Abstract
Andrographis (Andro) has been identified as an anti-cancer herbal. This study was to explore its underlying regulatory routes regarding cisplatin (DDP) resistance in lung cancer. The impacts of Andro on cell viability in lung cancer cells and normal cells BEAS-2B were validated using CCK8 tests. Then, cell viability and apoptosis analysis was performed in the cells after DDP, Andro, or combined treatment. RT-qPCR was applied for evaluating miR-155-5p and SIRT1 mRNA expressions, while western blot was for evaluating SIRT1 protein expressions. Binding sites between SIRT1 and miR-155-5p were predicted on TargetScan and were confirmed using luciferase reporter assays. Xenograft animal models were established for in vivo validation of the regulatory function of Andro in lung cancer. Andro decreased the cell viability in lung cancer cells but not normal cells BEAS-2B. The combined treatment with DDP and Andro induced the lowest viability and highest apoptosis in both A549 and A549/DDP cells. MiR-155-5p expression was suppressed, and SIRT was promoted by the Andro treatment, while overexpression of miR-155-5p reversed effects of Andro in cells, which was further counteracted by SIRT1 activation. SIRT1 was verified to be a target of miR-155-5p in A549/DDP cells. Moreover, Andro synergized with DDP in mice with lung cancer via miR-155-5p/SIRT1. Andro modulates cisplatin resistance in lung cancer via miR-155-5p/SIRT1 axis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung carcinoma is ranked as the first leading cause of cancer-related death all over the world (Romaszko and Doboszyńska 2018). People died from lung cancer because it is usually diagnosed at an advanced stage. Hence, it is necessary to use the low-dose computed tomography in lung cancer screening in early stage (Toumazis et al. 2020). Risk factors for lung cancer are many, but smoking is still one of the top and others are age, environmental exposure, air pollution, etc. (Bade and dela Cruz 2020; de Groot and Munden 2012). For lung cancer treatment, surgery is the most applicable in early stage, which is also the best curative option (Hoy et al. 2019). Besides that, chemotherapy has been established for lung cancer treatment in different stages (El-Hussein et al. 2021). Moreover, chemotherapy based on platinum is the mainstay of first-line therapy for lung cancer, including carboplatin- and cisplatin (DDP)-based chemotherapy (Griesinger et al. 2019). Cisplatin is widely used for treating a variety of malignancies due to its effects on interfering DNA repair mechanisms in cancer cells (Romani 2022). Side effects after DDP treatment include emesis, nephrotoxicity, decreased immune response to infections, and neurotoxicity, which further requires reduction in drug doses and even stopping administration (Qi et al. 2019). Drug resistance is another inherent challenge for DDP, decreasing efficacy of DDP (Ghosh 2019).
Clinically, the combination of DDP with other drugs is administered to enhance its effectiveness in cancer treatment. Increasing studies reveal that traditional Chinese medicines (TCM) promoted chemotherapy effects via decreasing drug resistance or alleviating organic damages (Chan et al. 2020). Andrographis (Andro) is commonly used as an anti-bacteria and anti-inflammation Chinese herbal medicine, and the major active component is andrographolide (Dai et al. 2019; Soo et al. 2019). Besides that, it was discovered that the combination of Oligomeric Proanthocyanidins and Andro enhanced anticancer capacity via suppressing cell growth and inducing cell apoptosis in colorectal cancer(Shimura et al. 2021). Andro could accelerate ferroptosis, apoptosis and decrease cell proliferation in gastric cancer cells (Ma et al. 2021). Andro water extract inhibited CD81 expression in esophageal cancer cells and mouse models dose dependently, and it could inhibit metastasis in vivo (Yue et al. 2022). Andro reduced autophagy in lung cancer cells through inhibiting STAT3 phosphorylation, and it decreased tumor growth in xenograft mice (Wang et al. 2022). Andro has been discovered to decrease 5-fluorouracil resistance in colorectal cancer cells via downregulating Dickkopf-1 (Zhao et al. 2021). Moreover, previous studies reported that Andro promoted the effects of DDP on inducing apoptosis of ovarian cancer cells (Yunos et al. 2013). Andro enhanced colorectal cancer cell apoptosis to sensitize the cytotoxicity of DDP (Lin et al. 2014). Previous research revealed that in A549 cells, Andro inhibited the epithelial-mesenchymal transition via activation of SIRT1/FOXO3 pathway, while in Mycobacterium tuberculosis-infected macrophages, Andro suppressed the pyroptosis via downregulating miR-155-5p and activating Nrf2 pathway (Fu et al. 2022; Li et al. 2020). Nevertheless, studies about the effect and related molecular mechanisms of Andro in regulating DDP resistance in lung cancer are rare. In this study, we predicted the bindings between miR-155-5p and SIRT1 in homo sapiens using Targetscan online database. Herein, we aimed to investigate effects of Andro on modulating efficacy of DDP in lung cancer, and underlying regulatory axis, miR-155-5p/SIRT1, was examined as well.
