Abstract
Long-wave sensitive (LWS) is a G protein-coupled receptor expressed in the retina, and zebrafish is a better model organism for studying vision, but the role of LWS1 in vision-guided behavior of larvae fish has rarely been reported. In this study, we found that zebrafish lws1 and lws2 are tandemly replicated genes, both with six exons, with lws1 being more evolutionarily conserved. The presence of Y277F in the amino acid sequence of lws2 may have contributed to the shift of λmax to green light. We established a lws1 knockout zebrafish model using CRISPR/Cas9 technology. Lws1−/− larvae showed significantly higher levels of feeding and appetite gene (agrp) expression than WT, and significantly lower levels of anorexia gene (pomc, cart) expression. In addition, green light gene compensation was observed in lws1−/− larvae with significantly increased expression levels of rh2-1. The light-dark movement test showed that lws1−/− larvae were more active under light-dark transitions or vibrational stimuli, and the expression of phototransduction-related genes was significantly up-regulated. This study reveals the important role of lws1 gene in the regulation of vision-guided behavior in larvae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Organisms use their sensory systems to obtain information from the environment and then integrate that information to produce relevant behavior (Privat et al. 2019). In visual system signalling, evolutionary and functional studies of optogenetic genes are often used to infer fundamental properties of the visual system (Horth 2007). Vertebrate visual pigmentation consists of opsins and light-sensitive chromogenic groups (11-cis-retinal or 11-cis-3,4-dehydroretinal), which confer different spectral sensitivities on cone receptors and play important roles in colour vision and signal transduction (Nathans 1990). There are five main subfamilies of opsins, one rod opsin (RH1, λmax ≈ 478–510 nm) that perceives dark or low light, and four cones opsins that perceive bright or strong light: shortwave-sensitive opsin 1 (SWS1, λmax ≈ 360–440 nm), shortwave-sensitive opsin 2 (SWS2, λmax ≈ 400–430 nm), medium-wave-sensitive opsin (RH2, λmax ≈ 450–530 nm), and long wavelength sensitive opsin (LWS, λmax ≈ 510–560 nm) (Chinen et al. 2003; Terakita 2005). Opsins play an important role in physiological functions such as feeding, growth, behavior and mate choice in the organism. (Escobar-Camacho et al. 2017; Musilova et al. 2021).
During biological evolution, genome-wide or tandem duplication and sequence divergence of opsins have facilitated the expansion of opsin family members in many species (Lin et al. 2017, Hofmann and Carleton 2009). When replicated subgenes are identical, functional redundancy occurs and one of them usually becomes a pseudogene during the evolutionary process (Zhang 2003), or a rare evolutionary equilibrium occurs in which the genes diverge functionally to allow for the stable conservation of both subgenes (Nowak et al. 1997). There are five important amino acid sites in opsin, the mutation site S180A, H197Y, Y277F, T285A, A308S (based on the human LWS retinoid number) is a major predictor of LWS/RH2/MWS opsin (with vitamin A1 as the chromor) λmax in the range of 512 ∼ 560 nm (Yokoyama and Radlwimmer 2001). Many studies have shown that the number of lws copies, protein expression and functional differentiation between subtypes varies by species (Musilova et al. 2021), and there is controversy as to whether or not fish copies of the lws co-facilitate visual function.
Humans having two reciprocal lws genes, the absence of one of which causes red-green color blindness (Cole 2002). There was no lws expression in the early evolutionary history of finch seabream (Lepidosteus platystomus) (Stieb et al. 2023). Lws1 and lws2 in medaka (Oryzias latipes) were 98.9% similar and had basically identical absorbance maxima (Matsumoto et al. 2006). The medaka lws1/lws2 single mutant has no behavioral impairment in response to red light sensitivity, indicating functional redundancy across paralogous homologous genes (Harada et al. 2019). Both guppy (Poecilia reticulata) and bluefin killifish (Lucania goodei) have four lws genes with different λmax, and the four homologous lws genes of guppy have undergone evolutionary diversification (neofunctionalisation) (Kawamura et al. 2016), the four lws genes of Bluefin Killifish exhibited significant differences in expression levels (Chang et al. 2021).
Visual retinoids bind to chromatophores to absorb light, initiating a visual transduction cascade response to perceive different colors and intensities of light, which guides the organism to produce light behaviors (Hardie and Juusola 2015; Macias-Muñoz et al. 2019). Instinctive visually guided behaviors are essential for the survival of larvae fish (Brandt et al. 1987; Fuiman et al. 2006; Muto and Kawakami 2013). Zebrafish is a model organism in the field of vision research with a wide range of visually guided behaviors, such as photodynamic responses, phototropic behaviors and feeding behaviors (Portugues et al. 2013; Orger and de Polavieja 2017), but there are limited reports on the evolutionary development of the zebrafish lws1 and lws2 genes and the effect of the lws1 gene on visually guided behaviors in larvae fish. It is with this aim in mind that the present experiment was conducted.
