Abstract
LTBP1 is closely related to TGF-β1 function as an essential component, which was unclear in gastric cancer (GC). Harbin Medical University (HMU)-GC cohort and The Cancer Genome Atlas (TCGA) dataset were combined to form a training cohort to calculate the connection between LTBP1 mRNA expression, prognosis and clinicopathological features. The training cohort was also used to verify the biological function of LTBP1 and its relationship with immune microenvironment and chemosensitivity. In the tissue microarrays (TMAs), immunohistochemical (IHC) staining was performed to observe LTBP1 protein expression. The correlation between LTBP1 protein expression level and prognosis was also analyzed, and a nomogram model was constructed. Western blotting (WB) was used in cell lines to assess LTBP1 expression. Transwell assays and CCK-8 were employed to assess LTBP1's biological roles. In compared to normal gastric tissues, LTBP1 expression was upregulated in GC tissues, and high expression was linked to a bad prognosis for GC patients. Based on a gene enrichment analysis, LTBP1 was primarily enriched in the TGF-β and EMT signaling pathways. Furthermore, high expression of LTBP1 in the tumor microenvironment was positively correlated with an immunosuppressive response. We also found that LTBP1 expression (p = 0.006) and metastatic lymph node ratio (p = 0.044) were independent prognostic risk factors for GC patients. The prognostic model combining LTBP1 expression and lymph node metastasis ratio reliably predicted the prognosis of GC patients. In vitro proliferation and invasion of MKN-45 GC cells were inhibited and their viability was decreased by LTBP1 knockout. LTBP1 plays an essential role in the development and progression of GC, and is a potential prognostic biomarker and therapeutic target for GC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As the fifth most common malignant tumor in the world, gastric cancer (GC) has more than 1.09 million new patients every year. Although the number of patients has decreased, it is still a digestive tract malignant tumor that cannot be ignored (Sung et al. 2021). The traditional treatment of GC is radical operation combined with chemotherapy, which has achieved good clinical effects. However, more than 60% of advanced GC patients have experienced postoperative recurrence and metastasis, and the five-year survival rate of advanced GC patients in China is lower than 40% (Wang et al. 2023). As molecular targets gradually become an important means of adjuvant clinical therapy, the rapid development of multiple sequencing technologies can also assist bioinformatics and clinical experts to focus their research on tumor sequencing data, so as to more conveniently and extensively search for potential biological markers and explore the potential research value of genes (Sheikholeslami et al. 2015; Dahlmann and Stein 2021).
LTBP, as a potential binding protein, is involved in the activation and secretion of TGF-β as a propeptide complex (Kanzaki et al. 1990; Miyazono et al. 1991; Saharinen et al. 1999; Robertson and Rifkin 2013). LTBP1 and TGF-β1 co-regulate tumor cell proliferation, apoptosis, and EMT(Cai et al. 2020; Fu et al. 2020). Among them, EMT not only promotes the separation of primary cancer cells and their distant metastasis, but also plays an important role in the occurrence of some malignant tumors (Nieto et al. 2016). TGF-β induces EMT in tumor cells through both Smad and non-Smad pathways and regulates other EMT-related processes through a variety of complexes (Hao et al. 2019). The EMT process of tumors is closely related to the TGF-β pathway, so LTBP1 deserves further exploration. In addition, LTBP1 induces EMT and regulates TGF-β activity promotes the progression of esophageal squamous cell carcinoma and gliomaSmad (Cai et al. 2020; Tritschler et al. 2009). The functional significance and predictive utility of LTBP1 in GC remain uncertain, and it is important to evaluate its expression level and prognostic significance in GC.
Materials and methods
Tissue samples and patients
A total of 269 patients underwent radical gastrectomy at the HMU Cancer Hospital were selected, after obtaining the patients' written, informed permission. The clinical data of these patients were also acquired to create the HMU-GC cohort. RNA isolation, library construction and mRNA sequencing were performed by Novogene Biotech Co. Ltd. (Beijing, China). The data have been deposited in the Gene Expression Omnibus (GEO) repositories (GSE184336 and GSE179252). In addition, gene expression and clinical data of GC samples were retrieved from GEO (GSE15459 and GSE62254) and The Cancer Genome Atlas (TCGA)-stomach adenocarcinoma (STAD) databases. The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee from Harbin Medical University Cancer Hospital, China (Approval Number: SHGC-1029). Informed consent was obtained from all subjects involved in the study.
Data processing
Subsequent analysis may be impacted by batch effects in distinct datasets. The Combat method is predicated on estimating the previous parameter distribution, individually estimating each gene's batch mean and variance, and making the necessary adjustments. It has been demonstrated that applying the Combat algorithm to remove batch effects in differential expression analysis reduces dependence, stabilizes error rate estimations, and enhances reproducibility (Leek et al. 2012). Using the ComBat algorithm in the "sva" package, the high-throughput sequencing data from the HMU-GC and TCGA-STAD cohorts were transformed to transcripts per kilobase million (TPM) values and corrected for batch effects from non-biotechnical biases. The two cohorts were merged as the training cohort. The original microarray CEL files of the GSE15459 and GSE62254 datasets were downloaded to calculate the absolute mRNA expression level, corrected and merged using the ComBat algorithm in the "sva" package as the validation cohort.
Bioinformatics analysis
Based on the median LTBP1 mRNA expression as the cutoff, patients in the training cohort, patients were split into LTBP1 high and LTBP1 low groups. Using the "limma" program, with | Fold change (FC) |> 2 and false discovery rate (FDR) < 0.05 as the criteria, the differentially expressed genes (DEGs) between the two groups were screened. Gene ontology (GO) annotation for the DEGs was identified using the org.Hs.eg.db package. The significantly enriched pathways of the DEGs were identified by Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis (https://www.kegg.jp/kegg/rest/keggapi.html). Gene set enrichment analysis (GSEA) was performed for the LTBP1 high group using "clusterProfiler" package, with | normalized enrichment score (NES) |> 1, nominal (NOM) p-value < 0.05 and FDR q-value < 0.25 as the filters. The protein–protein interaction (PPI) network for LTBP1 was constructed using the search tool for retrieving interacting genes (STRING), version 11.5 (https://string-db.org/). The minimum required interaction score was set to 0.9. The CIBERSORT and TIMER algorithms were used to analyze the immune cell composition of each tumor sample. Based on the ratio of immune cells to stromal cells, the ESTIMATE algorithm was used to generate the immune score, stromal score, ESTIMATE score, and tumor purity. Somatic mutation data of the TCGA-STAD cohort was downloaded from GDCquery_Maf (pipelines = "mutect2") using TCGA "biolinks" package, and the "maftools" package was used to analyze the top 20 individual cell variants with the highest mutation frequency in both groups. The EMT score for each tumor sample was calculated from the expression profile using the ssGSEA algorithm. Cox regression analysis and proportional risk hypothesis testing were carried out using the survival package, and a nomogram was constructed using the RMS package. The data was analyzed using the timeROC tool, and the timeROC was displayed using ggplot2. For the purpose of calibration analysis and visualization, utilize the rms package and the survival package. Using the survival package to fit the prognostic model and STDCA on R files to carry out DCA analysis. Chemosensitivity was analyzed based on PRISM Public drug sensitivity database (Corsello et al. 2020). The pan-cancer analysis of LTBP1 expression was performed using the Sangerbox 3.0 analysis software (Shen et al. 2022).