Methods
Cell culture
A549 and cisplatin (DDP)-resistant A549 (A549/DDP) cells, H460, H1299, and BEAS-2B cells were bought from Procell (Hubei, China). Cells were grown in DMEM with 10% FBS, 100 mg/mL streptomycin, and 100 U/mL penicillin (Gibco, USA) at 37°C, 5% CO2 in a humidified incubator. Cisplatin and Andrographis (Sigma-Aldrich, USA) were dissolved in dimethyl sulfoxide and diluted to 10, 20,30, 50, 100 μ M respectively before use. SRT1720 and EX527 were purchased from Selleck (China) and were adjusted to 100 nmol/L before use.
Cell transfection
The miR-155-5p mimics/inhibitor and their controls miR-NC mimics/inhibitor were all synthesized by GenePharma (China). The transfection reagent, Lipofectamine 3000, was purchased from Invitrogen. A549/DDP cells were used for the transfection of miR-155-5p mimics/inhibitor and miR-NC mimics/inhibitor respectively. The transfection efficiency was detected 48h later after transfection. On the other hand, to further secure the animal experiments, the lentiviral vectors from GenePharma were used for stable upregulation of miR-155-5p in A549/DDP cells.
RT-qPCR
Total RNA was extracted from cells using Beyozol reagent (Beyotime, China). High-Capacity cDNA Reverse Transcription and TaqMan Advanced miRNA cDNA synthesis kits were used in synthesizing cDNA (Applied Biosystems, USA). Thereafter, RT-qPCR was performed using the PowerUp™ SYBR™ Green Master Mix (Applied Biosystems) and CFX Opus 96 Real-Time PCR System (Bio-Rad, USA). Sequences of primers were listed as follows, which were: miR-155-5p, 5′-GGGTGTCGTATCCAGTGCAA-3′ (Forward) and 5′-GTCGTATCCAGTGCGTGTCG-3′ (Reverse) (Yang et al. 2020); SIRT1, 5′-TAGCCTTGTCAGATAAGGAAGGA-3′ (Forward) and 5′-ACAGCTTCACAGTCAACTTTGT-3′ (Reverse) (Lu et al. 2021); U6, 5′-GAGAAAGTTAGCACGGCTTCTG-3′ (Forward) and 5′-CAAAATATGGAATGCTTCAAAGAG-3′ (Reverse) (Yang et al. 2020) and GAPDH, 5′-TGACCTCAACTACATGGTCTACA-3′ (Forward) and 5′-CTTCCCATTCTCGGCCTTG-3′ (Reverse) (Zhou et al. 2018). U6 and GAPDH were internal controls for quantifying miR-155-5p or SIRT1 mRNA levels. Relative RNA expression levels were analyzed by the 2−ΔΔCt method.
CCK-8
As cells were treated by DDP, Andro (0, 10, 20, 30, 50, and 100 μ M) and their combination for 48 h, cell viabilities were examined. Cells were seeded in 96-well plates with 5000 cells per well. And 10 μ L of CCK-8 (Beyotime) was added and incubated with cells for 1 h. Then, absorbance values were examined using VANTAstar microplate reader (BMG LABTECH, Germany) at 450 nm.
Flow cytometry
After transfection and treatment, cells were washed and resuspended using PBS. Afterwards, cells (50,000 cells per well) were resuspended in Annexin V-FITC binding buffer (Beyotime). Subsequently, cells were collected and stained using 5 μ L Annexin V-FITC and 10 μ L propidium iodide (PI) for 15 min in dark room. Then, cells were detected for apoptosis rates using BD Accuri C6 (BD Bioscieces, USA).
Luciferase reporter gene test
After the binding sites of miR-155-5p with SIRT1 were predicted on TargetScan online tool (https://www.targetscan.org/vert_72/), the bindings were validated in A549/DDP cells. Wild or mutated type of SIRT1 was inserted into pmirGLO vector and was named as SIRT1-wt/mt (Promega, USA). Thereafter, SIRT1-wt/mut was transfected into A549/DDP cells with NC mimics or miR-155-5p mimics. The fluorescence was detected using Dual-Glo Luciferase Assay System kit (Promega).