In this study, we found that the lws1 in zebrafish is more conserved in evolution. According to the “five-site rule”, the presence of S180A and Y277F in the amino acid sequence of lws2 may cause λmax to shift towards green light We generated a lws1 knockout zebrafish model (lws1−/−) using CRISPR/Cas9 technology. We found that rh2-1 expression was significantly elevated in lws1−/− larvae, suggesting possible photocompensativeness. Enhanced visually guided behaviors (feeding and light sensitivity) in lws1−/− larvae may be associated with the up-regulation of phototransduction-related genes. In addition, we observed that lws1−/− larvae were also significantly more sensitive to vibratory stimuli. These findings suggest that lws1 knockout not only increases visually guided behaviors and upregulates phototransduction-related genes, but may also play an important role in regulating environmental sensitivity.
Results
Phylogenetic analysis of the zebrafish lws1 and lws2 genes
The evolutionary tree shows that the LWS is divided into two branches, with mammals clustered into a separate branch and zebrafish lws1 more closely related to the carp family (Fig. 1). Zebrafish (Danio rerio), medaka, turbot (Scophthalmus maximus), grass carp (Ctenopharyngodon Idella), yellow perch (Perca flavescens) and asian seabass (Lates calcarifer) all have lws1 and lws2 located adjacent to each other on the same chromosome, with strong conservation of neighboring genes, whereas human, cattle, nile rat, western clawed frog and brown-marbled grouper all have only one lws gene (Fig. 2). Compared to the lws2 gene, the zebrafish lws1 gene has higher amino acid sequence similarity to other species. The amino acid sequence similarity between zebrafish lws1 and lws2 genes was 91.6%. The similarity of zebrafish lws1 to mammals ranged from 75.6–79% and with fish from 77.6–94.4%, while the similarity of lws2 with mammals ranged from 75-76.7% and with fish from 76.4–91.3%. (Fig. S1). Sequence comparison revealed that amino acid at position 277 of lws2 was mutated from Y to F. According to the law of loci, the λmax of LWS2 will probably be shifted towards green light, lower than that of lws1 opsin (Fig. 3). Both zebrafish lws1 and lws2 genes have six exons and five introns, with the first intron of lws1 being longer and the 5’ flanking region shorter than that of lws2 (Fig. S2).
Phylogenetic evolutionary tree of lws1 and lws2 genes in selected mammals and fish. The maximum likelihood technique was used to infer evolutionary tree analysis based on the LG+G model, and the evolutionary tree reliability scores were generated by repeating the study 500 times. The number shown at each branch node indicates the bootstrap value (%). Red emphasizes the LWS1 and LWS2 in zebrafish. Sequences are from the following species: Danio rerio (zebrafish), Oryzias latipes (Medaka), Scophthalmus maximus (Turbot), Ctenopharyngodon Idella(Grass carp), Lates calcarifer (Asian seabass), Perca flavescens (Yellow perch), Cyprinus carpio (Common carp), Epinephelus fuscoguttatus (Hybrid grouper), Homo sapiens (Human), Bos taurus (Cattle), Arvicanthis niloticus (Nile rat), Xenopus tropicalis (Western Clawed Frog). Information on species is shown in Table S1
Lws1 and lws2 gene sequence alignment. The amino acid sequences of several representative vertebrates lws1 and lws2 are aligned, and the red shade represents completely conserved residues. The green boxes represent five important loci in the visual genes, and the mutant loci S180A, H197Y, Y277F, T285A, and A308S (based on the human LWS retinoid number) are the main predictors of λmax in the 512 ~ 560 nm range for LWS/RH2/MWS optical proteins (with vitamin A1 as the chromor)
Establishment of lws1 mutant zebrafish
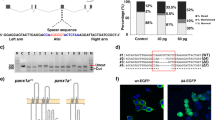
We employed a dual-target knockout technique, placing both targets on exon 1, which altered the base sequence of lws1, thereby blocking protein translation (Fig. 4A). The lws1-pure mutant was missing 11 bp of bases as compared to WT (Fig. 4B), and all of these deletions led in the premature termination of LWS1 protein translation with just 13 amino acids (Fig. 4C). In addition, we designed a pair of full-length primers to differentiate zebrafish lws1 expression differences in lws1−/− mutant and WT larvae (Fig. S3). Next, we sequenced the total length of lws1 gene in lws1−/− and WT larvae, which showed that the lws1 mRNA level of the lws1−/− mutant was missing by 11 bp (Fig. S4).
