Abstract
The increased interest in the application of lasers in neuro-oncology prompted us to present our experience of using the laser technologies in the treatment of cerebral gliomas. The aim of the study was to evaluate the clinical efficacy of image-guided laser surface thermal therapy (LSTT) and its influence on survival of patients with glioblastoma (GBM).
Data of 91 patients (49 males, 42 females, mean age 51.4 years, range 23–70 years) with supratentorial GBMs located in close vicinity to or within the eloquent brain areas were retrospectively analyzed.
All patients were divided into two groups: LSTT group (n = 28) and control group (n = 63). There were no significant differences by gender, age, Karnofsky Performance Scale (KPS) score, and tumor location between groups. Total removal in the LSTT group was performed in 67.9%, in the control group—31.7% (p < 0.01); on the contrary, subtotal removal prevailed in the control group—52.4%; in the LSTT group, it was 32.1%. In postoperative period, there was no significant difference in KPS score between the groups (p = 0.89). A higher degree of resection provided an increase in survival rates (p < 0.01). The median overall survival was 15.5 ± 10.5 months, in the LSTT group 18.4 ± 11.7 and in the control group 14.3 ± 9.1 (p = 0.03). The application of image-guided LSTT in patients with GBMs of eloquent brain areas allowed the high rate of complete resection and improved overall survival without the negative effect on the functional status after surgery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The inability of radical surgical resection of primary brain tumors caused by infiltrative growth pattern and extensive invasion into the eloquent brain areas explains early relapse following treatment with the tendency to malignant transformation [3, 8]. The decrease in the quality of life and life expectancy after surgery with adjuvant therapy can be observed both in patients with low-grade and high-grade gliomas [10, 12, 28]. The modern approach to the treatment of gliomas is multidisciplinary and should necessarily consider the pathohistological and genetic aspects of the tumor process. However, in patients with high-grade gliomas, the prognosis is commonly poor, and the overall median survival in patients with glioblastoma (GBM) even after the treatment in compliance with modern protocols slightly exceeds 1 year [2, 4, 8, 14].
From this perspective, most studies adhere to the tactics of the maximum possible resection of gliomas within the functionally reasonable limits [5, 25]. The impact of the extent of resection (EOR) maximizing, as a factor contributing to survival, could be accurately estimated when the better neurological and surgical outcomes were assured [2, 4, 14]. The desire for the total and supratotal tumor removal should be logically justified and conducted taking into account the specific features of the tumor process expansion and eloquent brain area location, as well as the patient’s initial neurological status [6, 31].
The option of safe mini-traumatic surgical resection of brain tumors has a direct connection with the application of intracerebral orientation methods, techniques of intraoperative identification of the tumor tissue, and determination of the spatial correlation of the tumor with intact brain structures [11, 25, 28].
In recent years, a return to the issue of using lasers in neuro-oncology is observed. The various laboratory and clinical findings confirm that high-energy laser radiation could be used as an instrument of mini-traumatic contactless and controlled destructive effect on tumors in the areas of vital and eloquent brain structures [20, 27, 30]. The laser thermal action on biological tissues results in effects of laser ablation, laser dissection, and laser coagulation. The dose of laser radiation could be continuously monitored in real time to obtain the required result [20, 29].
The improvement of laser devices and methods for delivering of laser radiation, as well as the emergence of neuronavigation systems with the possibility of detailed 3D planning, has allowed to expand indications for the use of lasers in neuro-oncology and to introduce new methods of laser action [7, 15,16,17,18].
In this study, we present the results of our experience on laser technology application in the high-grade glioma surgery using the image-guided laser surface thermal therapy (LSTT).
Materials and methods
Patient population
A retrospective study included all patients with supratentorial GBM in eloquent brain areas who were operated from June 2008 to September 2016 at Romodanov Neurosurgery Institute (Kiev, Ukraine) and registered in the National Cancer Registry of Ukraine (Kiev, Ukraine).