RNA-binding protein immunoprecipitation (RIP)
Magna RIP kit was purchased (Millipore, MA, USA). RIPA lysis was used for cell lysis, which were then incubated in RIP buffer and magnetic beads conjugated with mouse IgG (as a negative control) or human anti-Ago2 antibody (Abcam, Shanghai, China). The RNA after purification was then examined using RT-qPCR.
Western blot
Total proteins were extracted from cells and tumor tissues using RIPA lysis buffer (Beyotime). Protein was quantified using BCA assay kit (Beyotime). After that, protein was separated and transferred to PVDF membranes using 10% SDS-PAGE (Bioss, Beijing, China). After blocked by 5% non-fat milk, primary antibodies after dilution were incubated with PVDF membranes at 4°C overnight. Antibodies used were anti-SIRT1 (1:500, ab110304) and anti-GAPDH (1:500, ab9848). Later, membranes were cultivated with goat anti-rabbit IgG (HRP) antibody (1:1000, ab7090). BeyoECL Plus (Beyotime) was applied for developing, and gray bands of proteins were analyzed by Image J (NIH, USA).
Animal experiments
The animal experiments were approved by the Animal Ethical and Welfare Committee of Tianjin Medical University Cancer Institute and Hospital (Approved no.: LWFB-AE-2021029). Female BALB/c nude mice (6 weeks old, n=12) from Vital River Company (Beijing, China) were used for in vivo experiments. Mice were raised regularly in lab conditions (22±2°C, 55±10% humidity, and 12h to 12h light–dark cycle) and fed with sterilized food and water. At first, A549/DDP cells with stable overexpression of miR-155-5p were injected into the right flank of mice, while control cells were injected into the left flank of mice. Tumor volumes were examined every 4 days using calipers. The formula for calculating volume was volume (mm3) = 4/3×π×radius (mm3). After the tumor volumes in mice were larger than 100 mm3, mice were injected intraperitoneally with Andro (25 mg/kg) every other 2 days for 3 weeks and DDP (2.5 mg/kg/week) for 3 weeks. Mice were anesthetized using 4% isoflurane inhalation and humanely sacrificed on day 28.
Statistical methods
Student’s t test was used for difference evaluation in two groups, while the Brown-Forsythe and Welch analysis of variance (ANOVA) method was used for multiple comparisons. The two-way ANOVA was applied in cell viability detections. Results were considered statistically meaningful when P<0.05.
Results
Andrographis reduced the cisplatin resistance in lung cancer cells
First, the effect of Ando on the cell viability of lung cancer cells and normal lung epithelial cells BEAS-2B was examined using CCK8 method, which showed that Andro does not reduce the BEAS-2B cell viability but inhibits the cell viability of lung cancer cells, especially A549 cells (Fig. 1A). Thereafter, the cisplatin-resistant cell line, A549/DDP, was introduced and results showed that Andro could reduce the cell viability of A549/DDP (Fig. 1B). A549/DDP cells showed more significant resistance to DDP than A549 cells (Fig. 1C). To investigate impacts of Andro on modulating DDP resistance in lung cancer cells, parental A549 and A549/DDP cells were treated with 30 μ M DDP and combined groups (30 μ M DDP and 30 μ M Andro) for 48 h. Results showed that the combined group showed the lowest viability compared to Andro and Cisplatin treatment groups in both cell lines (Fig. 1D–E). Further, apoptosis assays revealed that the combined treatment enhanced the cell apoptosis in both A549 and A549/DDP cell lines compared to the DDP treatment alone (Fig. 2). These results showed that Andro could reduce the cisplatin resistance in lung cancer cells.