Immunohistochemistry
A total of 180 randomly selected tumor and para-tumor tissues were fixed, embedded in paraffin, and sectioned to build paraffin-embedded tissue microarrays (TMAs). The TMAs were heated at 62 °C for 2h in an oven, soaked in xylene for 30 min, dehydrated through an ethanol gradient (100%, 95% and 85% ethanol for 5 min each), and washed with water for 5 min. After washing thrice with PBS (5 min each time), the slides were immersed in EDTA antigen retrieval solution (pH 8.0) for 3 min at 120℃, and again washed thrice with PBS (10 min each time). The endogenous peroxidase was quenched by treating the sections with 0.3% H2O2 for 30 min. To block non-specific binding, the sections were incubated with goat serum (Boster, USA) for 1h, followed by overnight incubation with anti-LTBP1 antibody (Affinity, DF10191, 1:150) at 4 °C. After washing with PBS, the sections were incubated with the goat anti-rabbit IgG at room temperature for 40 min. The color was developed using diaminobenzidine (DAB), and the hematoxylin was used to counterstain the sections. Two pathologists examined the TMAs and divided into high and low expression groups by 50% of the positive area.
Cell culture
Procell Life Science & Technology Co., Ltd. (Wuhan, CN) supplied the GC cell lines AGS, HGC-27, and MKN-45 as well as the stomach epithelial cell line GES-1. The other cells were cultured in RPMI-1640 (Procell, CN), while AGS cells were cultured in Ham's F-12 (Procell, CN). 10% fetal bovine serum and 1% penicillin/streptomycin solution were added to all mediums. Every cell was cultured at 37°C with 5% CO2.
Western blotting
Cell lines were lysed with RIPA buffer (Beyotime BioTechnology, CN). The lysates were centrifuged at 13000 rpm for 15 min, and the protein concentration in the supernatants was measured with the Biochemical Acid (BCA) Protein Determination Kit (Beyotime BioTechnology, CN). Equal amounts of protein per sample were separated by 8% sodium dodecyl sulfate–polyacrylamide gels (SDS-PAGE), and transferred to PVDF membrane (Merck Millipore Ltd). After blocking with 5% skim milk for 2h, the blots were incubated overnight with anti-LTBP1 (Affinity, DF10191, 1:500) and anti-GAPDH (Abcam, ab8245, 1:5000) antibodies at 4 °C. The blots were then incubated with horseradish peroxidase-labeled secondary antibody at room temperature for 1h. The PVDF film was exposed to ECL reagent (Meilunbio, CN) to develop the positive bands.
Transient transfection
siRNA is from Sangon Biotech Co., Ltd. Shanghai, PR China, each tube of reagent was mixed thoroughly with 20ul of DEPC water. MKN-45 cells were laid in a six-well plate with a density of about 50%, then replaced with a 1.5 ml 10% culture medium without penicillin streptomycin, and added with a mixture of 200ul of jetPRIME®buffer, 5ul of siRNA and 4ul of jetPRIME® reagent (Polyplus-transfection, 101,000,046–1.5ml, FR). The cell lines were identified as siLTBP1-si1, LTBP1si2 and NC, respectively, after 24 h of replacement.
CCK8 assay
Cell proliferation was measured by Cell Counting Kit-8 (CCK-8) (Dalian Bergolin Biotechnology Co.,Ltd. Dalian, CN). Cells were inoculated into 96-well plates (3 × 103 per well). After cell adhesion, the cells were incubated in 10ul CCK-8 and 90ul culture medium for 40 min away from light, and fluorescence and cell counting were performed at 450nm with a miniature flat plate reader at low speed. The same experimental scheme was carried out at intervals of 24 h, 48 h and 72 h.
Transwell assays
800μl medium containing 10% FBS was added to the lower Transwell chambers. For the invasion assay, 50,000 cells suspended in serum-free media were introduced to the upper Transwell chambers. The cells that had moved or invaded the lower chambers did so after growing for twenty-four hours. After 30 min of crystal violet staining, the chambers were examined under an inverted microscope (100 ×) to count the cells.
Statistical analyses and bioinformatics analyses
The mean ± SD from at least three independent repeating measurements serves as a representation of the data acquired from the experiment. The R statistical software (v4.0.2) was used for all statistical analyses. Mann–Whitney U/Wilcoxon signed rank sum test was used to compare continuous variable data. Chi-square test was used to analyze the correlation between LTBP1 mRNA/protein expression and the clinicopathological characteristics. The survival package in R was used to estimate the risk ratio (HR) and 95% confidence interval (CI) through the Cox regression model. Survival curves were plotted using the Kaplan–Meier method. A two-tailed p-value < 0.05 was considered statistically significant for all tests.
Results
LTBP1 is correlated with the prognosis of patients.
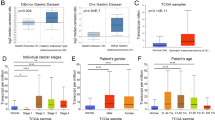
We showed the flow chart of this research (Fig. 1). In the training cohort, the higher LTBP1 patients median survival was only 36.1 months compared to 57.4 months in the LTBP1 low patients (p = 0.014) (Fig. 2A). In the GEO cohort, the mRNA expression is still related to prognosis. High LTBP1 patients correlated to worse prognosis in the GEO cohort as well (p = 0.001) (Fig. 2B). Furthermore, LTBP1 mRNA of GC tissues was substantially higher, LTBP1 mRNA of the adjacent gastric tissues was substantially lower (p < 0.001) in the training cohort (Fig. 2C). We also analyzed at the correlation between clinicopathological characteristics and LTBP1 mRNA expression. While there was no significant correlation of LTBP1 expression with gender and M stage (p > 0.05), it was significantly associated with age (p = 0.0062), T stage, N stage,pTNM stage (all p < 0.001) (Fig. 2D-G). In patients older than 60 years, high LTBP1 expression portended poor prognosis (p = 0.014). Furthermore, LTBP1 mRNA levels increased with tumor progression, i.e., from T3 and T4, N1-N3, and stage I-III. In patients with N1-N3, T3-T4, and stage III-IV, high expression LTBP1 patients have poor prognosis, although there was no statistical significance (Fig. 2H-O).