Generation and characterization of lws1 and lws2 mutant zebrafish. (A, B) Design of dual targets for lws1 based on CRISPR/Cas9 technology. Exons are indicated by yellow boxes, and single guide RNAs (sgRNAs) are labelled in exons in green and red. sgRNA sequences are highlighted in green and red boxes, and -11 bp deletions are indicated by sequencing validation. (C) Illustration of the structure of the deduced proteins of the WT and lws1 mutants, with numbers representing the number of amino acids coding for the protein
Transcriptional expression of phototransduction genes and opsin genes
Real-time fluorescence quantitative PCR was used to compare the relative mRNA expression of opsin genes and phototransduction genes in WT and lws1−/−larvae. The lws1−/− larvae had higher expression of the lws2 gene, significantly higher expression of rh2-1 and rho than in the WT, and significantly lower expression of rh2-2 than in the WT (p < 0.05) (Fig. 5A). To investigate whether the lws1 gene influences the phototransduction pathway, the results showed that the expression of all phototransduction pathway genes was significantly higher in the lws1−/− larvae than in the WT (p < 0.05) (Fig. 5B).
Feeding tests and transcript levels of appetite-related genes
Lws1−/− zebrafish ingested significantly more paramecia than the WT (Fig. 6A, p < 0.05), the agrp gene had significantly greater relative expression than WT (Fig. 6B, p < 0.05), and the relative expression of the npy, pomc, and cart genes displayed significantly lower than the WT (Fig. 6C, p < 0.05).
Feeding and appetite gene expression in LWS1-/- and WT larvae. (A) Relative levels of ingested paramecia in the digestive tract over a 30-minute period. (B) Expression of orexigenic genes. (C) Expression of anorexigenic genes. The data is expressed as: mean ± standard error (mean ± SEM, n=24), with a marker (*) reflecting statistically significant differences (p < 0.05)
Behavioral testing for alternating light and dark cycles
To confirm whether the lws1−/− mutation affects visually guided behavior in zebrafish, in this paper, we chose the cumulative distance moved per minute to describe the movement trend of zebrafish larvae during a 30 min behavioral test time and examined the movement of zebrafish during three alternating light-dark cycles. The results showed that the cumulative distance travelled by lws1−/− and WT larvae was small in darkness, and that the cumulative distance travelled in light increased sharply and then gradually returned to the level of the dark period (Fig. 7A). The cumulative distance moved by lws1−/− and WT larvae was significantly higher in the light period than the dark period (Fig. 7B, p < 0.05). The total travel distance of lws1−/− larvae within 30 min was significantly lower than that of WT (Fig. 7C, p < 0.05).
Photoperiodic movements of lws1-/- and WT larvae. (A) Cumulative movement of lws1-/- and WT larvae per minute of the light cycle. (B) Cumulative distance travelled by lws1-/-and WT larvae during the light or dark cycle (5 min). (C) Cumulative distance travelled over a 30 min period. The data is expressed as: mean ± standard error (mean ± SEM, n=24), with a marker (*) reflecting statistically significant differences (p < 0.05)
Behavioral response of zebrafish larvae during light-dark transitions
This experiment’s photoperiod consisted of three light-on and two light-off periods, and we tracked the movements of lws1−/− and WT larvae for 5 s before and after light transitions (light-on or light-off). The results showed that after the lights were turned on or off, the distance traveled by zebrafish increased and then gradually slowed down. Lws1−/− and WT larvae became active at the instant of light on, but both were not significant (Fig. 8A). However, the cumulative distance travelled by lws1−/− larvae was significantly higher than that of WT when the light was switched off twice (Fig. 8B, p < 0.05).
Behavioral response of zebrafish larvae during light-dark transitions. Lws1-/- and WT larvae’s distance travelled curves for 5s before and after switching the light on (A) or off (B). The red arrow represents the moment when the light is switched on or off. The data is expressed as: mean ± standard error (mean ± SEM, n=24), with a marker (*) reflecting statistically significant differences (p < 0.05)
Behavioral responses of zebrafish larvae to vibratory stimuli
Lws1−/− and WT larvae were treated to three vibrating stimuli during darkness and light, respectively, and moved aggressively at initially, before gradually returning to stillness. During darkness and light, lws1−/− larvae were substantially more sensitive (cumulative distance travelled) to vibratory stimuli than WT larvae (Fig. 9, p < 0.05).