The inclusion criteria were as follows: (1) age ≥ 18 years, (2) newly diagnosed supratentorial GBM (WHO grade IV), (3) tumor location within or in close vicinity of eloquent brain areas, and (4) follow-up and survival data availability. Exclusion criteria were as follows: (1) history of any other malignant tumor and (2) incomplete clinical and neuroimaging datasets.
Evaluation of the functional status of patients was carried out according to clinical observation in dynamics in the preoperative and postoperative period using the Karnofsky Performance Scale (KPS) by a board-certified neurosurgeon or physiotherapist. All patients received standardized perioperative care.
Imaging studies
MRI studies were carried out on an MR scanner with a magnetic field induction of 0.2 T MAGNETOM Concerto (Siemens, Germany) as first-line diagnostic imaging tool and 1.5 T Philips Intera (Philips, Netherlands) according to the radiological study protocol for the cranial program of navigation station.
3D T1-weighted with Gd-enhancement, T2-weighted, T2-FLAIR, and DWI sequence were used for presurgery planning. The SENSE parallel scanning technology was applied to obtain diffusion-weighted MRI images. The diffusion gradient was formed in 15 directions with a b-factor of 800 s/mm2.
Postoperative computed tomography was performed on Somatom AR STAR PLUS (Siemens, Germany) or Aquilion ONE (Toshiba, Japan) within 24 h of surgery to assess the extent of performed surgical resection and identification of complication signs. The EOR was characterized as total when no residual tumor on postoperative CT was visualized; the subtotal resection estimated the presence of less than 10% of tumor tissue. Otherwise, the partial resection was reported.
Image processing
Data processing of the DWI series was carried out using Extended MR Workspace (Philips Medical Systems, Netherlands). The diffusion tensor imaging fiber tracking (DTI-FT) was used to localize corticospinal tract and arcuate fasciculus. Subcortical tracts were visualized depending on the principal diffusion vector for each voxel in the retrograde and orthograde direction. The index of fractional anisotropy was 0.1–0.15, and the minimum fiber length (50–70 mm) and the rotation angle (20°–55°) were used as a criterion for the tract segmentation. Regions of interest were determined in the white matter around the tract passage zones based on the data of the previous MRI-T1 study and were located perpendicular to the tract fibers.
MR venography was performed based on a spatial reconstruction of contrast-enhanced MRI-T1 series using StealthStation Application Software Cranial 5 (Medtronic, USA). This data processing technique provided 3D visualization of convexital venous collectors, as well as smaller veins in the projection of surgical approach.
Series of MRI-T1, MRI-Т2, FLAIR, MR venography, and DTI-FT data were combined and displayed on the planning station monitor as an overlaid image in different combinations, depending on the informative value of each data series.
Spatial 3D-modeling and sequential preoperative planning included segmentation of the tumor, the identification of tumor invasion areas, contouring of the perifocal edema, and topographic reconstruction of the cerebral hemispheres with subcortical tracts, cerebral ventricles, and convexital vessels. The spatial location of the tumor to eloquent brain areas was defined on 3D models using Sawaya scale [22]. According to these data, the total tumor volume available for the removal, as well as the residual part of the tumor, was separately calculated. The optimal surgical approach boundaries were defining preoperative on the 3D model.
Image-guided laser surface thermal therapy
LSTT was used to maximize the EOR of the tumor remains in the eloquent brain areas. The surgical action of laser radiation on tumor tissue was committed with ablative and deep thermal destruction effect. We performed laser radiation using the semiconductor surgical lasers with wavelength 808 and 1470 nm (Fotonica-Plus, Ukraine).
The optimal parameters of the continuous and pulsed regimes of laser irradiation were obtained in a laboratory experiment on the intact rabbit brain then were tested on the model of transfused malignant glioma (strain 101.8) in rats. In order to elucidate the nature of laser-induced changes both in the tumor tissue and normal brain, the series of light-optical and electron-microscopic studies were carried out to assess the possibility of effective laser application in surgical neuro-oncology.