Flow chart. (GC: Gastric cancer; HMU: Harbin medical university; TCGA: The cancer genome atlas; STAD: Stomach adenocarcinoma; TMAs: Tissue microarrays; ROC: Receiver operating characteristic curve; AUC: Area under receiver operating characteristic curve; qPCR: Quantitative real-time PCR; MSI: Microsatellite instability; TMB: Tumor mutation burden)
The association between LTBP1 mRNA expression and the clinical features and prognosis of gastric cancer patients. (A) Overall survival (OS) of LTBP1 high and LTBP1 low patients in the HMU-TCGA cohort. (B) OS of LTBP1 high and LTBP1 low patients in the GEO cohort. (C) The differential expression of LTBP1 mRNA in GC (619 cases) and normal tissues (300 cases). (D-G) The association between LTBP1 expression and clinical features in the HMU-TCGA cohort. (H,I) OS of LTBP1 high and LTBP1 low patients in the ≤ 60 and > 60 age groups. (J,K) OS of LTBP1 high and LTBP1 low patients in T3 and T4 stages. (L,M) OS of LTBP1 high and LTBP1 low patients in N0 and N1-N3 stages. (N,O) OS of LTBP1 high and LTBP1 low patients in stage III and stage IV groups. *p < 0.05, **p < 0.01,***p < 0.001. (OS, Overall survival; GC, gastric cancer; HMU, Harbin Medical University; TCGA, the Cancer Genome Atlas, GEO, gene expression omnibus)
The biological functions of LTBP1
Based on the HMU-TCGA training cohort, we separated the patients into two groups based on their LTBP1 median value and performed limma analysis. We obtained (2,098) up-regulated genes and (6,584) down-regulated genes (Fig. 3A). The LTBP1-related DEGs were significantly enriched in biological processes (BP) including regulation of signaling and organelle organization and so on(Fig. 3B), cellular components (CC) such as nuclear part, endomembrane system, vesicle, nucleoplasm, and organelle membrane (Fig. 3C), and molecular functions (MF) like enzyme binding and GTPase binding (Fig. 3D). According to KEGG analysis, the DEGs were significantly associated with the MAPK, TNF, AMPK, cGMP-PKG and Rap1 pathways (Fig. 3E). Using GSEA was to further explore the potential mechanism of LTBP1. High LTBP1 expression was significantly correlated with the IL-6/JAK/STAT3, apoptosis, TGF-beta, Hedgehog and EMT, which play essential roles in tumor progression (Fig. 3F). We then analyzed the PPI network of LTBP1, and found TGF-β1, TGF-β3, FN1, THBS1 and ADAMTSL2 interact most with LTBP1 (Fig. 3G). In addition, high LTBP1 expression had a higher EMT score, suggesting that LTBP1 may promote the EMT and metastasis of GC cells (Fig. 3H).
The biological functions of LTBP1. (A) The DEGs between the LTBP1 high and LTBP1 low groups in HMU-TCGA cohort were screened using limma. GO enrichment analysis of LTBP1 and its related genes included the following: (B) Biological processes (BP), (C) Cellular components (CC) and (D) Molecular functions. (E) KEGG enrichment analysis of LTBP1 and its related in HMU-TCGA cohort. (F) GSEA enrichment analysis of LTBP1 in HMU-TCGA cohort. (G) PPI analysis of LTBP1-associated proteins. (H) EMT score of LTBP1 high and LTBP1 low groups. ***p < 0.001. (HMU, Harbin Medical University; TCGA, the Cancer Genome Atlas; DEGs: Differentially Expressed Genes. PPI: Protein–protein interaction network)
Relationship between LTBP1 and immunity
According to the ESTIMATE algorithm, the immune score, stromal score and ESTIMATE score of the LTBP1 high group were significantly higher than that of LTBP1 low group (all p < 0.05; Fig. 4A-C), which indicated that high expression of LTBP1 may induce an immunosuppressive tumor microenvironment (TME) and promote malignant progression. Moreover, tumor purity was significantly lower in the LTBP1 high group versus the LTBP1 low group (p < 0.05; Fig. 4D). In addition, we analyzed the abundance of infiltrating immune cells in the TME using the CIBERSORT and TIMER algorithms. According to Fig. 4E, there was a strong correlation (p < 0.001) between the infiltration of plasma cells, activated CD4 + T cells, activated NK cells, M2 macrophages, and mast cells and LTBP1 expression in the CIBERSORT algorithm. According to the results of TIMER, the infiltrations of T cells, neutrophils, macrophages, and DC cells were significantly higher in the LTBP1 high group Fig. 4F).
Relationship between LTBP1 and immunity. LTBP1 expression correlated with (A) immune score, (B) ESTIMATE score, (C) stromal score and (D) tumor purity as per the ESTIMATE algorithm. (E) CIBESORT analysis and (F) TIMER analysis showed the relationship between LTBP1 expression and immune cells in the tumor microenvironment. *p < 0.05, **p < 0.01,***p < 0.001, ****p < 0.0001
LTBP1 expression correlates with mutation burden and chemosensitivity
According to the mRNA expression of LTBP1, we analyzed its sensitivity to chemotherapy drugs. As shown in Fig. 5A, high LTBP1 expression correlated significantly with the sensitivity to docetaxel (p = 0.04), paclitaxel (p = 0.03) and cisplatin (p < 0.001), indicating that these drugs may have better therapeutic outcomes in high GC expression patients. Furthermore, PCLO and ZFHX4 were the two most frequently altered genes in TCGA-STAD cohort, and the mutation rate was lower in the LTBP1 high group (Fig. 5B).
Relationship between LTBP1 protein expression and prognosis.
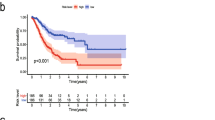
To further explore the clinical potential of LTBP1, we performed immunohistochemical staining on the TMAs (Fig. 6A), and analyzed the survival data of 100 patients who had been followed up for three years. High expression of LTBP1 was associated to a poor prognosis, as illustrated in Fig. 6B (p = 0.001; HR: 3.62, 95% CI: 1.69–7.74). In addition, LTBP1 protein expression was correlated to the pTNM stage and tumor location. High LTBP1 expression patients have a later tumor stage (Table 1). Univariate Cox regression analysis further showed that LTBP1 expression (p < 0.001), lymph node metastasis rate (p < 0.001) and tumor location (p = 0.003) were significantly correlated to the prognosis of GC (Table 2), and multivariate Cox regression analysis identified LTBP1 expression (p = 0.006) and lymph node metastasis rate (p = 0.044) as independent risk factors for GC.
