Abstract
In eukaryotes, the genome does not emerge in a specific shape but rather as a hierarchial bundle within the nucleus. This multifaceted genome organization consists of multiresolution cellular structures, such as chromosome territories, compartments, and topologically associating domains, which are frequently defined by architecture, design proteins including CTCF and cohesin, and chromatin loops. This review briefly discusses the advances in understanding the basic rules of control, chromatin folding, and functional areas in early embryogenesis. With the use of chromosome capture techniques, the latest advancements in technologies for visualizing chromatin interactions come close to revealing 3D genome formation frameworks with incredible detail throughout all genomic levels, including at single-cell resolution. The possibility of detecting variations in chromatin architecture might open up new opportunities for disease diagnosis and prevention, infertility treatments, therapeutic approaches, desired exploration, and many other application scenarios.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The DNA within the nucleus of mammalian cells is hierarchically packed to form chromatin fibers, and the genome’s 3D structure is crucial in a variety of biological processes (Hug, et al. 2018; Bortle and Corces 2012). For instance, high-order chromatin patterns are commonly related to gene regulation over a long distance and consequently control cell fate and development (Beagrie et al. 2017). Moreover, chromatin decondensation and condensation are necessary for correcting chromosomal division throughout meiosis and mitosis (Hagstrom and Meyer 2003); errors in a high-order chromatin structure might result in clinical illnesses and developmental abnormalities (Lupiáñez, et al. 2016). In animal development, the shape of the 3D genome is highly complex, particularly during notable lineage determination, gametogenesis, and early embryonic growth. Throughout mammalian species, primordial germinal cells (PGCs) undergo a number of processes at meiosis I (MI) and meiosis II (MII) to produce mature gametes (1n) (Schlecht et al. 2004). In males, spermatogonia quickly undergo two rounds of meiotic division and the M-phase before producing haploid spermatids. Throughout spermiogenesis, spermatids are driven to develop mature sperm through multilateral chromatin condensation and genome-wide transcription silencing (Schagdarsurengin and Steger 2016); meanwhile, protamine replaces the majority of histones and serves as a DNA-binding protein (Ooi and Henikoff 2007; Gatewood et al. 1990). PGCs stay at the diplotene stage of meiosis I for days to weeks in female mice but take relatively long in humans (Hilscher et al. 1974). A small proportion of oocytes leaving the diplotene phase are paused to start meiosis I after being stimulated by hormones, including follicle-stimulating hormone and luteinizing hormone, only to be halted again during the M-phase of meiosis II prior to fertilization (MacLennan et al. 2015). The two parental nuclei fuse after fertilization to form a totipotent zygote; this process implies severe epigenetic reprogramming (Xu and Xie 2018). In oocytes and sperms, DNA methylation and histone modifications are altered in distinct manners (Hales et al. 2011). Transcription is triggered at a specific moment during the conception of zygote genome activation (ZGA). The change from maternal-to-zygotic is known as a maternal-to-zygotic transition (Langley et al. 2014). ZGA develops in two-cell stages in mice and between four and eight-cell stages in humans (Lee et al. 2014a). Epigenomic architecture undergoes extensive remodeling throughout this event (Eckersley-Maslin et al. 2018). Before ZGA, topologically associating domain (TAD) structures are largely disorganized, and TADs are re-established during ZGA (Hug et al. 2017). Owing to the shortage of techniques for exploring the 3D genome, the molecular basis of the chromatin architecture that enables these crucial developmental events remains difficult to resolve (Sigal et al. 2018). In the last several years, substantial progress has been achieved in the development of cutting-edge technology (FISH) for studying chromatin structures (Levsky and Singer 2003). In combination with CRISPR-Cas genome editing techniques, microscopy has enabled the dynamic spatiotemporal imaging of genomic areas in living cells (Wu et al. 2019). Furthermore, chromosomal conformation capture, capture-C, 3C, and its derived techniques (including circularized chromosome conformation capture [4C], chromosome conformation capture carbon copy [5C], and Hi-C) are effective in identifying 3D chromatin structures at the DNA level (Hughes et al. 2014). These new technologies have greatly expanded the number of techniques available for exploring the 3D genome and improved our understanding of high-order chromatin structure.