Behavioral response of zebrafish larvae to vibratory stimuli. Curves of the distance travelled by zebrafish litters during the dark phase (A) and the light phase (B) as a function of time, i.e., the distance travelled from 5s before to 5 s after the onset of each tapping stimulus. Red arrows represent the duration of vibratory stimulation in light or dark conditions. The data is expressed as: mean ± standard error (mean ± SEM, n=24), with a marker (*) reflecting statistically significant differences (p < 0.05)
Materials and methods
Lws gene sequence analysis in vertebrates
Species information for the vertebrates used in this experiment was obtained from the National Center for Biotechnology Information (NCBI) (https://www.ncbi.nlm.nih.gov/genbank) or Ensembl (http://asia.ensembl.org/index.html), and species included: zebrafish, medaka, turbot, grass carp, asian seabass, yellow perch, common carp, hybrid grouper, human, cattle, nile rat, western clawed frog.
The names and accession numbers of the lws gene for all species are shown in (Table S1). Multiple sequence alignment was performed on all gene sequences using the Clustal W tool in the MEGA X software with alignment and manual adjustments (Kumar et al. 2018). The maximum likelihood technique was used to infer evolutionary tree analysis based on the LG + G model (Lv et al. 2019), and the evolutionary tree reliability scores were generated by repeating the study 500 times. Homology analysis between genomes was performed by the online software Genomicus (http://www.dyogen.ens.fr/genomicus) (Dupré and Tostivint 2014). Zebrafish lws1 and lws2 gene structures were analyzed by Gene Structure Display Server 2.0(http://gsds.gao-lab.org/?(Hu et al 2015).
Construction of lws1 mutant zebrafish by CRISPR/Cas9 technology
Lws1 mutant zebrafish were constructed by CRISPR/Cas9 technology. Sequencing results for single-guide RNAs (sgRNAs) and PCR primers are shown in Supplementary Table S2. sgRNA were cloned into the pMD-18T vector and synthesized using the TranscriptAid T7 High Yield Transcription Kit (Thermo Fisher Scientific, Waltham, MA, USA) for synthesis. Compounds of sgRNA (50 ng/μL) and Cas9 protein (New England Biolabs, Ipswich, MA, USA) were co-injected into wild-type embryos at the first or second cell stage of development. F0 generation zebrafish were crossed with wild type to obtain F1 generation zebrafish. Individuals with lws1 heterozygous mutations were detected using the T7 Nucleic Acid Endonuclease 1 Assay (Vazyme, Nanjing, China) according to the manufacturer’s instructions, followed by sequencing (Fig. S3-4). F1 heterozygous individuals were crossed to produce F2 pure individuals, and all experiments were performed with F3 pure individuals. Lws1 mutant zebrafish named lws1−/− zebrafish.
Feeding experiments with zebrafish larvae
Lipophilic tracer 4-(4-(di-decylamino) styryl)-N-methylpyridinium iodide (4-Di-10-ASP; Invitrogen, Carlsbad, CA, USA) was used for fluorescent labeling of paramecia cultured (Shimada et al. 2012) in our laboratory. The paramecia were suspended in 1 mL of distilled water, and then 25 mg/mL of 4-Di-10-ASP was added. After 1 h of staining, the paramecia were washed by centrifugation to remove residual 4Di-10-ASP. At 6 days post hatching (6 dph), the lws1−/− and WT larvae were fed with 4-Di-10-ASP labeled paramecia for 30 min. The intra-abdominal fluorescence signal was measured with a fluorescein filterset using the Victor2 fluorescent plate reader (PerkinElmer, Boston, MA, USA) in well scan mode. Each point’s total was specified as being paramecia to the amounts of paramecia provided to each zebrafish (Shimada et al. 2012). After WT and lws1−/− zebrafish swallowed non-fluorescent paramecia for 30 min, samples were collected and analyzed for appetite gene expression.
Behavioral testing experiments
WT and lws1−/− larvae were placed in 24-well plates (wells 15.74 mm), 1 tail/well, and 12 parallel samples were set up for WT and lws1−/− larvae, and after waiting for the larvae to acclimate for 12 h, the data were processed by a behavioral monitor, the DanioVision TM (Noldus, The Netherlands), and by the accompanying EthoVision XT 14 video tracking software to process the behavioral data of larvae (Jiang et al. 2018). The behavioral test procedure of the instrument started with 5 min of dark adaptation, followed by three cycles of 5 min of darkness and 5 min of light, and one rapid and strong vibratory stimulus was given 1 min before every light transition, the surroundings were kept quiet, and the room temperature was controlled at 28 ± 0.5 °C. The distance and speed of the movement of the fish were recorded every 0.1 s, and the experiment was repeated two times.