Relationship between LTBP1 expression and prognosis. (A) Representative immunohistochemical and HE staining images of gastric cancer TMAs (G3:poorly-differentiated, G1:well-differentiated, MC: mucinous carcinoma) showing LTBP1 expression (magnification: 50 × and 400x). (B) Kaplan–Meier survival curve based on LTBP1 expression levels. (C) Nomogram prediction model. (D) Kaplan–Meier survival curves based on the nomogram prediction model. (E) ROC curve and (F) calibration curve of the nomogram. (G,H) Decision curve analysis for 2 and 3 years. *p < 0.05, **p < 0.01; NS: no significance; ROC: Receiver operating characteristic
Following the results of multivariate analysis, nomograph model was made to predict the prognosis of GC patients (Fig. 6C). The total score was calculated for each patient, and the cohort was divided into the low-risk and high-risk groups based on the median risk score. As shown in Fig. 6D, patients in the high-risk group had worse prognosis compared to those in the low-risk group (p = 0.001; HR: 5.62, 95% CI: 2.27–13.94). In addition, the area under the receiver operating characteristic curve (ROC) (AUC) for predicting 1-, 2- and 3-year prognosis of GC patients was 0.53 (0.258–0.801), 0.776 (0.646–0.906) and 0.755 (0.633–0.876) respectively (Fig. 6E), indicating that the prognosis model has good clinical application in evaluating the long-term prognosis of patients. The satisfactory prediction value of the model was further confirmed by the calibration curve (C-index:0.702 (0.652–0.752)) (Fig. 6F). The 2- and 3-year decision curve (DCA) analysis showed that combining the expression of LTBP1 with the lymph node metastasis rate can clinically evaluate the prognosis of GC patients (Fig. 6G-H).
LTBP1 restrains cell viability and invasion in vitro
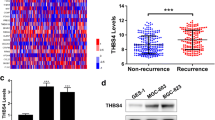
Using the western blot assays, we were able to observe the expression of LTBP1 in the normal gastric epithelial cell line (GES-1) and several GC cell lines (AGS, HGC-27, and MKN-45). It was discovered that MKN-45 cell lines had the highest expression of LTBP1 (Fig. 7A). In MKN-45 cell lines, we instantly downregulated the expression of LTBP1 (Fig. 7B). According to CCK-8 assays, the viability of GC cells with LTBP1 knockdown was lower than that of the negative control group (Fig. 7C). Transwell assays suggested that LTBP1 expression reduction might substantially prevent GC cell invasion (Fig. 7D). This implies that LTBP1 may facilitate the development of GC cells.
LTBP1 restrains cell viability and invasion in vitro. (A) LTBP1 expression in normal gastric epithelium and different gastric cancer cell lines by western blot assays. (B) The siRNA effects (NC, si-1 and si-2) were examined by western blot assays. (C) The relative proliferation rates of MKN-45 cells were measured in cells with the NC or transiently silenced LTBP1 (si-1 and si-2). (D) Invasive abilities were detected with Transwell assays without matrigel, in cells with the NC or LTBP1 transiently silenced (si-1 and si-2)
Analysis of LTBP1 in other cancers
LTBP1 is highly expressed in various cancers (Fig. 8A). In addition, high LTBP1 expression was correlated with the poor OS of 11 types of cancer, including glioma (GBMLGG), brain lower grade glioma (LGG), adrenocortical carcinoma (ACC) and kedney renal papillary cell carcinoma (KIRP) (p < 0.001; Fig. 8B). This suggests that LTBP1 is significant in other malignancies as well, and warrants further research. The TIMER algorithm showed a positive correlation between LTBP1 and infiltrating immune cells (Fig. 8C). Thus, LTBP1 is associated with multiple immune cells in various cancers, and may affect the immune landscape in tumors. Nevertheless, its role in the tumor immune microenvironment needs to be studied for each individual cancer. Furthermore, LTBP1 was positively correlated with immune checkpoints in most tumors such as pancreatic cancer (PAAD), pan-kidney cohort (KIPAN), breast invasive carcinoma (BRCA) and so on (Fig. 8D). These results suggest that LTBP1 may regulate the tumor immune microenvironment, and influence patient response to immunotherapy. Analysis of tumor purity, microsatellite instability (MSI) and the tumor mutation burden (TMB) further showed that LTBP1 expression is a potential biomarker for guiding targeted treatment against acute myeloid leukemia (LAML), adrenocortical carcinoma (ACC), mesothelioma (MESO), pancreatic adenocarcinoma (PAAD), BRCA, KICH and other tumors (Fig. 8E-G). However, LTBP1 expression was negatively correlated with TMB and MSI in most cancers. In addition, LTBP1 expression showed a negative correlation with most tumor stem cells, which are essential factors in tumor progression. Taken together, LTBP1 expression is closely related to the development of multiple tumors and deserves further investigation.
Analysis of LTBP1 expression in other cancers. (A) Expression of LTBP1 in various cancers. (B) Overall survival of LTBP1 in various cancers. (C)TIMER analysis showed that the relationship between LTBP1 expression and immune cell infiltration in various tumor types. (D) The LTBP1-related immune checkpoints of various cancers. (E) Tumor purity analysis, (F) TMB analysis, (G)MSI analysis of various cancer. MSI: Microsatellite Instability; TMB: Tumor Mutation Burden. *p < 0.05, **p < 0.01,***p < 0.001, ****p < 0.0001
Discussion
GC has a high incidence and mortality rate in developing countries (Sung et al. 2021), which is one of the serious public health burdens in the world. Although targeted therapy for GC has made some progress in recent years (Doi et al. 2017), its survival benefits for GC patients are still limited. Therefore, there is still a need to improve treatment strategies for GC by finding new and effective targets for treatment.
Drug resistance is driven by a number of factors, particularly TME, which has recently attracted much attention. Mesenchymal cells in the TME promoting tumor progression and inducing drug resistance have been validated in various tumors (Falcomata et al. 2022; Affo et al. 2021; Ham et al. 2021). As a major component of tumor stroma, CAFs induce fibrous proliferation, which can directly lead to reduced drug perfusion and thus reduce the effectiveness of chemotherapy (Provenzano et al. 2012). The pathogenesis of fibrous proliferation associated with oncology treatment has not been well studied. Related studies have shown that TGF-β is considered a key mediator in triggering the pathogenesis of fibrosis development (Caja et al. 2018). TGF-β primarily operates biologically by way of the Smad pathway. The TGF-β Receptor II (TβRII) binds to TGF-β, allowing TβRII to phosphorylate the TβRI on serine and threonine, hence initiating TβRI catalytic activity. The catalytically active TβRI phosphorylates the receptor-activated (R-) Smads' C-terminal serine residues through Smad2 and Smad3. They attach to the trimeric protein complex made up of Smad and other transcription factors when they are phosphorylated, which can either stimulate or inhibit the translation of a gene (Yu et al. 2008). This is the simplest explanation of the Smad pathway. Moreover, TGF-β activates non-Smad pathways. These non-Smad proteins can control the canonical Smad pathway in addition to their involvement in TGF-β-mediated biological responses (Hong et al. 2011). The biological function of TGF-β is to activate the proliferation of fibroblasts, initiate EMT, and increase the synthesis of extracellular matrix (ECM) (Caja et al. 2018). As LTBP1 may be closely related to TGF-β (Fu et al. 2020), we hope to determine the possibility of LTBP1 as a potential target for GC treatment by analyzing its expression level and prognostic correlation in GC.