In all clinical cases, the tumor infiltration areas in close vicinity or within eloquent brain were segmented and marked on the 3D model. These areas were identified intraoperatively using neuronavigation system StealthStation TREON Plus (Medtronic, USA) and then irradiated with a beam of 1–3 mm in diameter. The ablative effect that aimed the distinct destruction of the compact areas of tumor invasion was possible due to a higher water absorption of 808-nm wave light (impulse/pause = 50 ms/10 ms, 8–12 W). The effect of deep thermal destruction of tumor cells was performed using 1047-nm laser light by irradiation of all surface of resection cavity with a wide beam of 5–10 mm in diameter using continuous regime of irradiation with a power of 5–7 W. The total dose of radiation depended on the dimensions of radiation area.
Statistical analysis
The continuous variables were analyzed using descriptive statistics and stated as mean. The standard deviation and frequency of distribution were calculated for categorical variables.
The chi-square test was used to compare distributions of categorical variables, and two-sample t test was used to compare the groups for continuous variables. The McNemar test was used to analyze the depending samples. Pearson correlation coefficients were computed to examine the relationship between the variables.
Overall survival (OS) was estimated by the Kaplan-Meier method. Survival curve comparison for the various subgroups was performed using the log-rank (Mantel-Cox) test. OS was calculated as a function of time for the period from histological diagnosis onset until death or last follow-up if censored. The multivariable Cox proportional hazards model was used to examine six variables: age, largest diameter of the tumor, Sawaya grade, EOR, using of LSTT, and neuronavigation. A p value of < 0.05 was considered significant.
Statistical analysis was performed using the Deducer package (Java GUI extensions to statistical programming platform R licensed under the GNU).
Results
The study population included 42 (46.2%) women and 49 (53.8%) men with a mean age of 51.4 years (range 23–70 years). Tumors affected eloquent brain areas (Sawaya III) in 24 (26.4%) and located adjacent to eloquent areas (Sawaya II) in 67 (73.6%) cases. Large tumors (> 50mm) were observed in 41 (45.1%) patients, medium size tumors (30–50 mm) in 40 (44.0%), and small (< 30mm) in 10 (10.9%).
The average KPS score was 65.6 in the preoperative period, and the number of patients with KPS scores less than 70 was 81 (89.0%). Demographic and clinical characteristics of patients in study groups are presented in Table 1.
The reliable relationship of the functional status in the preoperative period with tumor size (R = − 0.02, p = 0.84) and localization (R = − 0.15, p = 0.53) has not been estimated.
Total resection was carried out in 39 (42.8%) cases; in other cases, the high risk of eloquent brain injury allowed to perform subtotal removal in 42 (46.2%) patients, and partial in 10 (11.0%). The clinical and surgical outcomes are summarized in Table 2.
The EOR was slightly negative correlated with Sawaya grade (R = 0.18, p = 0.01) and did not have a reliable relationship with the tumor size (R = − 0.19, p = 0.06). Neuronavigation was used for surgical planning and intraoperative support in 80 (87.9%) patients. The use of neuronavigation promoted the higher grade of tumor resection (R = 0.28, p < 0.01).
Laser thermal therapy was performed after the microsurgical resection of the tumor in 28 (31.9%) cases. Ablative effect of laser radiation was used for additional elimination of tumor tissue in 19 (20.9%) patients where extensive tumor invasion of eloquent brain areas was identified. At the same time, the concomitant coagulating effect provided gentle hemostasis. The average radiation dose was 3406 (700–7000) J, with an average power of 5 (4–7) W (Fig. 1).
Total resection of a newly diagnosed right motor area glioblastoma operated on with using of LSTT. a Preoperative T2 MRI shows a mass lesion compressing precentral gyrus (Sawaya grade III). b Preoperative T1Gd MRI reveals canvas enhancement and signs of intratumoral hemorrhage. c DTI MRI data demonstrates distortion of the corticospinal tract. d Intraoperative images of LSTT in areas along corticospinal tract. e, f Early (24 h) postoperative CT confirms the absence of residual tumor. g After 3 months, CT shows no contrast enhancement in the operative site
There were no statistically significant differences between study groups in sex, age, Sawaya grade, preoperative KPS score, and adjuvant therapy scheme.
