgical clipping of ruptured cerebral aneurysms: 18 year follow-up of the UK cohort of the International Subarachnoid Aneurysm Trial (ISAT). Lancet 385:691–697
Polin RS, Coenen VA, Hansen CA, Shin P, Baskaya MK, Nanda A, Kassell NF (2000) Efficacy of transluminal angioplasty for the management of symptomatic cerebral vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg 92:284–290
Samuels O, Webb A, Culler S, Martin K, Barrow D (2011) Impact of a dedicated neurocritical care team in treating patients with aneurysmal subarachnoid hemorrhage. Neurocrit Care 14:334–340
Sarpong Y, Nattanmai P, Schelp G, Bell R, Premkumar K, Stapleton E, McCormick A, Newey CR (2017) Improvement in quality metrics outcomes and patient and family satisfaction in a neurosciences intensive care unit after creation of a dedicated neurocritical care team. Crit Care Res Pract 2017:6394105
Wachter D, Hans F, Kreitschmann-Andermahr I, Rohde V (2011) Lower incidence of transcranial Doppler and symptomatic vasospasm after aneurysmal subarachnoid hemorrhage and aneurysm clipping in the elderly patient? Neurosurgery 69:261–266
Wachter D, Kreitschmann-Andermahr I, Gilsbach JM, Rohde V (2011) Early surgery of multiple versus singular aneurysms after subarachnoid hemorrhage—an increased risk for cerebral vasospasm? J Neurosurg 114:935–941
Wärme PE, Bergstrom R, Persson I (1991) Neurosurgical intensive care improves outcomes after head injury. Acta Neurochir 110:57–65
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was reviewed by the local ethics committee (ethics committee Georg-August-University Göttingen), but did not need approval because it was a retrospective analysis of anonymous data.
Informed consent
Not needed because of anonymous patient data
Additional information
The manuscript is submitted on behalf of all authors and all named authors have participated in this work.
Rights and permissions
About this article
Cite this article
Mielke, D., Malinova, V., Moerer, O. et al. Does the subspecialty of an intensive care unit (ICU) has an impact on outcome in patients suffering from aneurysmal subarachnoid hemorrhage?. Neurosurg Rev 42, 147–153 (2019). https://doi.org/10.1007/s10143-018-0973-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-018-0973-x