Abstract
Patients presenting with spontaneous, non-aneurysmal subarachnoid hemorrhage (SAH) achieve better outcomes compared to patients with aneurysmal SAH. Nevertheless, some patients develop shunt-dependent hydrocephalus during treatment course. We therefore analyzed our neurovascular database to identify factors determining shunt dependency after non-aneurysmal SAH. From 2006 to 2016, 131 patients suffering from spontaneous, non-aneurysmal SAH were admitted to our department. Patients were stratified according to the distribution of cisternal blood into patients with perimesencephalic SAH (pSAH) versus non-perimesencephalic SAH (npSAH). Outcome was assessed according to the modified Rankin Scale (mRS) at 6 months and stratified into favorable (mRS 0–2) versus unfavorable (mRS 3–6). A multivariate analysis was performed to identify predictors of shunt dependency in patients suffering from non-aneurysmal SAH. Overall, 18 of 131 patients suffering from non-aneurysmal SAH developed shunt dependency (14%). In detail, patients with npSAH developed significantly more often shunt dependency during treatment course, when compared to patients with pSAH (p = 0.02). Furthermore, patients with acute hydrocephalus, presence of intraventricular hemorrhage, presence of clinical vasospasm, and anticoagulation medication prior SAH developed significantly more often shunt dependency, when compared to patients without (p < 0.0001). However, “acute hydrocephalus” was the only significant and independent predictor for shunt dependency in all patients with non-aneurysmal SAH in the multivariate analysis (p < 0.0001). The present study identified acute hydrocephalus with the necessity of CSF diversion as significant and independent risk factor for the development of shunt dependency during treatment course in patients suffering from non-aneurysmal SAH.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Spontaneous subarachnoid hemorrhage (SAH) is usually caused by a rupture of an intracranial aneurysm. However, in approximately 15% of patients suffering from SAH, identification of the bleeding source is not possible [18, 24]. This subgroup has been described as patients suffering from non-aneurysmal, non-traumatic, spontaneous subarachnoid hemorrhage previously. Patients with SAH without a bleeding source are considered to have a more benign course of disease compared to patients suffering from aneurysmal SAH [8, 14, 24]. Nevertheless, these patients can develop chronic post-hemorrhagic hydrocephalus with the need of cerebrospinal fluid (CSF) diversion leading to the necessity of insertion of a ventriculoperitoneal shunt (VPS) [11].
We therefore analyzed our institutional data in order to identify possible risk factors associated with the development of a chronic hydrocephalus during treatment course.
Materials and methods
Patients
Between January 2006 and August 2016, 814 patients suffering from spontaneous SAH were admitted to our institution. SAH was diagnosed by computed tomography (CT) or lumbar puncture. All patients with spontaneous SAH underwent four-vessel digital subtraction angiography (DSA); following 2012, three-dimensional reconstructions were additionally performed. All patients with confirmed bleeding source (intracranial aneurysm, vascular malformation) or traumatic origin of SAH were excluded from further analysis. In patients with angiogram-negative SAH, a spinal magnetic resonance imaging (MRI) scan was performed in order to rule out any spinal bleeding source of SAH. Furthermore, patients underwent a second four-vessel DSA 6 weeks after ictus, if a potential bleeding source was not identified during the first diagnostic workup. Patients with spontaneous, non-traumatic SAH without identification of a bleeding source after diagnostic screening were then included for further analysis in the present study.