The EOR was significantly different between the LSTT group and control group (p < 0.01). In the postoperative period, KPS scores did not differ between the study groups (р = 0.89). The average KPS score increased from 65.6 to 80.9, and the number of patients with KPS scores less than 70 decreased from 81 (89.0%) to 17 (18.7%). The KPS score in the postoperative period was significantly different from the preoperative (p < 0.01). There was no reliable relationship between the KPS score in the postoperative period and the tumor size (R = 0.03, p = 0.75), localization (R = − 0.09, p = 0.37) of the tumor, and the EOR (R = − 0, 06, p = 0.52). Complications associated with the use of LSTT were not revealed.
Radiotherapy was conducted in 78 (85.7%) patients, in 13 (14.3%) of which it was supplemented with chemotherapy (lomustine or temozolomide); chemotherapy alone was in 3 (3.3%) patients.
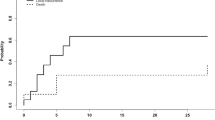
Significant differences were found (p = 0.03) comparing the survival rates among patients in the LSTT group and control group. The average life expectancy for all patients was 15.5 ± 10.5 months with a median of 13 months, in the LSTT group 18.4 ± 11.7 with a median of 16 months, and in the control group 14.3 ± 9.1 with a median of 12 months (Fig. 2).
The Kaplan-Meier survival analysis showed a difference between groups of patients, depending on the adjuvant therapy (p = 0.03). At the same time, tumor size (p = 0.72), localization (p = 0.16), postoperative KPS (p = 0.1), and using of neuronavigation (p = 0.17) have no significant effect on survival.
The overall survival differed between the groups of patients with varying degrees of resection (p < 0.01), with the median survival 7.4 ± 4.0 months in the group with the partial resection that was significantly (p < 0.01) lower than that in groups of subtotal (16.7 ± 10.9) and total resection (16.3 ± 9.5).
The Cox proportional hazards modeling (Table 3) revealed that the greater EOR provided a benefit to prognosis and partial resection had a hazard rate (HR) of 3.34 (95% CI 1.48–7.57, p < 0.005). An additional factor with a negative effect on survival was a postoperative KPS score less than 70 (HR 1.74, 95% CI 0.96–3.18; p = 0.07). The using of LSTT was a predictive of better survival (HR 1.66, 95% CI 0.96–2.84; p = 0.068).
Discussion
The goal of treatment in patients with glial brain tumors is to improve survival while maintaining high rates of health-related quality of life [5, 6, 10, 14]. The results achieved in prior studies have shown that the better prognosis is promoted by the greater EOR and by the absence of a postoperative neurological deficit [4, 8, 12, 28]. For tumors invading eloquent brain areas, it is of great importance not to strive for the maximum removal, but to optimize the EOR, taking into account the risk of neurological deterioration [3, 5, 13, 31].
One of the solutions for the treatment of patients with tumors of the eloquent brain areas was found at the junction of stereotactic and laser technologies. The influence of high-energy laser radiation provokes strictly local changes occurring in the normal brain and tumor tissue. These characteristics of the microscopic pattern are inherent exclusively to laser-induced thermal effects [1, 19, 21]. In the center of the radiation focus at the area of high power density, the structural components of the tumor tissue are coagulated and evaporated, resulting in the formation of a distinctly defined coagulation channel surrounded by a zone of perifocal necrobiosis and edema. Electron microscopy reveals the roughly damaged tumor cells in the perifocal zone with the destruction of intercellular contacts, ruptures of nuclear and cytoplasmic membranes, and karyopyknosis with the distortion of cytoplasmic organelles [23, 32]. Our experimental data suggest that the severity of destructive changes in tumor tissue increases in 24–48 h, revealing the detached effect of the laser irradiation. The findings of electron microscopy indicated the irreversibility of laser-induced thermal damage to tumor cells in the perifocal area of the radiation focus. Simultaneously in the necrobiosis zone, the neurons with signs of reparative regeneration in the form of intranuclear condensation of ribonucleoprotein granules, an increase in the number of ribosomes and polysomes, and hypertrophy of the endoplasmic reticulum could be found.