RNA isolation and quantitative RT-PCR
Using a multi-function microplate reader, the amount of RNA was measured (Biotek, USA). Following the manufacturer’s instructions, 1 μg of total RNA was reverse-transcribed using the HiScript Q RT SuperMix for qPCR (+ gDNA wiper) kit from Vazyme, Nanjing, China. The PCR products were detected using 1.2% agarose gel electrophoresis, which was carried out for 20 min at 120 V. The Gel Imaging System was then used to take pictures of the gel under ultraviolet light. In the following investigations, real-time PCR was used to analyse the level of gene expression. Table S3 contained details regarding relative gene primers. β-actin was a housekeeping gene in organisms (Lu et al. 2023). The AceQ® qPCR SYBR Green-Master Mix kit was used for the qPCR (Vazyme). The Ct (2−ΔΔCt) value method was used to calculate the relative gene expression (Livak and Schmittgen 2001).
Data analysis
Statistical analysis was performed with SPSS 25.0 software. All data were presented as mean ± S.E.M (standard error of the mean). Differences between the three groups were analysed using one-way ANOVA and Duncan’s multiple range test, and comparisons between the two groups were made using Student’s T-test, with p < 0.05 considered statistically significant.
Discussion
The phylogeny and function of lws gene have been more comprehensively studied in many species, but the function played by the lws1 gene in the phototransduction behavior of zebrafish larvae has been rarely reported. By comparing lws gene sequences from different species, we can see the duplication and selection events that lws gene go through during evolution. Lws is strongly conserved in fish, with distinct homologies occurring in mammals and toads. The zebrafish lws1 gene has more homologous similarity to other species, and lws2 appears as Y277F in the “five-site rule” (Yokoyama and Radlwimmer 2001), with its λmax potentially shifted towards green light. The mRNA expression of the green light gene (rh2-1) was significantly increased when lws1 was knocked down, suggesting that the lws1 gene may be involved in the green light-sensitive response, and that when the lws1 gene was knocked down, the expression of rh2-1 was greatly increased due to light compensatory. Previous studies have found that osteoglossus lineages have lost the typical vertebrate green-sensitive opsin gene (rh2) and replaced it with a copy of the green-sensitive LWS opsin(Liu et al. 2019). The loss of LWS by Noctua reduces the effect of green light aiming(Liu et al. 2018; Zaccardi et al. 2006).
For zebrafish, the ability to catch prey is highly dependent on the rapid development of the visual system (Bianco et al. 2011; Muto and Kawakami 2013). In feeding behavior studies, a significant increase in feeding was found in lws1−/− larvae. The increase in feeding may be related to a significant increase in the expression of green light gene (rh2-1) and phototransduction genes in lws1−/−, suggesting that lws1−/− has high photosensitivity, which improves larval feeding success. Previous studies have found that green light promotes growth in red-finned Eastern leatherback turtles (Takifugu rubripes) and feeding and survival rates in haddock larvae (Mellanogrammus aeglefinus), while red light inhibits growth (Kim et al. 2016; Villamizar et al. 2011). In addition, the up-regulation of the appetite gene (agrp) and the down-regulation of the anorexia gene compared to WT further suggests that LWS1 plays an important role in the regulation of feeding in zebrafish larvae (Karakatsouli et al. 2007). Light exposure is inextricably linked to changes in hormone regulation and body metabolism in fish (Head and Malison 2000). The significantly lower npy gene in lws1−/− larvae than in WT may be the result of adaptive adjustments in the digestive and metabolic systems of the fish (increased secretion of digestive enzymes and hormones in response to higher intake) resulting from chronic overfeeding in lws1−/− larvae.
Light and dark motion tests and responses to light stimuli are effective methods for reflecting the integrated function of the visual pathway (Zhang 2021, Cai 2018, Guo 2021). We assessed the photosensitive behavior of the larvae after knocking down the lws1 gene using an alternating light/dark behavioral test method (Hu et al. 2019). During alternating light and darkness, zebrafish larvae exhibit a range of different behavioral swimming patterns in response to environmental factors (Kalueff et al. 2013).
The cumulative distance travelled by zebrafish during the 30 min of behavioral testing was significantly lower in the lws1−/− larvae than in the WT. The cumulative distance travelled by zebrafish larvae during the dark phase was significantly lower than during the light phase, and previous studies have also found a preference for dark environments in adults (Serra et al. 1999; Tuz-Sasik et al. 2022), which larvae will strongly avoid (Steenbergen et al. 2011). It has also been observed that when zebrafish larvae are placed to light, their swimming steering angle rises as the light fades over time (Burgess and Granato 2007). As the darkness persist, this overactive steering angle levels off and the larvae begin to swim normally (Burgess and Granato 2007; MacPhail et al. 2009). However, previous experiments have reported that the cumulative distance travelled by zebrafish larvae in behavioral tests was higher in the dark and relatively lower in the light (Jarema et al. 2015; Steele et al. 2018; Leuthold et al. 2019). Cumulative differences in swimming distances between dark and light conditions in zebrafish may be related to the experimental procedure or the developmental stage of the fish, but specific factors need to be further investigated.