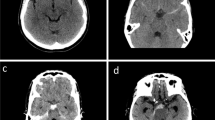
Information including patient characteristics on admission and during treatment course, radiological features, and functional neurological outcome were collected and entered into a computerized database (SPSS, version 24, IBM Corp., Armonk, NY). The World Federation of Neurological Surgeons (WFNS) scale was used in order to grade patients on admission [19, 22]. Patients suffering from spontaneous SAH were divided into good grade (WFNS grades I–III) versus (vs.) poor grade (WFNS grades IV–V) on admission. Patients with SAH were stratified into two groups according to the subarachnoid blood distribution into (1) patients suffering from perimesencephalic SAH (pSAH, Fig. 1a) and (2) patients suffering from non-perimesencephalic SAH (npSAH, Fig. 1b) [17]. In addition, further radiological features were identified and collected, including the Barrow Neurological Institute (BNI) scale [27], presence of intraventricular hemorrhage (IVH), and new cerebral ischemia during treatment course. Diagnosis of acute hydrocephalus was based on radiological criteria and relevant clinical symptoms within the acute phase after ictus. Patients with acute hydrocephalus (AH) were treated by ventriculostomy for external CSF diversion [21]. All patients with spontaneous SAH received nimodipine from the day of clinical admission. Screening for cerebral vasospasm (CVS) was performed daily using neurological examination and transcranial Doppler ultrasound (TCD) measurements. If CVS was suspected on the basis of TCD or delayed ischemic neurological deficit (DIND), CT-angiography/-perfusion (CT-A, CT-P) was performed in order to confirm CVS. Presence of radiological CVS was defined as vessel narrowing in CT-A. Presence of clinical CVS was defined as CVS-associated DIND and/or CVS-related perfusion deficit in CT-P. In cases of onset of clinical CVS, hypertension was induced with catecholamines during treatment course [16]. Delayed cerebral ischemia (DCI) was defined as occurrence of new ischemic lesions on any radiological imaging during treatment course compared to the initial CT scan on admission. The necessity of VPS surgery was considered when elevation of the drip chamber of the external ventricular drainage (EVD) resulted in neurological deterioration or increased ventricular size. In case of shunt-dependent hydrocephalus during treatment, a VPS was placed, primarily in the right frontal horn as previously reported [20]. Indication for VPS surgery in patients suffering from shunt-dependent hydrocephalus were based on clinical symptoms, radiological features of hydrocephalus, or patients not tolerating CSF drainage weaning [1]. Outcome was assessed according to the modified Rankin Scale (mRS) after 6 months and stratified into favorable (mRS 0–2) versus unfavorable (mRS 3–6).
Statistics
Data analyses were performed using the computer software package SPSS (version 24, IBM Corp., Armonk, NY). Unpaired t test was used for parametric statistics. Categorical variables were analyzed in contingency tables using Fisher’s exact test. Results with p < 0.05 were considered statistically significant.
Furthermore, a multivariate analysis was performed to find independent predictors of chronic hydrocephalus in patients suffering from non-aneurysmal SAH using binary logistic regression analysis to find confounding factors between potentially independent predictors. Variables with significant p values in the univariate analysis were considered as potentially independent variables in a multivariate analysis. A backward stepwise method was used to construct a multivariate logistic regression model in relation to chronic hydrocephalus as a dependent variable with an inclusion criterion of a p value < 0.05.
Results
Patient characteristics
Between January 2006 and August 2016, 814 patients suffering from spontaneous SAH were admitted to our institution, and in 131 patients of which no bleeding source was found (16%). In detail, 56 of 131 patients suffered from pSAH (43%), and 75 patients suffered from npSAH (57%).
Patient characteristics, including age, clinical admission status, angiographic and radiological findings, presence of acute hydrocephalus, information concerning the use of anticoagulation agents prior SAH, shunt dependency, and clinical outcome of the present series, are shown in Table 1.