The emergence of laser devices with radiation in the near-infrared part of the spectrum, which penetrates the brain tissue to a depth of 10 mm, has justified the use of lasers for interstitial thermal therapy [15, 24, 29]. The method of laser interstitial thermal therapy (LITT) is considered as a method of the selective technique of cytoreduction, which due to the difference in thermal conductivity between pathological and normal tissues allows initiating thermal changes in tumor cells around the stereotactically introduced optical fiber. The procedure of LITT requires a constant thermometry to prevent a sharp increase in temperature that leads to a decrease in the optical permeability of tissues due to their carbonation [16,17,18].
For intraoperative LITT control, the technology of MRI thermometry was offered that helped to visualize intracerebral thermal gradients during the irradiation process. After the procedure of LITT, the combination of processes of primary and secondary tissue injury induces the increase of perifocal edema with a peak on the third or fourth day after the surgery. Expanding of edema causes the high risks of neurological disorders and could require additional intervention aimed at the internal or external decompression [7, 9, 17, 26].
In our study, the use of the image-guided LSTT technique as an addition to the traditional microsurgical resection of gliomas in eloquent brain areas was applied with the purpose of maximizing the EOR while preventing the development of a neurological deficit and achieving better oncological outcomes.
Preoperative 3D planning and multimodal neuronavigation control of the entire surgical procedure was mandatory with the location of tumors close to or directly in the eloquent brain areas to reduce the risk of neurological disorders. The use of multimodal neuroimaging data, such as DTI-FT, allowed visualizing the subcortical tracts and then calculating the maximum possible resection volume within the FLAIR abnormality area. MR venography played a crucial role in the selection of the transcortical access zone and served data used as reliable cortical landmarks. The multimodal preoperative planning and intraoperative neuroimaging allowed ensuring the high KPS scores and better neurological outcomes in both study groups that could be comparable to previously published results in patients with gliomas [4, 6, 11, 14]. As regards survival rates, in control group, they were similar to Filippini et al. [4].
The increase in the overall survival rates of patients with GBM in our research could be associated primarily with the greater EOR in the eloquent brain areas due to the direct laser ablation of residual tumor tissue and/or formation of the necrobiosis zone with unviable tumor tissue. In addition, wide irradiation of all resection cavity surfaces provided the thermal destruction of tumor cells invading the perifocal zone due to the penetration of laser radiation deep into the brain tissue.
LSTT is a procedure that can be performed as a supplement to the traditional surgical resection of brain gliomas without a significant risk of neurologic disorders. We show that the use of image-guided LSTT increases both the EOR and the survival rates of operated patients, which was statistically significant.
Among the limitations, first of all, it is necessary to point out the retrospective nature of the study that explains the lack of prognostic marker data (IDH mutation and MGMT methylation status) and ununified diagnostic algorithms (in particular, a prevalence of CT for postoperative verification of the EOR). Also, the study groups of patients were different in the same criteria for the use of neuronavigation or the largest tumor size. Although further there was no difference in survival between groups of patients with GBMs of different sizes, at the same time, the use of neuronavigation significantly contributed to better results. Nevertheless, some restrictions proceed from the small number of patients included in the study, which can be eliminated with further controlled prospective studies.
The subsequent development of the image-guided LSTT technique is seen in the intraoperative control of the irradiation process using direct thermal therapy. It is also quite promising to use laser-induced fluorescence and application of photodynamic therapy to increase the volume of tumor cytoreduction.
Conclusions
In our study, the combination of multimodal neuroimaging methods, with image-guided laser techniques in patients with GBMs of eloquent brain areas, provides an increase of the degree of resection, while maintaining high KPS score, and is associated with a survival advantage.