LTBPs are an essential component of the ECM that promote the formation of the matrix, and maintain its strength and elasticity (Davis et al. 2014). LTBPs are also required for the assembly and secretion of TGF-β (Miyazono et al. 1991), and regulate the bioavailability of the TGF-β superfamily proteins (Robertson et al. 2011). LTBP1 was the first protein of this family to be discovered, and is highly expressed in the lungs, heart, spleen, kidneys and stomach (Davis et al. 2014; Tsuji et al. 1990). It regulates cardiovascular development, cerebral tubule formation and various neurological functions (Beaufort et al. 2014; Horiguchi et al. 2015). In addition, among different malignant tumors, LTBP1 may be functioning. Cai et al. (Cai et al. 2020) found that LTBP1 promoted the ability of esophageal squamous cell carcinoma (ESCC) cells to invade and migrate to adjacent tissues. Knocking down LTBP1 in the ESCC cells inhibited EMT via downregulation of TGF-β. Likewise, Tritschler et al. (Tritschler et al. 2009) found that LTBP1 protein levels in glioblastoma (GBM) tissues correlated positively with the severity of malignancy, and overexpression of LTBP1 in GBM cell lines upregulated TGF-β and increased the levels of phosphorylated Smad2. Taki et al. (Taki et al. 2020) used immunocore-deposition to show that LTBP1 interacts with and upregulates CD109 in various tumors, and increases TGF-β levels in the matrix. In this study, LTBP1 was found to be highly expressed in the GC tissue of the HMU-TCGA training cohort. High mRNA and protein levels of LTBP1 were strongly associated with a more aggressive clinical phenotype and poorer prognosis characteristics in GC patients. Furthermore, we confirmed by means of in vitro functional studies that LTBP1 has the ability to stimulate GC cell proliferation, invasion, and migration, consequently facilitating GC development. Thus, LTBP1 can be used as a potential biological indicator to assess the prognosis of GC patients. Nevertheless, the biological function and clinical application significance of LTBP1 in GC are still unknown.
Tumor occurrence and development require tumor cells to increase metabolic flux through various metabolic pathways, activate signaling pathways and transcription networks to meet the increased the energy and biosynthesis requirements for maintaining proliferation and survival (Martinez-Reyes and Chandel 2021). GO analysis showed that LTBP1 and its correlated genes may play a role in protein metabolism and modification, which will be the focus of our subsequent study. GSEA further showed the involvement of LTBP1 in the TGF-β signaling and EMT pathways. Through the reversible process of epithelial-mesenchymal transition (EMT), mesenchymal cells acquire new characteristics and express different cytoskeleton and cell adhesion molecules. Epithelial and mesenchymal tumor cells have a greater chance of survival, metastasis, and organ colonization in distal regions (Pal et al. 2021). The regulation network of EMT involves several signaling pathways. Studies show that the RAS-MAPK and TGF-β pathways can trigger EMT via Smad and RREB1 transcription factors (Su et al. 2020; Horiguchi et al. 2009). Consistent with this, KEGG showed LTBP1 and its correlated genes were enriched in the MAPK signaling pathway, which is involved in cell growth, development, differentiation, apoptosis, as well as tumor occurrence (Keshet and Seger 2010; Yu et al. 2015; Fang and Richardson 2005). In addition, the PPI network of LTBP1 also revealed that TGF-β1, TGF-β3 and FN1 are its main interacting genes. Lin et al. showed that knocking down LTBP in natural killer/T cell tumors downregulated p-Smad2, TGF-β1, p-p38 and p-Smad3, and this inhibitory effect was reversed with exogenous TGF-β1 (Lin et al. 2021). LTBP1 assembles in the ECM with the help of fibronectin (FN1) (Dallas et al. 2005), and inhibiting LTBP1 expression in ESCC cells downregulates FN1 (Cai et al. 2020). In addition, there was evident that FN1 promotes EMT of ovarian cancer cells (Liang et al. 2020). Our study also found that LTBP1 is highly correlated with molecules related to the EMT pathway. Thus, the oncogenic role of LTBP1 in gastric tumors may be dependent on the MAPK and TGF-β signaling pathways, and LTBP1 may interact with TGF-β1 and FN1 to promote EMT of GC cells.
In this study, we found that high expression of LTBP1 was associated with higher immune score and higher stromal score by ESTIMATE algorithm. Higher immune score indicated that LTBP1 might promote immune infiltration of tumor cells. However, it may play an immunosuppressive role, resulting in the immune escape and distant metastasis of GC cells, leading to poor prognosis. This study explored the correlation between LTBP1 and immune cell infiltration by CIBERSORT algorithm. High LTBP1 expression correlated with increased infiltration of DCs, mast cells, plasma cells, M2 macrophages and sthers. The M2 macrophages promote angiogenesis and inhibit anti-tumor immune responses, and are associated with poor prognosis of GC (Yin et al. 2017). While infiltration of the cytotoxic CD8+ T cells, NK cells and CD4+ T cells portends favorable prognosis, a greater abundance of mast cells, plasma cells and regulatory T cells (Treg) is associated with poor prognosis of GC (Wei et al. 2018; Oya et al. 2020; Kindlund et al. 2017). Thus, we can surmise that LTBP1 helps establish an immunosuppressive TME, which allows the tumor cells to escape immune surveillance and metastasize to distant organs, eventually resulting in poor prognosis. The complexity of immune microenvironment components in GC make the immune microenvironment plays different functions in different stages of the same tumor. Our study on LTBP1 and GC cell immunity must be verified by follow-up experiments. In addition, high LTBP1 expression was associated with a higher stromal score. This results further confirm our previous hypothesis that LTBP1 and TGF-β1 play a synergistic role in promoting the biological function of EMT in GC, thereby increasing the mesenchymal ratio and fibrosis development in GC.
To further validate the hypothesis that LTBP1 may enhance chemotherapy drug resistance by increasing the degree of GC fibrosis, we analyzed the sensitivity of GC patients with different levels of LTBP1 expression to chemotherapy drugs. The results suggest that docetaxel, pemetrexed and cisplatin are suitable for patients with high LTBP1 expression. Thus, the most appropriate chemotherapeutic drugs can be selected for GC patients based on LTBP1 expression levels. Furthermore, we plan to conduct more in vitro and in vivo experiments to verify whether it is possible to improve the sensitivity of GC patients to chemotherapy by antagonizing the biological behavior of LTBP1 and TGF-β1 synergistically promoting EMT.