Blood distribution in patients with non-aneurysmal SAH
Overall, favorable outcome was achieved in 114 patients suffering from non-aneurysmal SAH in the present series (86%). In detail, all patients with pSAH achieved favorable outcome versus (vs.) 58 patients suffering from npSAH (100 vs. 77%; p < 0.0001, OR 33.8, 95% CI 1.9–576). Details on BNI grading of blood distribution are given in Table 1. Patients with npSAH suffered significantly more often from intraventricular hemorrhage when compared to patients with pSAH (37 vs. 16%; p = 0.01, OR 3.1, 95% CI 1.3–7.3). Significantly more patients with npSAH revealed radiological CVS during treatment course compared to patients with pSAH (16 vs. 2%; p = 0.007, OR 10.5, 95% CI 1.3–83.2). However, presence of clinical CVS or DCI did not differ significantly between both groups. Furthermore, patients with pSAH were significantly younger and presented in better clinical condition when compared to patients suffering from npSAH (p = 0.0003; p = 0.005; Table 1). However, no significant differences between both groups were observed concerning anticoagulation medication prior SAH (p = 0.06).
Acute hydrocephalus
Overall, 34 patients (26%) underwent ventriculostomy due to onset of AH in the early stages of treatment. AH occurred significantly more often in patients suffering from npSAH when compared to patients with pSAH (39 vs. 9%; p = 0.0001, OR 6.4, 95% CI 2.3–18).
Shunt-dependent hydrocephalus
Overall, 18 of 131 patients (14%) with non-aneurysmal SAH developed shunt-dependent hydrocephalus during treatment course (Table 2). Patients suffering from npSAH developed shunt-dependent hydrocephalus significantly more often compared to patients with pSAH (20 vs. 5%; p = 0.02, OR 4.4, 95% CI 1.2–16.1). Furthermore, patients with initial AH developed significantly more often shunt dependency compared to patients without initial AH (44 vs. 3%; p < 0.0001, OR 24.7, 95% CI 6.5–93.9). Patients with non-aneurysmal SAH and IVH suffered significantly more often from shunt-dependent hydrocephalus compared to patients without (p = 0.045, OR 3.0, 95% CI 1.1–8.4). Patients with non-aneurysmal hemorrhage and the occurrence of clinical CVS during treatment course developed significantly more often shunt dependency compared to patients without (p = 0.02, OR 6.2, 95% CI 1.5–25.7). Additionally, patients with use of anticoagulation agents prior non-aneurysmal SAH suffered significantly more often from shunt dependency compared to patients without (p = 0.03, OR 3.3, 95% CI 1.2–9.4). Favorable outcome did not differ significantly between patients who developed no shunt dependency throughout the treatment course and patients with development of a shunt dependency after SAH (89 vs. 78%, p = 0.3).
Multivariate analysis
We performed a multivariate logistic regression analysis of those variables significantly associated with shunt dependency in patients suffering from non-aneurysmal SAH. The multivariate regression model did illustrate the variable “acute hydrocephalus” (p < 0.0001, OR 24.7, 95% CI 6.5–93.9) as independent and significant predictor for shunt dependency in patients suffering from non-aneurysmal SAH (Nagelkerke’s R2 = 0.39, Table 3).
Discussion
Development of a shunt-dependent hydrocephalus after aneurysmal SAH is a known cause for unfavorable outcome and cognitive deficit [6]. Several risk factors for shunt dependency in patients suffering from aneurysmal SAH have previously been reported [4, 5, 10, 26]. In patients with spontaneous SAH without a bleeding source, a better prognosis compared to patients with aneurysmal SAH has been reported [3, 9, 24]. However, previously published studies suggested that several factors might influence potential favorable outcome in patients with non-aneurysmal SAH [2, 9, 12, 15, 25]. Despite initial subarachnoid blood distribution, chronic hydrocephalus might influence functional outcome in patients with non-aneurysmal SAH. However, great variability concerning the rate of development of shunt-dependent hydrocephalus in patients with non-aneurysmal SAH has been reported. In detail, Duong et al. reported chronic hydrocephalus in 8% of patients with non-aneurysmal SAH, leading to necessity of VPS surgery in 3% [7]. Konczalla et al. reported that shunt-dependent hydrocephalus occurred in 10% of patients suffering from non-aneurysmal SAH [13]. The present series reports a rate of 14% for development of shunt-dependent hydrocephalus after non-aneurysmal SAH. Differences in shunt dependency rate in patients suffering from non-aneurysmal SAH might be caused by different proportions of patients with pSAH and diffuse blood distribution (npSAH) in the previously mentioned studies.