Andrographis increased the cisplatin sensitivity in lung cancer in vitro. A, B: Cells (BEAS-2B, H460, H1299, A549, and A549/DDP) were treated with 10, 20, 30, 50, 100 μ M Andro for 48 h, and then CCK8 method was used to measure the cell viability at 450 nm. C. A549 and A549/DDP cells were treated with 10, 20, 30, 50, 100 μ M DDP for 48 h, and then CCK8 assays were performed to compare the changes in viability. D, E: A549 and A549/DDP cells were treated with 30 μ M DDP and combined groups (30 μ M DDP and 30 μ M Andro) for 48 h, and CCK8 assays were then performed.****P < 0.0001
Andrographis decreased the cisplatin resistance by downregulating miR-155-5p in A549/DDP cells
To explore whether miR-155-5p was modulated by Andro in lung cancer cells, we have examined miR-155-5p expression in A549/DDP cells after Andro treatment (0, 10, 20, and 30 μ M for 48 h). RT-qPCR results revealed that miR-155-5p expressions were downregulated dose-dependently after Andro treatment (Fig. 3A). Afterwards, miR-155-5p expressions were detected in A549/DDP cells after treated by DDP (30 μ M) or the combination of 30 μ M DDP and 30 μ M Andro for 48 h. Results revealed that DDP treatment decreased miR-155-5p expression, but the combined group showed lower miR-155-5p expression level in A549/DDP cells (Fig. 3B). Thereafter, miR-155-5p expression levels were regulated by transfection method in A549/DDP cells, and RT-qPCR results validated that miR-155-5p inhibitor suppressed miR-155-5p expression while miR-155-5p mimics elevated miR-155-5p expression in A549/DDP cells (Fig. 3C). Furthermore, the cells with miR-155-5p modulation were treated using 30 μ M DDP, together with or without 30 μ M Andro treatment for 48 h. CCK8 results showed that upregulation of miR-155-5p could promote the cell viability and partly reverse the inhibitory effect of Andro on A549/DDP cells, while the inhibition of miR-155-5p could add to the suppressive function of Andro on cell viability in A549/DDP (Fig. 3E). Correspondingly, apoptosis results showed that Andro further synergized with DDP, which enhanced the cell apoptosis rates, but miR-155-5p mimics could reverse this, and the downregulation of miR-155-5p could add to the effect of Andro treatment in A549/DDP cells (Fig. 3F).
Andrographis inhibited miR-155-5p regulation in A549/DDP cells. A: RT-qPCR method was used to detect the miR-155-5p expression in A549/DDP cells after Andro treatment (0, 10, 20, 30 μ M) for 48 h. B: A549/DDP cells were treated with 30 μ M DDP or 30 μ M DDP and 30 μ M Andro together for 48 h, and then the miR-155-5p expression was detected. C, D: A549/DDP cells were transfected with miR-155-5p inhibitor/mimics and their controls. RT-qPCR was used for miR-155-5p expression. E–F: Cells after transfection were exposed to 30 μ M DDP treatment with or without 30 μ M Andro treatment for 48 h. Then, CCK8 and flow cytometry methods were applied. * P < 0.0332, ** P < 0.0021, *** P < 0.0002, **** P < 0.0001
miR-155-5p could target and regulate SIRT1 in A549/DDP cells
To further investigate potential regulatory route of miR-155-5p in A549/DDP cells, we predicted the potential target of miR-155-5p, SIRT1 on TargetScan (https://www.targetscan.org/vert_72/). The binding sites were shown in Fig. 4A. Then, the dual luciferase reporter assays were performed, and results showed that the relative luciferase activity was lower in the cell group transfected with miR-155-5p mimics with SIRT1-wt (Fig. 4B). RIP assays results showed that miR-155-5p and SIRT were enriched in Ago2-containing beads compared to the IgG control (Fig. 4C). These findings suggest that SIRT1 was a target of miR-155-5p. Furthermore, we found that SIRT1 mRNA and protein expressions were decreased after miR-155-5p upregulation and were enhanced by knockdown of miR-155-5p in A549/DDP cells (Fig. 4C–F).
miR-155-5p could target and regulate SIRT1 in A549/DDP cells. A: Predicted bindings between miR-155-5p and SIRT1 in homo sapiens according to Targetscan online tool. B: Luciferase reporter assays. C: RIP assays. D–G: The A549/DDP cells were transfected with miR-155-5p mimics/inhibitor and their controls. RT-qPCR was used to measure relative mRNA expression for SIRT1, while western blot assays were for the protein levels. * P < 0.0332, ** P < 0.0021, *** P < 0.0002, **** P < 0.0001
Andrographis modulated DDP resistance in lung cancer cells via miR-155-5p/SIRT1 axis
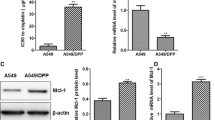
Andro treatment could further inhibit the cell viability, synergizing with DDP, and miR-155-5p upregulation could reverse the inhibitory effect of Andro, while the activation of SIRT1 by the agonist SRT1720 partly suppressed the cell viability in A459/DDP cells; on the other hand, miR-155-5p downregulation could further decrease the cell viability, adding to the coeffect of Andro and DDP, while the inhibition of SIRT1 by EX527 could reverse the inhibitory effect of DDP, Andro, and miR-155-5p inhibitor (Fig. 5A). Similarly, the apoptosis assays revealed the opposite effect of SRT1720 to miR-155-5p upregulation as well as the inhibitory effect of EX527 on cell apoptosis, which was against the DDP, Andro, and miR-155-5p inhibitor in A549/DDP cells (Fig. 5B–C). SIRT1 mRNA and protein expression levels were increased by Andro treatment; SRT1720 further activated SIRT1, but EX527 inhibited SIRT1 in A549/DDP cells (Fig. 6A–C).