Recently, the crucial assessment worth somatic mutation for chemosensitivity had been confirmed by more and more studies (Rodler et al. 2023; Konstantinopoulos et al. 2019). Therefore, we analyzed somatic mutation data from the TCGA-STAD cohort and found that PCLO and ZFHX4 were the two most common mutated genes, and the mutation rate was lower in the high LTBP1 group. Other studies have also found the application value of PCLO gene mutations and ZFHX4 gene mutations in evaluating chemotherapy sensitivity in other solid tumors. Qiu et al. found that patients with extensive stage small cell lung cancer with PCLO single gene mutations exhibit strong resistance to etoposide treatment (Qiu et al. 2019). Al et al. also found that ZFHX4 has predictive value for the responsiveness of neoadjuvant epirubicin/cyclophosphamide therapy in breast cancer (Al Amri et al. 2020). This result provided new insights into the mechanism by which LTBP1 affects chemotherapy sensitivity in GC patients.
After IHC, advanced GC patients had higher LTBP1 expression with poor prognosis, suggesting that LTBP1 is strongly correlated with tumor progression. Furthermore, LTBP1 expression and lymph node metastasis rate were identified as independent risk factors of GC, and the nomogram constructed with these two variables showed good predictive performance and clinical benefit. However, it was limited by the number of patients and follow-up time. We still need to supplement patients and follow-up time to further explore the potential of its clinical application.
We also investigated the role of LTBP1 in cancers. LTBP1 was overexpressed in most cancers and was associated with the prognosis of 14 cancer types. Furthermore, LTBP1 can predict the immunotherapy response in most cancers, and may even be a key therapeutic target. In addition, immunotherapy is increasingly being used in the treatment of certain types of cancer. In some countries, PD1 and PDL-1 inhibitors have been approved for treating patients with recurrent GC and advanced GC (Kang et al. 2017; Fuchs et al. 2018), as well as for the immunotherapy of patients with GC. Likewise, LTBP1 can guide targeted therapy in LAML, ACC, MESO, GBM, testicular germ cell tumors (TGCT), BRCA, PAAD and KICH. Currently, only a few targeted therapies have been approved clinically. For instance, trastuzumab is used to treat HER2 overexpressing tumors, and ramucirumab inhibits tumor angiogenesis by targeting vascular endothelial growth factor receptor 2 (VEGFR2) (Seeneevassen et al. 2021). However, these two targeted therapies can only satisfy certain types of cancer, so we can consider further studies of LTBP1 in guiding targeted therapies. In addition, LTBP1 is negatively correlated with TMB and MSI in the majority of tumors. Since both are determinants of the efficacy of immunotherapy (Cristescu et al. 2018; Lin et al. 2020; van Velzen et al. 2020), suggesting that LTBP1 has a good application prospect in guiding patients' immunotherapy, predicting the effect of immunotherapy, and guiding targeted therapy. Although LTBP1 was also found to be associated with immune cell infiltration in various tumors, its role in the immune microenvironment of each cancer remains to be studied.
There are some limitations to this study that ought to be considered. First, we established a prognostic model based on the combination of clinicopathological features. Although LTBP1 showed good predictive performance, less than 180 patients were followed up for 3 years. Therefore, we will have to validate the prognostic efficacy of LTBP1 on a larger cohort. Second, this study did not elaborate the mechanisms through which LTBP1 promotes GC occurrence and development, which will also have to be analyzed by more functional in vitro and in vivo assays.
Conclusions
In conclusion, we verified the reliability of LTBP1 for predicting GC prognosis. Our findings show that the abnormal expression of LTBP1 may promote EMT in GC cells by affecting TGF-β pathway, which can be further studied as a important factor of TGF-β pathway.
Data availability
Harbin Medical University (HMU)- gastric cancer (GC) cohort were stored in the Gene Expression Omnibus (GEO) repository (GSE184336 and GSE179252). Patients’ data were saved in the Gastric Cancer Information Management System v1.2 of Harbin Medical University Cancer Hospital (Copyright No.2013SR087424, http: www. sgihmu.com).
Abbreviations
- GC:
-
Gastric cancer
- HMU:
-
Harbin medical university
- TCGA:
-
The cancer genome atlas
- WB:
-
Western blotting
- IHC:
-
Immunohistochemical
- CAFs:
-
Cancer-associated fibroblasts
- GEO:
-
Gene expression omnibus
- STAD:
-
Stomach adenocarcinoma
- DEGs:
-
Differentially expressed genes
- FDR:
-
False discovery rate
- GO:
-
Gene ontology
- KEGG:
-
Kyoto encyclopedia of genes and genomes
- GSEA:
-
Gene set enrichment analysis
- NES:
-
Normalized enrichment score
- NOM:
-
Nominal
- PPI:
-
Protein-protein interaction
- STRING:
-
Search tool for retrieving interacting genes
- TMAs:
-
Tissue microarrays
- DAB:
-
Diaminobenzidine
- GES:
-
Gastric epithelial cell line
- BCA:
-
Biochemical Acid
- SDS-PAGE:
-
Sodium dodecyl sulfate–polyacrylamide gels
- CI:
-
Confidence interval
- BP:
-
Biological processes
- CC:
-
Cellular components
- MF:
-
Molecular functions
- TME:
-
Tumor microenvironment
- ROC:
-
Receiver operating characteristic curve
- AUC:
-
Area under receiver operating characteristic curve
- DCA:
-
Decision curve analysis
- GBMLGG:
-
Glioma
- LGG:
-
Brain lower grade glioma
- ACC:
-
Adrenocortical carcinoma
- KIRP:
-
Kedney renal papillary cell carcinoma
- BLCA:
-
Bladder urothelial carcinoma
- CHOL:
-
Cholangiocarcinoma
- HNSC:
-
Neck squamous cell carcinoma
- KICH:
-
Kidney chromophobe
- PAAD:
-
Pancreatic cancer
- KIPAN:
-
Pan-kidney cohort
- BRCA:
-
Breast invasive carcinoma
- MSI:
-
Microsatellite instability
- TMB:
-
Tumor mutation burden
- LAML:
-
Acute myeloid leukemia
- MESO:
-
Mesothelioma
- PAAD:
-
Pancreatic adenocarcinoma
- ESCC:
-
Esophageal squamous cell carcinoma
- GBM:
-
Glioblastoma
- Treg:
-
Regulatory T cells
- TGCT:
-
Testicular germ cell tumors
- VEGFR2:
-
Vascular endothelial growth factor receptor 2
- BMI:
-
Body mass index
- INFa:
-