The cumulative distance travelled by WT and lws1−/− larvae did not change significantly between 5 s before and after switching on the light, and the distance travelled by zebrafish larvae did not change much at the moment of switching on the light because, once acclimatised to darkness, larvae show a “freezing” response to the sudden introduction of light, which reduces their swimming distance and speed (MacPhail et al. 2009). The cumulative distance of lws1−/− larvae was significantly higher than that of WT when the light was turned off, which may be due to the greater sensitivity of lws1−/−larvae to the light perception of the environment.
In addition, motor responses to stimuli in juvenile zebrafish are co-mediated by lateral hair cells and highly myelinated Mautener neurons at the back of the brain (Kohashi 2012, Mekdara 2018, Wang 2017). Under light or dark conditions, the cumulative distance of lws1−/− larvae after vibration stimulation (Banerjee et al. 2022) was significantly higher than that of WT, suggesting that lws1 knockout may promote the function of lateral hair cells and highly myelined Mautener neurons in the rear of the brain of larva Showed increased sensitivity to the environment, but further study is needed.
In conclusion, we revealed the evolution of the zebrafish lws gene and the first construction of a zebrafish lws1 mutation model, and further investigated the effect of the lws1 gene on the visual behavior of larvae. These studies help to reveal the role of lws1 genes in the regulation of behavior and may also expand the understanding of zebrafish as a model organism.
Data availability
No datasets were generated or analysed during the current study.
References
Banerjee S, Ranspach LE, Luo X, Cianciolo LT, Fogerty J, Perkins BD, Thummel R (2022) Vision and sensorimotor defects associated with loss of Vps11 function in a zebrafish model of genetic leukoencephalopathy. Sci Rep 12(1):3511
Bianco IH, Kampff AR, Engert F (2011) Prey capture behavior evoked by simple visual stimuli in larval zebrafish. Front Syst Neurosci 5:101
Brandt SB, Mason DM, Macneill DB, Coates T, Gannon JE (1987) Predation by alewives on larvae of yellow perch in Lake Ontario. Trans Am Fish Soc 116(4):641–645
Burgess HA, Granato M (2007) Modulation of locomotor activity in larval zebrafish during light adaptation. J Exp Biol 210(14):2526–2539
Cai S, Chen Y, Shang Y, Cui J, Li Z, Li Y (2018) Knockout of zebrafish interleukin 7 receptor (IL7R) by the CRISPR/Cas9 system delays retinal neurodevelopment. Cell Death Dis 9(3):273
Chang CH, Catchen J, Moran RL, Rivera-Colón AG, Wang YC, Fuller RC (2021) Sequence analysis and ontogenetic expression patterns of cone opsin genes in the bluefin killifish (Lucania goodei). J Hered 112(4):357–366
Chinen A, Hamaoka T, Yamada Y, Kawamura S (2003) Gene duplication and spectral diversification of cone visual pigments of zebrafish. Genetics 163(2):663–675
Cole BL (2002) Protan colour vision deficiency and road accidents. Clin Exp Optom 85(4):246–253
Dupré D, Tostivint H (2014) Evolution of the gastrin-cholecystokinin gene family revealed by synteny analysis. Gen Comp Endocrinol 195:164–173
Escobar-Camacho D, Ramos E, Martins C, Carleton KL (2017) The opsin genes of amazonian cichlids. Mol Ecol 26(5):1343–1356
Fuiman LA, Rose KA, Cowan JH Jr et al (2006) Survival skills required for predator evasion by fish larvae and their relation to laboratory measures of performance. Anim Behav 71(6):1389–1399
Guo R, Li F, Lu M, Ge K, Gan L (2021) Shang, D. LIM Homeobox 9 knockdown by morpholino does not affect zebrafish retinal development. Biol Open 10:bio056382
Harada Y, Matsuo M, Kamei Y, Goto M, Fukamachi S (2019) Evolutionary history of the medaka long-wavelength sensitive genes and effects of artificial regression by gene loss on behavioral photosensitivity. Sci Rep 9(1):2726
Hardie RC, Juusola M (2015) Phototransduction in drosophila. Curr Opin Neurobiol 34:37–45
Head AB, Malison JA (2000) Effects of lighting spectrum and disturbance level on the growth and stress responses of yellow perch Perca flavescens. J World Aquacult Soc 31(1):73–80