Andrographis modulated DDP resistance in lung cancer cells via miR-155-5p/SIRT1. A: A549 cells after transfection were treated using 30 μ M DDP, 30 μ M Andro for 48 h and with addition of SRT1720 or EX527. Then, CCK8 method was used to detect the cell viability. B–C: Apoptosis assays. && vs DDP + Andro group. * P < 0.0332, ** P < 0.0021, *** P < 0.0002, **** P < 0.0001. & P < 0.0332, && P < 0.0021, &&& P < 0.0002
Andrographis reduced DDP resistance in mice with lung cancer via miR-155-5p/SIRT1
A549/DDP cells with stable transfection of miR-155-5p mimics were injected into mice subcutaneously, and after the tumor volumes were bigger than 100 mm3, DDP (2.5 mg/kg/week) was administered with or without Andro (25 mg/kg). The results from the animal experiments showed that tumor volumes and weight were reduced in the Andro group, indicating that Andro synergized with DDP to inhibit the tumor growth; miR-155-5p upregulation could partly reverse the inhibitory effect of Andro treatment (Fig. 7A–C). In addition, the SIRT1 protein expression was inhibited by miR-155-5p upregulation but was enhanced after Andro treatment in tumors (Fig. 5D–E).
Andrographis synergized with DDP in mice with lung cancer via miR-155-5p/SIRT1. A549/DDP cells with stable upregulation of miR-155-5p or without transfection were injected into 12 mice. After the tumor volumes in mice were larger than 100 mm3, mice were injected intraperitoneally with Andro (25 mg/kg) every other 2 days and DDP (2.5 mg/kg/week) for 3 weeks. A: Tumor volumes after DDP/Andro administration. B. Tumors extracted from mice. C. Tumor weight. D–E: SIRT1 protein levels in tumor tissues. * P < 0.0332, ** P < 0.0021, *** P < 0.0002, **** P < 0.0001
Discussion
Accumulating evidence has revealed that Chinese herbal medicines might be facilitative in malignancies as adjunctive treatments to traditional therapeutical approaches (Yang et al. 2012). Andro sensitized human laryngeal cancer cells to carboplatin via enhancing carboplatin-induced cell apoptosis and suppressing cell viability (Mao et al. 2019). Andro could synergize with paclitaxel as anti-cancer agents in lung cancer cells A549 (Yuan et al. 2016). Previously, the study of Zhang, J., et al. showed that Andro inhibited the proliferation and promoted apoptosis of lung cancer cells (Zhang et al. 2021a). In this study, we showed that Andro reduced resistance of lung cancer cells to DDP, enhancing apoptosis and inhibiting proliferation via mediating miR-155-5p and SIRT1 in vitro and in vivo, which is in consistent with the previous finding that Andro could add to the DDP sensitivity in lung cancer cells (Yuwen et al. 2017). In addition, this study revealed a new regulatory mechanism, miR-155-5p/SIRT1, beneath Andro in reducing DDP resistance in lung cancer.