Risk factors for shunt dependency in non-aneurysmal SAH
Despite well-known risk factors for shunt dependency in patients suffering from aneurysmal SAH, data on risk factors for VPS-dependent hydrocephalus in patients with non-aneurysmal SAH is scarce.
The univariate analysis of the present study revealed that patients suffering from npSAH and therefore diffuse blood distribution developed significantly more often shunt-dependent hydrocephalus compared to patients with pSAH (p = 0.02). In line with these results, a recent published study did identify diffuse blood distribution in patients with non-aneurysmal SAH as predictor for development of shunt dependency during treatment course [11]. Presence of IVH is known as a significant predictor for development of shunt-dependent hydrocephalus in patients suffering from aneurysmal SAH [5]. Likewise, presence of IVH in patients suffering from non-aneurysmal SAH was significantly associated with the necessity of VPS implantation in the present series (p = 0.045). Similarly, occurrence of clinical CVS has previously been associated with shunt dependency in patients suffering from aneurysmal SAH [6]. In the present series, development of clinical CVS in patients with non-aneurysmal SAH was significantly associated with shunt dependency during treatment course (p = 0.02). The amount of subarachnoid blood might influence the risk of CVS and chronic hydrocephalus, which might explain the association between both entities [6]. Additionally, we identified acute hydrocephalus as significantly associated with higher shunt dependency in the univariate analysis of the present series (p < 0.0001). Acute hydrocephalus with the necessity of a CSF diversion and increased daily CSF output has been identified as predictor for chronic shunt dependency in patients with aneurysmal SAH [23]. Despite the above-mentioned results of the univariate analysis for potential risk factors, the presence of initial AH with necessity of CSF diversion was the only significant and independent risk factor for shunt dependency in patients with non-aneurysmal SAH in the multivariate analysis of the present series (p < 0.0001).
Limitations
The present study has several limitations. Statistical analysis and data collection was retrospective and the present data represent only a single-center experience. Furthermore, there are only a small number of shunted patients in the present patient collective, which might also influence statistical results.
Conclusions
The present study identified acute hydrocephalus with the necessity of CSF diversion as significant and independent risk factor for the development of shunt dependency during treatment course in patients suffering from non-aneurysmal SAH. This should be taken in consideration during treatment of patients with non-aneurysmal SAH.
References
Adams H, Ban VS, Leinonen V, Aoun SG, Huttunen J, Saavalainen T, Lindgren A, Frosen J, Fraunberg M, Koivisto T, Hernesniemi J, Welch BG, Jaaskelainen JE, Huttunen TJ (2016) Risk of shunting after aneurysmal subarachnoid hemorrhage: a collaborative study and initiation of a consortium. Stroke 47:2488–2496. https://doi.org/10.1161/STROKEAHA.116.013739
Beseoglu K, Pannes S, Steiger HJ, Hanggi D (2010) Long-term outcome and quality of life after nonaneurysmal subarachnoid hemorrhage. Acta Neurochir 152:409–416. https://doi.org/10.1007/s00701-009-0518-8