Expanding growth and a distinct border with the surrounding tissue
- INFc:
-
Infiltrating growth and an indistinct border with the surrounding tissue
- INFb:
-
In-between INFa and INFc
- CEA:
-
Carcinoembryonic antigen
- CA19-9:
-
Carbohydrate antigen 19–9
- CA72-4:
-
Carbohydrate antigen 72–4
- HER2:
-
Human Epidermal Growth Factor Receptor 2
- IQR:
-
Interquartile range
References
Affo S, Nair A, Brundu F, Ravichandra A, Bhattacharjee S, Matsuda M, Chin L, Filliol A, Wen W, Song X et al (2021) Promotion of cholangiocarcinoma growth by diverse cancer-associated fibroblast subpopulations. Cancer Cell 39(6):866-882.e811
Al Amri WS, Baxter DE, Hanby AM, Stead LF, Verghese ET, Thorne JL, Hughes TA (2020) Identification of candidate mediators of chemoresponse in breast cancer through therapy-driven selection of somatic variants. Breast Cancer Res Treat 183(3):607–616
Beaufort N, Scharrer E, Kremmer E, Lux V, Ehrmann M, Huber R, Houlden H, Werring D, Haffner C, Dichgans M (2014) Cerebral small vessel disease-related protease HtrA1 processes latent TGF-beta binding protein 1 and facilitates TGF-beta signaling. Proc Natl Acad Sci U S A 111(46):16496–16501
Cai R, Wang P, Zhao X, Lu X, Deng R, Wang X, Su Z, Hong C, Lin J (2020) LTBP1 promotes esophageal squamous cell carcinoma progression through epithelial-mesenchymal transition and cancer-associated fibroblasts transformation. J Transl Med 18(1):139
Caja L, Dituri F, Mancarella S, Caballero-Diaz D, Moustakas A, Giannelli G, Fabregat I (2018) TGF-beta and the Tissue Microenvironment: Relevance in Fibrosis and Cancer. Int J Mol Sci 19(5):1294
Corsello SM, Nagari RT, Spangler RD, Rossen J, Kocak M, Bryan JG, Humeidi R, Peck D, Wu X, Tang AA et al (2020) Discovering the anti-cancer potential of non-oncology drugs by systematic viability profiling. Nat Cancer 1(2):235–248
Cristescu R, Mogg R, Ayers M, Albright A, Murphy E, Yearley J, Sher X, Liu XQ, Lu H, Nebozhyn M et al (2018) Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 362(6411)
Dahlmann M, Stein US (2021) A Bioinformatic Pipeline to Identify Biomarkers for Metastasis Formation from RNA Sequencing Data. Methods Mol Biol 2294:221–238
Dallas SL, Sivakumar P, Jones CJ, Chen Q, Peters DM, Mosher DF, Humphries MJ, Kielty CM (2005) Fibronectin regulates latent transforming growth factor-beta (TGF beta) by controlling matrix assembly of latent TGF beta-binding protein-1. J Biol Chem 280(19):18871–18880
Davis MR, Andersson R, Severin J, de Hoon M, Bertin N, Baillie JK, Kawaji H, Sandelin A, Forrest AR, Summers KM et al (2014) Transcriptional profiling of the human fibrillin/LTBP gene family, key regulators of mesenchymal cell functions. Mol Genet Metab 112(1):73–83
Doi T, Shitara K, Naito Y, Shimomura A, Fujiwara Y, Yonemori K, Shimizu C, Shimoi T, Kuboki Y, Matsubara N et al (2017) Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody-drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose-escalation study. Lancet Oncol 18(11):1512–1522
Falcomata C, Barthel S, Widholz SA, Schneeweis C, Montero JJ, Toska A, Mir J, Kaltenbacher T, Heetmeyer J, Swietlik JJ et al (2022) Selective multi-kinase inhibition sensitizes mesenchymal pancreatic cancer to immune checkpoint blockade by remodeling the tumor microenvironment. Nat Cancer 3(3):318–336
Fang JY, Richardson BC (2005) The MAPK signalling pathways and colorectal cancer. Lancet Oncol 6(5):322–327
Fu X, Zhang P, Song H, Wu C, Li S, Li S, Yan C (2020) LTBP1 plays a potential bridge between depressive disorder and glioblastoma. J Transl Med 18(1):391
Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges JP et al (2018) Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol 4(5):e180013
Ham IH, Lee D, Hur H (2021) Cancer-Associated Fibroblast-Induced Resistance to Chemotherapy and Radiotherapy in Gastrointestinal Cancers. Cancers 13(5):1172
Hao Y, Baker D, Ten Dijke P (2019) TGF-beta-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int J Mol Sci 20(11):2767
Hong M, Wilkes MC, Penheiter SG, Gupta SK, Edens M, Leof EB (2011) Non-Smad transforming growth factor-beta signaling regulated by focal adhesion kinase binding the p85 subunit of phosphatidylinositol 3-kinase. J Biol Chem 286(20):17841–17850
Horiguchi K, Shirakihara T, Nakano A, Imamura T, Miyazono K, Saitoh M (2009) Role of Ras signaling in the induction of snail by transforming growth factor-beta. J Biol Chem 284(1):245–253
Horiguchi M, Todorovic V, Hadjiolova K, Weiskirchen R, Rifkin DB (2015) Abrogation of both short and long forms of latent transforming growth factor-beta binding protein-1 causes defective cardiovascular development and is perinatally lethal. Matrix Biol 43:61–70
Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK et al (2017) Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 390(10111):2461–2471
Kanzaki T, Olofsson A, Moren A, Wernstedt C, Hellman U, Miyazono K, Claesson-Welsh L, Heldin CH (1990) TGF-beta 1 binding protein: a component of the large latent complex of TGF-beta 1 with multiple repeat sequences. Cell 61(6):1051–1061
Keshet Y, Seger R (2010) The MAP kinase signaling cascades: a system of hundreds of components regulates a diverse array of physiological functions. Methods Mol Biol 661:3–38
Kindlund B, Sjoling A, Yakkala C, Adamsson J, Janzon A, Hansson LE, Hermansson M, Janson P, Winqvist O, Lundin SB (2017) CD4(+) regulatory T cells in gastric cancer mucosa are proliferating and express high levels of IL-10 but little TGF-beta. Gastric Cancer 20(1):116–125
Konstantinopoulos PA, Waggoner S, Vidal GA, Mita M, Moroney JW, Holloway R, Van Le L, Sachdev JC, Chapman-Davis E, Colon-Otero G et al (2019) Single-Arm Phases 1 and 2 Trial of Niraparib in Combination With Pembrolizumab in Patients With Recurrent Platinum-Resistant Ovarian Carcinoma. JAMA Oncol 5(8):1141–1149
Leek JT, Johnson WE, Parker HS, Jaffe AE, Storey JD (2012) The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 28(6):882–883
Liang H, Yu M, Yang R, Zhang L, Zhang L, Zhu D, Luo H, Hong Y, Yu T, Sun J et al (2020) A PTAL-miR-101-FN1 Axis Promotes EMT and Invasion-Metastasis in Serous Ovarian Cancer. Mol Ther Oncolytics 16:53–62
Lin A, Zhang J, Luo P (2020) Crosstalk Between the MSI Status and Tumor Microenvironment in Colorectal Cancer. Front Immunol 11:2039