Hofmann CM, Carleton KL (2009) Gene duplication and differential gene expression play an important role in the diversification of visual pigments in fish. Integr Comp Biol 49(6):630–643
Horth L (2007) Sensory genes and mate choice: evidence that duplications, mutations, and adaptive evolution alter variation in mating cue genes and their receptors. Genomics 90(2):159–175
Hu B, Jin J, Guo AY, Zhang H, Luo J, Gao G (2015) GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics 31(8):1296–1297
Hu Z, Dang Y, Liu C, Zhou L, Liu H (2019) Acute exposure to ustiloxin A affects growth and development of early life zebrafish, Danio rerio. Chemosphere 226:851–857
Jarema KA, Hunter DL, Shaffer RM, Behl M, Padilla S (2015) Acute and developmental behavioral effects of flame retardants and related chemicals in zebrafish. Neurotoxicol Teratol 52(Pt B):194–209
Jiang F, Liu J, Zeng X, Yu L, Liu C, Wang J (2018) Tris (2-butoxyethyl) phosphate affects motor behavior and axonal growth in zebrafish (Danio rerio) larvae. Aquat Toxicol 198:215–223
Kalueff AV, Gebhardt M, Stewart AM, Cachat JM, Brimmer M, Chawla JS, Craddock C, Kyzar EJ, Roth A, Landsman S, Gaikwad S, Robinson K, Baatrup E, Tierney K, Shamchuk A, Norton W, Miller N, Nicolson T, Braubach O, Gilman CP, Pittman J, Rosemberg DB, Gerlai R, Echevarria D, Lamb E, Neuhauss SC, Weng W, Bally-Cuif L, Schneider H (2013) Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish 10(1):70–86
Karakatsouli N, Papoutsoglou SE, Pizzonia G, Tsatsos G, Tsopelakos A, Chadio S, Kalogiannis D, Dalla C, Polissidis A, Papadopoulou-Daifoti Z (2007) Effects of light spectrum on growth and physiological status of gilthead seabream Sparus aurata and rainbow trout Oncorhynchus mykiss reared under recirculating system conditions. Aquac Eng 36(3):302–309
Kawamura S, Kasagi S, Kasai D, Tezuka A, Shoji A, Takahashi A, Imai H, Kawata M (2016) Spectral sensitivity of guppy visual pigments reconstituted in vitro to resolve association of opsins with cone cell types. Vis Res 127:67–73
Kim BH, Hur SP, Hur SW, Lee CH, Lee YD (2016) Relevance of light spectra to growth of the rearing tiger puffer takifugu rubripes. Dev Reprod 20(1):23–29
Kohashi T, Nakata N, Oda Y (2012) Effective sensory modality activating an escape triggering neuron switches during early development in zebrafish. J Neurosci 32(17):5810–5820
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35(6):1547–1549
Leuthold D, Klüver N, Altenburger R, Busch W (2019) Can environmentally relevant neuroactive chemicals specifically be detected with the locomotor response test in zebrafish embryos? Environ Sci Technol 53(1):482–493
Lin JJ, Wang FY, Li WH, Wang TY (2017) The rises and falls of opsin genes in 59 ray-finned fish genomes and their implications for environmental adaptation. Sci Rep 7(1):15568
Liu YJ, Yan S, Shen ZJ, Li Z, Zhang XF, Liu XM, Zhang QW, Liu XX (2018) The expression of three opsin genes and phototactic behavior of Spodoptera exigua (Lepidoptera: Noctuidae): evidence for visual function of opsin in phototaxis. Insect Biochem Mol Biol 96:27–35
Liu DW, Wang FY, Lin JJ, Thompson A, Lu Y, Vo D, Yan HY, Zakon H (2019) The cone opsin repertoire of osteoglossomorph fishes: gene loss in mormyrid electric fish and a long wavelength-sensitive cone opsin that survived 3R. Mol Biol Evol 36(3):447–457
Livak KJ, Schmittgen TDJm (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2– ∆∆CT method. Methods 25(4):402–408
Lu K, Liang XF, Tang SL et al (2023) Role of short-wave-sensitive 1 (sws1) in cone development and first feeding in larval zebrafish. Fish Physiol Biochem 49(5):801–813
Lv L-Y, Liang X-F, He S (2019) Genome-wide identification and characterization of olfactory receptor genes in Chinese perch, Siniperca chuatsi. Genes 10(2):178
Macias-Muñoz A, Rangel Olguin AG, Briscoe AD (2019) Evolution of phototransduction genes in lepidoptera. Genome Biol Evol 11(8):2107–2124
MacPhail RC, Brooks J, Hunter DL, Padnos B, Irons TD, Padilla S (2009) Locomotion in larval zebrafish: influence of time of day, lighting and ethanol. Neurotoxicology 30(1):52–58
Matsumoto Y, Fukamachi S, Mitani H, Kawamura S (2006) Functional characterization of visual opsin repertoire in Medaka (Oryzias latipes). Gene 371(2):268–278