References
Burke L, Rovin R, Cerullo L, Brown J (1985) Thermal effects of the Nd: YAG and carbon dioxide lasers on the central nervous system. Lasers Surg Med 5(1):67–71
Delgado-López P, Corrales-García E (2016) Survival in glioblastoma: a review on the impact of treatment modalities. Clin Transl Oncol 18(11):1062–1071. https://doi.org/10.1007/s12094-016-1497-x
Eyüpoglu I, Hore N, Savaskan N, Grummich P, Roessler K, Buchfelder M, Ganslandt O (2012) Improving the extent of malignant glioma resection by dual intraoperative visualization approach. PLoS One 7(9):e44885
Filippini G, Falcone C, Boiardi A et al (2008) Prognostic factors for survival in 676 consecutive patients with newly diagnosed primary glioblastoma. Neuro-Oncology 10(1):79–87
Gil-Robles S, Duffau H (2010) Surgical management of World Health Organization Grade II gliomas in eloquent areas: the necessity of preserving a margin around functional structures. Neurosurg Focus 28(2):E8. https://doi.org/10.3171/2009.12.FOCUS09236
González-Darder J, González-López P, Talamantes F, Quilis V, Cortés V, García-March G, Roldán P (2010) Multimodal navigation in the functional microsurgical resection of intrinsic brain tumors located in eloquent motor areas: role of tractography. Neurosurg Focus 28(2):E5. https://doi.org/10.3171/2009.11.FOCUS09234
Hawasli A, Kim A, Dunn G, Tran D, Leuthardt E (2014) Stereotactic laser ablation of high-grade gliomas. Neurosurg Focus 37(6):E1. https://doi.org/10.3171/2014.9.FOCUS14471
Hervey-Jumper S, Berger M (2014) Role of surgical resection in low- and high-grade gliomas. Curr Treat Options Neurol 16(4):284. https://doi.org/10.1007/s11940-014-0284-7
Jethwa P, Barrese J, Gowda A, Shetty A, Danish S (2012) Magnetic resonance thermometry-guided laser-induced thermal therapy for intracranial neoplasms. Oper Neurosurg 71:ons133–ons145
Keles G, Lamborn K, Berger M (2001) Low-grade hemispheric gliomas in adults: a critical review of extent of resection as a factor influencing outcome. J Neurosurg 95(5):735–745
Kurimoto M, Hayashi N, Kamiyama H, Nagai S, Shibata T, Asahi T, Matsumura N, Hirashima Y, Endo S (2004) Impact of neuronavigation and image-guided extensive resection for adult patients with supratentorial malignant astrocytomas: a single-institution retrospective study. Minim Invasive Neurosurg 47(5):278–283
Lacroix M, Abi-Said D, Fourney D et al (2001) A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 95(2):190–198
Lacroix M, Toms S (2014) Maximum safe resection of glioblastoma multiforme. J Clin Oncol 32(8):727–728. https://doi.org/10.1200/JCO.2013.53.2788
McGirt M, Chaichana K, Gathinji M, Attenello F, Than K, Olivi A, Weingart J, Brem H, Quiñones-Hinojosa A (2009) Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg 110(1):156–162. https://doi.org/10.3171/2008.4.17536
Missios S, Bekelis K, Barnett GH (2015) Renaissance of laser interstitial thermal ablation. Neurosurg Focus 38(3):E13. https://doi.org/10.3171/2014.12.FOCUS14762
Norred S, Johnson J (2014) Magnetic resonance-guided laser induced thermal therapy for glioblastoma multiforme: a review. Biomed Res Int 2014:1–9. https://doi.org/10.1155/2014/761312
Pisipati S, Smith K, Shah K, Ebersole K, Chamoun R, Camarata P (2016) Intracerebral laser interstitial thermal therapy followed by tumor resection to minimize cerebral edema. Neurosurg Focus 41(4):E13. https://doi.org/10.3171/2016.7.FOCUS16224
Rahmathulla G, Recinos PF, Kamian K, Mohammadi AM, Ahluwalia MS, Barnett GH (2014) MRI-guided laser interstitial thermal therapy in neuro-oncology: a review of its current clinical applications. Oncology 87(2):67–82. https://doi.org/10.1159/000362817