Lin R, Li X, Wu S, Qian S, Hou H, Dong M, Zhang X, Zhang M (2021) Suppression of latent transforming growth factor-beta (TGF-beta)-binding protein 1 (LTBP1) inhibits natural killer/ T cell lymphoma progression by inactivating the TGF-beta/Smad and p38(MAPK) pathways. Exp Cell Res 407(1):112790
Martinez-Reyes I, Chandel NS (2021) Cancer metabolism: looking forward. Nat Rev Cancer 21(10):669–680
Miyazono K, Olofsson A, Colosetti P, Heldin CH (1991) A role of the latent TGF-beta 1-binding protein in the assembly and secretion of TGF-beta 1. Embo J 10(5):1091–1101
Nieto MA, Huang RY, Jackson RA, Thiery JP (2016) Emt: 2016. Cell 166(1):21–45
Oya Y, Hayakawa Y, Koike K (2020) Tumor microenvironment in gastric cancers. Cancer Sci 111(8):2696–2707
Pal A, Barrett TF, Paolini R, Parikh A, Puram SV (2021) Partial EMT in head and neck cancer biology: a spectrum instead of a switch. Oncogene 40(32):5049–5065
Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR (2012) Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 21(3):418–429
Qiu Z, Lin A, Li K, Lin W, Wang Q, Wei T, Zhu W, Luo P, Zhang J (2019) A novel mutation panel for predicting etoposide resistance in small-cell lung cancer. Drug Des Devel Ther 13:2021–2041
Robertson IB, Rifkin DB (2013) Unchaining the beast; insights from structural and evolutionary studies on TGFbeta secretion, sequestration, and activation. Cytokine Growth Factor Rev 24(4):355–372
Robertson I, Jensen S, Handford P (2011) TB domain proteins: evolutionary insights into the multifaceted roles of fibrillins and LTBPs. Biochem J 433(2):263–276
Rodler E, Sharma P, Barlow WE, Gralow JR, Puhalla SL, Anders CK, Goldstein L, Tripathy D, Brown-Glaberman UA, Huynh TT et al (2023) Cisplatin with veliparib or placebo in metastatic triple-negative breast cancer and BRCA mutation-associated breast cancer (S1416): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 24(2):162–174
Saharinen J, Hyytiainen M, Taipale J, Keski-Oja J (1999) Latent transforming growth factor-beta binding proteins (LTBPs)–structural extracellular matrix proteins for targeting TGF-beta action. Cytokine Growth Factor Rev 10(2):99–117
Seeneevassen L, Bessede E, Megraud F, Lehours P, Dubus P, Varon C (2021) Gastric Cancer: Advances in Carcinogenesis Research and New Therapeutic Strategies. Int J Mol Sci 22(7):3418
Sheikholeslami MF, Sadraei J, Farnia P, ForozandehMoghadam M, EmadiKochak H (2015) Co-infection of Mycobacterium tuberculosis and Pneumocystis jirovecii in the Iranian Patients With Human Immunodeficiency Virus. Jundishapur J Microbiol 8(2):e17254
Shen W, Song Z, Zhong X, Huang M, Shen D, Gao P, Qian X, Wang M, He X, Wang T et al (2022) Sangerbox: A comprehensive, interaction-friendly clinical bioinformatics analysis platform. iMeta 1(3):36
Su J, Morgani SM, David CJ, Wang Q, Er EE, Huang YH, Basnet H, Zou Y, Shu W, Soni RK et al (2020) TGF-beta orchestrates fibrogenic and developmental EMTs via the RAS effector RREB1. Nature 577(7791):566–571
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 71(3):209–249
Taki T, Shiraki Y, Enomoto A, Weng L, Chen C, Asai N, Murakumo Y, Yokoi K, Takahashi M, Mii S (2020) CD109 regulates in vivo tumor invasion in lung adenocarcinoma through TGF-beta signaling. Cancer Sci 111(12):4616–4628
Tritschler I, Gramatzki D, Capper D, Mittelbronn M, Meyermann R, Saharinen J, Wick W, Keski-Oja J, Weller M (2009) Modulation of TGF-beta activity by latent TGF-beta-binding protein 1 in human malignant glioma cells. Int J Cancer 125(3):530–540
Tsuji T, Okada F, Yamaguchi K, Nakamura T (1990) Molecular cloning of the large subunit of transforming growth factor type beta masking protein and expression of the mRNA in various rat tissues. Proc Natl Acad Sci U S A 87(22):8835–8839
van Velzen MJM, Derks S, van Grieken NCT, Haj Mohammad N, van Laarhoven HWM (2020) MSI as a predictive factor for treatment outcome of gastroesophageal adenocarcinoma. Cancer Treat Rev 86:102024
Wang H, Fang T, Yin X, Lou S, Han B, Gao J, Wang Y, Wang X, Zhang D, Wang Y et al (2023) Prognostic importance of the preoperative New-Naples prognostic score for patients with gastric cancer. Cancer Med 12(2):1358–1375
Wei M, Shen D, Mulmi Shrestha S, Liu J, Zhang J, Yin Y (2018) The Progress of T Cell Immunity Related to Prognosis in Gastric Cancer. Biomed Res Int 2018:3201940
Yin S, Huang J, Li Z, Zhang J, Luo J, Lu C, Xu H, Xu H (2017) The Prognostic and Clinicopathological Significance of Tumor-Associated Macrophages in Patients with Gastric Cancer: A Meta-Analysis. PLoS ONE 12(1):e0170042
Yu J, Zhang L, Chen A, Xiang G, Wang Y, Wu J, Mitchelson K, Cheng J, Zhou Y (2008) Identification of the gene transcription and apoptosis mediated by TGF-beta-Smad2/3-Smad4 signaling. J Cell Physiol 215(2):422–433
Yu P, Ye L, Wang H, Du G, Zhang J, Zhang J, Tian J (2015) NSK-01105 inhibits proliferation and induces apoptosis of prostate cancer cells by blocking the Raf/MEK/ERK and PI3K/Akt/mTOR signal pathways. Tumour Biol 36(3):2143–2153
Acknowledgements
Not applicable
Funding
This work was partially supported by the Natural Science Foundation of Inner Mongolia Autonomous Region: 2022QR08003. This work was supported by Heilongjiang Provincial Leading Talent EchelonReserve Leader
Author information
Authors and Affiliations
Contributions
XJ, SY and XY jointly completed the writing of the manuscript, YW and TF completed the immunochemistry experiment and drew the picture, XJ and SY were responsible for the editing of the picture, LZ, XB, YX and YW participated in the review of the article and put forward suggestions for revision.
Corresponding author
Ethics declarations
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee from Harbin Medical University Cancer Hospital, China (Approval Number: SHGC-1029). Informed consent was obtained from all subjects involved in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, X., Yin, S., Yin, X. et al. A prognostic marker LTBP1 is associated with epithelial mesenchymal transition and can promote the progression of gastric cancer. Funct Integr Genomics 24, 30 (2024). https://doi.org/10.1007/s10142-024-01311-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10142-024-01311-4