Boswell S, Thorell W, Gogela S, Lyden E, Surdell D (2013) Angiogram-negative subarachnoid hemorrhage: outcomes data and review of the literature. J Stroke Cerebrovasc Dis 22:750–757. https://doi.org/10.1016/j.jstrokecerebrovasdis.2012.02.001
de Oliveira JG, Beck J, Setzer M, Gerlach R, Vatter H, Seifert V, Raabe A (2007) Risk of shunt-dependent hydrocephalus after occlusion of ruptured intracranial aneurysms by surgical clipping or endovascular coiling: a single-institution series and meta-analysis. Neurosurgery 61:924–933; discussion 933–924. https://doi.org/10.1227/01.neu.0000303188.72425.24
Diesing D, Wolf S, Sommerfeld J, Sarrafzadeh A, Vajkoczy P, Dengler NF (2017) A novel score to predict shunt dependency after aneurysmal subarachnoid hemorrhage. J Neurosurg 1–7. doi:https://doi.org/10.3171/2016.12.JNS162400
Dorai Z, Hynan LS, Kopitnik TA, Samson D (2003) Factors related to hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery 52:763–769 discussion 769–771
Duong H, Melancon D, Tampieri D, Ethier R (1996) The negative angiogram in subarachnoid haemorrhage. Neuroradiology 38:15–19
Elhadi AM, Zabramski JM, Almefty KK, Mendes GA, Nakaji P, McDougall CG, Albuquerque FC, Preul MC, Spetzler RF (2015) Spontaneous subarachnoid hemorrhage of unknown origin: hospital course and long-term clinical and angiographic follow-up. J Neurosurg 122:663–670. https://doi.org/10.3171/2014.10.JNS14175
Gupta SK, Gupta R, Khosla VK, Mohindra S, Chhabra R, Khandelwal N, Gupta V, Mukherjee KK, Tewari MK, Pathak A, Mathuriya SN (2009) Nonaneurysmal nonperimesencephalic subarachnoid hemorrhage: is it a benign entity? Surg Neurol 71:566–571; discussion 571,571-562,572. https://doi.org/10.1016/j.surneu.2008.04.021
Jabbarli R, Bohrer AM, Pierscianek D, Muller D, Wrede KH, Dammann P, El Hindy N, Ozkan N, Sure U, Muller O (2016) The CHESS score: a simple tool for early prediction of shunt dependency after aneurysmal subarachnoid hemorrhage. Eur J Neurol 23:912–918. https://doi.org/10.1111/ene.12962
Kang P, Raya A, Zipfel GJ, Dhar R (2016) Factors associated with acute and chronic hydrocephalus in nonaneurysmal subarachnoid hemorrhage. Neurocrit Care 24:104–109. https://doi.org/10.1007/s12028-015-0152-7
Konczalla J, Kashefiolasl S, Brawanski N, Lescher S, Senft C, Platz J, Seifert V (2016) Cerebral vasospasm and delayed cerebral infarctions in 225 patients with non-aneurysmal subarachnoid hemorrhage: the underestimated risk of Fisher 3 blood distribution. J Neurointerv Surg 8:1247–1252. https://doi.org/10.1136/neurintsurg-2015-012153
Konczalla J, Platz J, Schuss P, Vatter H, Seifert V, Güresir E (2014) Non-aneurysmal non-traumatic subarachnoid hemorrhage: patient characteristics, clinical outcome and prognostic factors based on a single-center experience in 125 patients. BMC Neurol 14:140. https://doi.org/10.1186/1471-2377-14-140
Konczalla J, Schmitz J, Kashefiolasl S, Senft C, Platz J, Seifert V (2016) Non-aneurysmal non-perimesencephalic subarachnoid hemorrhage: effect of rehabilitation at short-term and in a prospective study of long-term follow-up. Top Stroke Rehabil 23:261–268. https://doi.org/10.1080/10749357.2016.1149982
Konczalla J, Schmitz J, Kashefiolasl S, Senft C, Seifert V, Platz J (2015) Non-aneurysmal subarachnoid hemorrhage in 173 patients: a prospective study of long-term outcome. Eur J Neurol 22:1329–1336. https://doi.org/10.1111/ene.12762
Raabe A, Beck J, Keller M, Vatter H, Zimmermann M, Seifert V (2005) Relative importance of hypertension compared with hypervolemia for increasing cerebral oxygenation in patients with cerebral vasospasm after subarachnoid hemorrhage. J Neurosurg 103:974–981. https://doi.org/10.3171/jns.2005.103.6.0974