Rosomoff H, Carroll F (1966) Reaction of neoplasm and brain to laser. Arch Neurol 14(2):143–148. https://doi.org/10.1001/archneur.1966.00470080027004
Roux F, Devaux B, Merienne L, Cioloca C, Chodkiewicz J (1990) 1.32 μm Nd:YAG laser during neurosurgical procedures experience with about 70 patients operated on with the MC 2100 unit. Acta Neurochir 107(3–4):161–166
Roux F, Mordon S, Fallet-Bianco C, Merienne L, Devaux B, Chodkiewicz J (1990) Effects of 1.32-μm Nd-YAG laser on brain thermal and histological experimental data. Surg Neurol 34(6):402–407. https://doi.org/10.1016/0090-3019(90)90244-J
Sawaya R, Hammoud M, Schoppa D, Hess K, Wu S, Shi W, WiIdrick D (1998) Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery 42(5):1044–1055. https://doi.org/10.1097/00006123-199805000-00054
Schober R, Bettag M, Sabel M, Ulrich F, Hessel S (1993) Fine structure of zonal changes in experimental Nd:YAG laser–induced interstitial hyperthermia. Lasers Surg Med 13(2):234–241
Schulze P et al (2001) Correlation of neuropathologic findings and phase-based MRI temperature maps in experimental laser-induced interstitial thermotherapy. J Magn Reson Imaging 14(5):658–658. https://doi.org/10.1002/jmri.1232
Senft C, Forster M, Bink A, Mittelbronn M, Franz K, Seifert V, Szelényi A (2012) Optimizing the extent of resection in eloquently located gliomas by combining intraoperative MRI guidance with intraoperative neurophysiological monitoring. J Neuro-Oncol 109(1):81–90
Sloan A, Ahluwalia M, Valerio-Pascua J et al (2013) Results of the NeuroBlate System first-in-humans Phase I clinical trial for recurrent glioblastoma. J Neurosurg 118(6):1202–1219
Stellar S, Polanyi T, Bredemeier H (1970) Experimental studies with the carbon dioxide laser as a neurosurgical instrument. Med Biol Eng 8(6):549–558. https://doi.org/10.1007/BF02478229
Stummer W, Reulen H, Meinel T et al (2008) Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery 62(3):564–576
Svaasand L, Boerslid T, Oeveraasen M (1985) Thermal and optical properties of living tissue: application to laser-induced hyperthermia. Lasers Surg Med 5(6):589–602
Takizawa T (1984) The carbon dioxide laser surgical unit as an instrument for surgery of brain tumours—its advantages and disadvantages. Neurosurg Rev 7(2–3):135–144. https://doi.org/10.1007/BF01780696
Wolbers J (2014) Novel strategies in glioblastoma surgery aim at safe, supra-maximum resection in conjunction with local therapies. Chin J Cancer 33(1):8–15. https://doi.org/10.5732/cjc.013.10219
Yaroslavsky AN, Schulze PC, Yaroslavsky IV, Schober R, Ulrich F, Schwarzmaier HJ (2002) Optical properties of selected native and coagulated human brain tissues in vitro in the visible and near infrared spectral range. Phys Med Biol 47(12):2059–2073. https://doi.org/10.1088/0031-9155/47/12/305
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved and conducted within the guidelines of the institutional review board.
Informed consent
The written informed consent was obtained from each patient or appropriate family member before the surgery.
Rights and permissions
About this article
Cite this article
Rozumenko, A., Kliuchka, V., Rozumenko, V. et al. Image-guided resection of glioblastoma in eloquent brain areas facilitated by laser surface thermal therapy: clinical outcomes and long-term results. Neurosurg Rev 41, 1045–1052 (2018). https://doi.org/10.1007/s10143-018-0948-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-018-0948-y