MicroRNA (miRNA) is a class of approximately 22-nucleotide small noncoding RNA that might be involved in modulating DDP resistance in malignancies, mediating apoptosis, hypoxia, and proliferation, etc., through regulating the target genes (Wang et al. 2020; Zhang et al. 2021b). For instance, miR-140 could inhibit the DDP resistance in lung cancer through SIRT1/ROS/JNK signaling (Lin et al. 2020). MiR-155-5p has been reported to act as oncogene in many kinds of cancers. MiR-155-5p was elevated in gastric cancer cells, while resveratrol treatment suppressed miR-155-5p expression, inhibiting cell growth and enhancing cell apoptosis (Su et al. 2022). MiR-155-5p has been reported to be elevated with progression of lung cancer, and it facilitated A549 cell proliferation (Zhu et al. 2020; Yang et al. 2021). Moreover, miR-155 suppression upregulated Apaf-1 protein expressions, increasing sensitivity to DDP in lung cancer cells (Zang et al. 2012). Additionally, miR-155-5p was suppressed after Andro treatment, leading to elevated Nrf2 expressions, thereby reducing pyroptosis of macrophages (Fu et al. 2022). In our study, miR-155-5p expression was decreased after Andro treatment. Cell viability that was inhibited by DDP and Andro treatment was restored by miR-155-5p mimics. Therefore, Andro might modulate miR-155-5p to sensitize lung cancer cells to DDP.
SIRT1, a conserved NAD+-dependent deacetylase, belongs to mammalian silent information regulator 1 family (Ji et al. 2018). In the study of Guo, H., et al., SIRT1 protein expressions were elevated by Quercetin treatment, inducing A549 cell autophagy and apoptosis (Guo et al. 2021). Andro promoted SIRT1 transcription to prevent oxidative stress, inflammation, and mitochondrial dysfunction in macrophages (Zhang et al. 2020). In this study, Andro treatment enhanced SIRT1 protein expression, while miR-155-5p reversely modulated SIRT1 protein expression in A549/DDP cells. In addition, SIRT1 activation could restore the apoptosis rates reduced by miR-155-5p mimics, and SIRT1 inhibition could inhibit the apoptosis promoted by miR-155-5p inhibitor. Furthermore, we investigated the effect of Andro on mediating DDP sensitivity in vivo, showing that Andro treatment could further inhibit tumor volume and weight, but miR-155-5p upregulation could partly reverse the Andro effect. Compared with former studies, we have discovered that Andrographis could synergize with DDP in lung cancer via miR-155-5p/SIRT1 axis. This suggests that Andrographis might serve as an adjunctive approach for DDP-based chemotherapy in lung cancer.
The limitations of this research mainly include that among the popular chemotherapies, only DDP resistance was studied, and in addition, the molecular mechanism was limited to miR-155-5p/SIRT1. In the future, more cellular and animal studies regarding multi-drug resistance in lung cancer should be performed and more mechanisms related to Andrographis, such as autophagy, ferroptosis, metastasis, should be investigated.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bade BC, dela Cruz CS (2020) Lung cancer 2020: epidemiology, etiology, and prevention. Clin Chest Med 41:1–24
Chan DW, Yung MM, Chan YS, Xuan Y, Yang H, XU D, Zhan JB, Chan KK, Ng TB, Ngan HY (2020) MAP30 protein from Momordica charantia is therapeutic and has synergic activity with cisplatin against ovarian cancer in vivo by altering metabolism and inducing ferroptosis. Pharmacol Res 161:105157
Dai Y, Chen SR, Chai L, Zhao J, Wang Y, Wang Y (2019) Overview of pharmacological activities of Andrographis paniculata and its major compound andrographolide. Crit Rev Food Sci Nutr 59:S17–s29
de Groot P, Munden RF (2012) Lung cancer epidemiology, risk factors, and prevention. Radiol Clin North Am 50:863–876
El-Hussein A, Manoto SL, Ombinda-Lemboumba S, Alrowaili ZA, Mthunzi-Kufa P (2021) A review of chemotherapy and photodynamic therapy for lung cancer treatment. Anticancer Agents Med Chem 21:149–161