Rinkel GJ, Wijdicks EF, Hasan D, Kienstra GE, Franke CL, Hageman LM, Vermeulen M, van Gijn J (1991) Outcome in patients with subarachnoid haemorrhage and negative angiography according to pattern of haemorrhage on computed tomography. Lancet 338:964–968
Rinkel GJ, Wijdicks EF, Vermeulen M, Hasan D, Brouwers PJ, van Gijn J (1991) The clinical course of perimesencephalic nonaneurysmal subarachnoid hemorrhage. Ann Neurol 29:463–468. https://doi.org/10.1002/ana.410290503
Rosen DS, Macdonald RL (2004) Grading of subarachnoid hemorrhage: modification of the World Federation of Neurosurgical Societies scale on the basis of data for a large series of patients. Neurosurgery 54:566–575 discussion 575-566
Schuss P, Borger V, Güresir Á, Vatter H, Güresir E (2015) Cranioplasty and ventriculoperitoneal shunt placement after decompressive craniectomy: staged surgery is associated with fewer postoperative complications. World Neurosurg 84:1051–1054. https://doi.org/10.1016/j.wneu.2015.05.066
Schuss P, Wispel C, Borger V, Güresir Á, Vatter H, Güresir E (2017) Accuracy and safety of ventriculostomy using two different procedures of external ventricular drainage: a single-center series. J Neurol Surg Part A, Central Eur Neurosurg. https://doi.org/10.1055/s-0037-1606544
Teasdale GM, Drake CG, Hunt W, Kassell N, Sano K, Pertuiset B, De Villiers JC (1988) A universal subarachnoid hemorrhage scale: report of a committee of the World Federation of Neurosurgical Societies. J Neurol Neurosurg Psychiatry 51:1457
Tso MK, Ibrahim GM, Macdonald RL (2016) Predictors of shunt-dependent hydrocephalus following aneurysmal subarachnoid hemorrhage. World Neurosurg 86:226–232. https://doi.org/10.1016/j.wneu.2015.09.056
van Gijn J, van Dongen KJ, Vermeulen M, Hijdra A (1985) Perimesencephalic hemorrhage: a nonaneurysmal and benign form of subarachnoid hemorrhage. Neurology 35:493–497
Whiting J, Reavey-Cantwell J, Velat G, Fautheree G, Firment C, Lewis S, Hoh B (2009) Clinical course of nontraumatic, nonaneurysmal subarachnoid hemorrhage: a single-institution experience. Neurosurg Focus 26:E21. https://doi.org/10.3171/2009.2.FOCUS092
Wilson CD, Safavi-Abbasi S, Sun H, Kalani MY, Zhao YD, Levitt MR, Hanel RA, Sauvageau E, Mapstone TB, Albuquerque FC, McDougall CG, Nakaji P, Spetzler RF (2017) Meta-analysis and systematic review of risk factors for shunt dependency after aneurysmal subarachnoid hemorrhage. J Neurosurg 126:586–595. https://doi.org/10.3171/2015.11.JNS152094
Wilson DA, Nakaji P, Abla AA, Uschold TD, Fusco DJ, Oppenlander ME, Albuquerque FC, McDougall CG, Zabramski JM, Spetzler RF (2012) A simple and quantitative method to predict symptomatic vasospasm after subarachnoid hemorrhage based on computed tomography: beyond the Fisher scale. Neurosurgery 71:869–875. https://doi.org/10.1227/NEU.0b013e318267360f
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The present study was approved by the local ethics committee.
Informed consent
Informed consent was not sought as a retrospective study design was used.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Schuss, P., Hadjiathanasiou, A., Brandecker, S. et al. Risk factors for shunt dependency in patients suffering from spontaneous, non-aneurysmal subarachnoid hemorrhage. Neurosurg Rev 42, 139–145 (2019). https://doi.org/10.1007/s10143-018-0970-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-018-0970-0