Mekdara PJ, Schwalbe MAB, Coughlin LL, Tytell ED (2018) The effects of lateral line ablation and regeneration in schooling giant danios. J Exp Biol 221(Pt 8):jeb175166
Musilova Z, Salzburger W, Cortesi F (2021) The visual opsin gene repertoires of teleost fishes: evolution, ecology, and function. Annu Rev Cell Dev Biol 37:441–468
Muto A, Kawakami K (2013) Prey capture in zebrafish larvae serves as a model to study cognitive functions. Front Neural Circuits 7:110
Nathans J (1990) Determinants of visual pigment absorbance: identification of the retinylidene Schiff’s base counterion in bovine rhodopsin. Biochemistry 29(41):9746–9752
Nowak MA, Boerlijst MC, Cooke J, Smith JM (1997) Evolution of genetic redundancy. Nature 388(6638):167–171
Orger MB, de Polavieja GG (2017) Zebrafish behavior: opportunities and challenges. Annu Rev Neurosci 40:125–147
Portugues R, Severi KE, Wyart C, Ahrens MB (2013) Optogenetics in a transparent animal: circuit function in the larval zebrafish. Curr Opin Neurobiol 23(1):119–126
Privat M, Romano SA, Pietri T et al (2019) Sensorimotor transformations in the zebrafish auditory system. Curr Biol 29(23):4010–4023
Serra EL, Medalha CC, Mattioli R (1999) Natural preference of zebrafish (Danio rerio) for a dark environment. Braz J Med Biol Res 32(12):1551–1553
Shimada Y, Hirano M, Nishimura Y, Tanaka T (2012) A high-throughput fluorescence-based assay system for appetite-regulating gene and drug screening. PLoS ONE 7(12):e52549
Steele WB, Kristofco LA, Corrales J et al (2018) Comparative behavioral toxicology with two common larval fish models: exploring relationships among modes of action and locomotor responses. Sci Total Environ 640–641:1587–1600
Steenbergen PJ, Richardson MK, Champagne DL (2011) Patterns of avoidance behaviors in the light/dark preference test in young juvenile zebrafish: a pharmacological study. Behav Brain Res 222(1):15–25
Stieb SM, Cortesi F, Jardim de Queiroz L, Carleton KL, Seehausen O, Marshall NJ (2023) Long-wavelength-sensitive (lws) opsin gene expression, foraging and visual communication in coral reef fishes. Mol Ecol 32(7):1656–1672
Terakita A (2005) The opsins. Genome Biol 6(3):213
Tuz-Sasik MU, Boije H, Manuel R (2022) Characterization of locomotor phenotypes in zebrafish larvae requires testing under both light and dark conditions. PLoS ONE 17(4):e0266491
Villamizar N, Blanco-Vives B, Migaud H, Davie A, Carboni S, Sánchez-Vázquez FJ (2011) Effects of light during early larval development of some aquacultured teleosts: a review. Aquaculture 315(1–2):86–94
Wang C, Zhong Z, Sun P, Zhong H, Li H, Chen F (2017) Evaluation of the hair cell regeneration in zebrafish larvae by measuring and quantifying the startle responses. Neural Plast 2017:8283075
Yokoyama S, Radlwimmer FB (2001) The molecular genetics and evolution of red and green color vision in vertebrates. Genetics 158(4):1697–1710
Zaccardi G, Kelber A, Sison-Mangus MP, Briscoe AD (2006) Color discrimination in the red range with only one long-wavelength sensitive opsin. J Exp Biol 209(Pt 10):1944–1955
Zhang J (2003) Evolution by gene duplication: an update. Trends Ecol Evol 18(6):292–298
Zhang YT, Chen M, He S et al (2021) Microplastics decrease the toxicity of triphenyl phosphate (TPhP) in the marine medaka (Oryzias melastigma) larvae. Sci Total Environ 763:143040
Acknowledgements
No.
Funding
This work was financially supported the National Natural Science Foundation of China (31972809) and the Key Research & Development Program of Hubei Province (2022BBA0051).
Author information
Authors and Affiliations
Contributions
Di-Mei Xu supplement and analyzed experimental data, wrote the original draft. Fa-Rui Chai designed and performed the experiment, analyzed experimental data. Ke Lu participated in some of the experiments. Xu-Fang Liang designed and supervised the experiment and wrote and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, DM., Chai, FR., Liang, XF. et al. Knockout of lws1 in zebrafish (Danio rerio) reveals its role in regulating feeding and vision-guided behavior. Funct Integr Genomics 24, 62 (2024). https://doi.org/10.1007/s10142-024-01333-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10142-024-01333-y