Fu Y, Shen J, Liu F, Zhang H, Zheng Y, Jiang X (2022) Andrographolide suppresses pyroptosis in Mycobacterium tuberculosis-infected macrophages via the microRNA-155/Nrf2 axis. Oxid Med Cell Longev 2022:1885066
Ghosh S (2019) Cisplatin: The first metal based anticancer drug. Bioorg Chem 88:102925
Griesinger F, Korol EE, Kayaniyil S, Varol N, Ebner T, Goring SM (2019) Efficacy and safety of first-line carboplatin-versus cisplatin-based chemotherapy for non-small cell lung cancer: A meta-analysis. Lung Cancer 135:196–204
Guo H, Ding H, Tang X, Liang M, Li S, Zhang J, Cao J (2021) Quercetin induces pro-apoptotic autophagy via SIRT1/AMPK signaling pathway in human lung cancer cell lines A549 and H1299 in vitro. Thorac Cancer 12:1415–1422
Hoy H, Lynch T, Beck M (2019) Surgical treatment of lung cancer. Crit Care Nurs Clin North Am 31:303–313
Ji K, Sun X, Liu Y, Du L, Wang Y, He N, Wang J, Xu C, Liu Q (2018) Regulation of apoptosis and radiation sensitization in lung cancer cells via the Sirt1/NF-κB/Smac pathway. Cell Physiol Biochem 48:304–316
Li J, Liu J, Yue W, Xu K, Cai W, Cui F, Li Z, Wang W, He J (2020) Andrographolide attenuates epithelial-mesenchymal transition induced by TGF-β1 in alveolar epithelial cells. J Cell Mol Med 24:10501–10511
Lin HH, Shi MD, Tseng HC, Chen JH (2014) Andrographolide sensitizes the cytotoxicity of human colorectal carcinoma cells toward cisplatin via enhancing apoptosis pathways in vitro and in vivo. Toxicol Sci 139:108–120
Lin Z, Pan J, Chen L, Wang X, Chen Y (2020) MiR-140 Resensitizes cisplatin-resistant NSCLC cells to cisplatin treatment through the SIRT1/ROS/JNK pathway. Onco Targets Ther 13:8149–8160
Lu Z, Liu H, Song N, Liang Y, Zhu J, Chen J, Ning Y, Hu J, Fang Y, Teng J, Zou J, Dai Y, Ding X (2021) METTL14 aggravates podocyte injury and glomerulopathy progression through N(6)-methyladenosine-dependent downregulating of Sirt1. Cell Death Dis 12:881
Ma R, Shimura T, Yin C, Okugawa Y, Kitajima T, Koike Y, Okita Y, Ohi M, Uchida K, Goel A, Yao L, Zhang X, Toiyama Y (2021) Antitumor effects of Andrographis via ferroptosis-associated genes in gastric cancer. Oncol Lett 22:523
Mao W, He P, Wang W, Wu X, Wei C (2019) Andrographolide sensitizes Hep-2 human laryngeal cancer cells to carboplatin-induced apoptosis by increasing reactive oxygen species levels. Anticancer Drugs 30:e0774
Qi L, Luo Q, Zhang Y, Jia F, Zhao Y, Wang F (2019) Advances in toxicological research of the anticancer drug cisplatin. Chem Res Toxicol 32:1469–1486
Romani AMP (2022) Cisplatin in cancer treatment. Biochem Pharmacol 206:115323
Romaszko AM, Doboszyńska A (2018) Multiple primary lung cancer: a literature review. Adv Clin Exp Med 27:725–730
Shimura T, Sharma P, Sharma GG, Banwait JK, Goel A (2021) Enhanced anti-cancer activity of andrographis with oligomeric proanthocyanidins through activation of metabolic and ferroptosis pathways in colorectal cancer. Sci Rep 11:7548
Soo HL, Quah SY, Sulaiman I, Sagineedu SR, Lim JCW, Stanslas J (2019) Advances and challenges in developing andrographolide and its analogues as cancer therapeutic agents. Drug Discov Today 24:1890–1898
Su N, Li L, Zhou E, Li H, Wu S, Cao Z (2022) Resveratrol downregulates miR-155-5p to block the malignant behavior of gastric cancer cells. Biomed Res Int 2022:6968641
Toumazis I, Bastani M, Han SS, Plevritis SK (2020) Risk-based lung cancer screening: a systematic review. Lung Cancer 147:154–186
Wang S, Li MY, Liu Y, Vlantis AC, Chan JY, Xue L, Hu BG, Yang S, Chen MX, Zhou S, Guo W, Zeng X, Qiu S, van Hasselt CA, Tong MC, Chen GG (2020) The role of microRNA in cisplatin resistance or sensitivity. Expert Opin Ther Targets 24:885–897
Wang XR, Jiang ZB, Xu C, Meng WY, Liu P, Zhang YZ, Xie C, Xu JY, Xie YJ, Liang TL, Yan HX, Fan XX, Yao XJ, Wu QB, Leung EL (2022) Andrographolide suppresses non-small-cell lung cancer progression through induction of autophagy and antitumor immune response. Pharmacol Res 179:106198
Yang G, Li X, Li X, Wang L, Li J, Song X, Chen J, Guo Y, Sun X, Wang S, Zhang Z, Zhou X, Liu J (2012) Traditional chinese medicine in cancer care: a review of case series published in the chinese literature. Evid Based Complement Alternat Med 2012:751046
Yang J, Jia Y, Wang B, Yang S, Du K, Luo Y, Li Y, Zhu B (2021) Circular RNA CHST15 sponges miR-155-5p and miR-194-5p to promote the immune escape of lung cancer cells mediated by PD-L1. Front Oncol 11:595609
Yang N, Cheng H, Mo Q, Zhou X, Xie M (2020) miR-155-5p downregulation inhibits epithelial-to-mesenchymal transition by targeting SIRT1 in human nasal epithelial cells. Mol Med Rep 22:3695–3704
Yuan H, Sun B, Gao F, Lan M (2016) Synergistic anticancer effects of andrographolide and paclitaxel against A549 NSCLC cells. Pharm Biol 54:2629–2635
Yue GG, Gomes AJ, Saeed MEM, Tsui KY, Dawood M, Drif AI, Wong EC, Lee WF, Liu W, Chiu PW, Efferth T, Lau CB (2022) Identification of active components in Andrographis paniculata targeting on CD81 in esophageal cancer in vitro and in vivo. Phytomedicine 102:154183
Yunos NM, Mutalip SS, Jauri MH, Yu JQ, Huq F (2013) Anti-proliferative and pro-apoptotic effects from sequenced combinations of andrographolide and cisplatin on ovarian cancer cell lines. Anticancer Res 33:4365–4371
Yuwen D, Mi S, Ma Y, Guo W, Xu Q, Shen Y, Shu Y (2017) Andrographolide enhances cisplatin-mediated anticancer effects in lung cancer cells through blockade of autophagy. Anticancer Drugs 28:967–976
Zang YS, Zhong YF, Fang Z, Li B, An J (2012) MiR-155 inhibits the sensitivity of lung cancer cells to cisplatin via negative regulation of Apaf-1 expression. Cancer Gene Ther 19:773–778
Zhang J, Li C, Zhang L, Heng Y, Xu T, Zhang Y, Chen X, Hoffman RM, Jia L (2021a) Andrographolide induces noxa-dependent apoptosis by transactivating ATF4 in human lung adenocarcinoma cells. Front Pharmacol 12:680589
Zhang T, Zhang P, Li HX (2021b) CAFs-derived exosomal miRNA-130a confers cisplatin resistance of NSCLC cells through PUM2-dependent packaging. Int J Nanomedicine 16:561–577
Zhang XF, Ding MJ, Cheng C, Zhang Y, Xiang SY, Lu J, Liu ZB (2020) Andrographolide attenuates oxidative stress injury in cigarette smoke extract exposed macrophages through inhibiting SIRT1/ERK signaling. Int Immunopharmacol 81:106230
Zhao Y, Wang C, Goel A (2021) Andrographis overcomes 5-fluorouracil-associated chemoresistance through inhibition of DKK1 in colorectal cancer. Carcinogenesis 42:814–825
Zhou X, Yan T, Huang C, Xu Z, Wang L, Jiang E, Wang H, Chen Y, Liu K, Shao Z, Shang Z (2018) Melanoma cell-secreted exosomal miR-155-5p induce proangiogenic switch of cancer-associated fibroblasts via SOCS1/JAK2/STAT3 signaling pathway. J Exp Clin Cancer Res 37:242
Zhu HZ, Fang CJ, Guo Y, Zhang Q, Huang LM, Qiu D, Chen GP, Pang XF, Hu JJ, Sun JG, Chen ZT (2020) Detection of miR-155-5p and imaging lung cancer for early diagnosis: in vitro and in vivo study. J Cancer Res Clin Oncol 146:1941–1951
Funding
This study was funded by the Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-010A).
Author information
Authors and Affiliations
Contributions
CP, TZ, YC, CW designed the study and analyzed the data; BY, CC conducted the experiments and acquired the data; CP, TZ, YC, ZZ drafted the writing; CP, CW made revisions; and all authors approved to publish this version.
Corresponding author
Ethics declarations
Ethical approval
Animal experiments were approved by the Ethical Committee of Tianjin Medical University Cancer Institute and Hospital (Approved no.: LWFB-AE-2021029).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chong Pang, Tengyue Zhang, and Yulong Chen contribute equally to this paper.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pang, C., Zhang, T., Chen, Y. et al. Andrographis modulates cisplatin resistance in lung cancer via miR-155-5p/SIRT1 axis. Funct Integr Genomics 23, 260 (2023). https://doi.org/10.1007/s10142-023-01186-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10142-023-01186-x