TADs
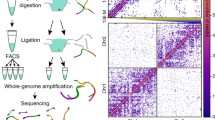
In interphase, mammalian chromatin is organized hierarchically, starting with an extremely flexible chromatin fiber that wraps DNA around a histone protein octamer complex (Chaffey et al. 2003) (H4, H2B, H2A, and H3) at the center of the nucleosome. Together with several other polypeptides, nucleosomes are arranged around a 10-nm “string of bead” fiber that determines the chromatin (Bajpai and Padinhateeri 2020). The chromatin features different layers of regulatory modifications, including DNA methylation and hydroxymethylation (Edwards et al. 2017), post-translational changes of exposing histone tails (Bannister and Kouzarides 2011), binding of chromatin remodelers (Bickmore and Steensel 2013), and chromatin-associated RNAs (Fig. 1) (Cech and Steitz 2014). Individual interphase chromosomes are usually located in distinct chromosomal regions (Cremer and Cremer 2010), as proven by microscopy-based techniques including chromosome painting and different C technologies (3C, 4C, and 5C) shown in Table 1 (Bolzer et al. 2005; Lieberman-Aiden et al. 2009; Nagano et al. 2017). TAD is a secondary chromosomal structure related to histone modifications (Dixon et al. 2012), gene expression (Dixon et al. 2015), lamina association, and DNA replication (Bolton et al. 1984; Bilodeau et al. 2009). The 3D genome is divided into layers that reflect the structural and functional basic components within a genomic organization, including TADs and basic compartments (Nora et al. 2012; Sexton et al. 2012). From a different perspective, insulated neighborhoods must be considered (Dowen et al. 2014). Mammalian genomes contain TADs that range in size from tens of kb to 1 or 2 Mb, with an average of about 800 kb. Mammalian TADs have two important aspects: relative invariance throughout differentiation and overall conservation of relative location across mice and man. A genome-wide Hi-C investigation in mouse and human cells and a 5C analysis of the X chromosome inactivation center (Xic) in differentiated cells and in mouse ESCs both provided the first evidence regarding TADs in mammals (Dekker and Heard 2015). Furthermore, CCCTC-binding factor (CTCF)-cohesin chromatin loops between CTCF convergent points are visible on mammalian Hi-C maps, which is an additional TAD characteristic (Szabo et al. 2019). The abundance of inverted CTCF regions across TAD borders was reported in zebrafish, indicating that this characteristic is conserved throughout vertebrate lineages. CTCF, on the other hand, was not detected in other organisms like Caenorhabditis elegans, yeast, or plants, as loop-anchored domains are not present in such species (Szabo et al. 2019). New research has shown the CTCF insulator protein plays a crucial role in forming the chromatin borders and loops that divide the topologically associated domain. Gene expression can be changed by genomic changes that eliminate CTCF-associated borders and promote abnormal enhancer-gene contacts (Flavahan et al. 2016a). Nevertheless, a mechanistic study is still required to fully understand the function of TADs at the single-cell level (Arzate-Mejía, et al. 2018) using Hi-C and other recent emerging novel techniques. Genomic regions within each chromosomal territory are not randomly placed and are linked through different transcriptional activations (Bickmore and Steensel 2013; Misteli 2007). Transcribable locus-rich areas are likely to be found at the boundaries of chromatin territories (Fig. 2) (Shah et al. 2018; Schoenfelder et al. 2010). Different chromosomal territories can interact with one another, especially near their borders (Branco and Pombo 2006). At the megabase level, genetic areas with comparable chromatin properties likely communicate with one another (Lieberman-Aiden et al. 2009).
TADS and transcriptionally active or inactive areas. The nucleus consists of multiple compartments, of which there are two basic compartments: the active compartment (the yellow areas within the nucleus) and the inactive compartment (the purple areas within the nucleus), which are randomly and continuously present in the nucleus (In nature, such compartmentalization is not present; however, at present, it is shown for the ease of understanding). Both compartments contain topologically associated domains (TADs), which act according to the characteristic of a certain compartment. The presence of a transcriptional activator (VP64) (denoted as a blue circle) and other transcriptional components such as nascent RNA, RNA polymerase II (pol II), and histone protein (H3K39 me3) in the active compartment make this region transcriptionally active, and the insertion of the transcriptional repressor (SV39H1) (denoted as a yellow circle) in the inactive compartment makes this region transcriptionally inactive (created with BioRender.com)
3D Genomic explanation of chromatin features. A Using different imaging-based methods, the chromosomes within the nucleus were found to inhabit distinctive territories. These technologies also describe these territories as a region with an elevated rate of intra-chromosomal interactions. B Inside the nucleus, the DNA is arranged into active (A) and inactive (B) regions. The A region (a green cluster of DNA) is basically at the center, and the B region (a purple cluster of DNA) mostly surrounds the nucleolus and nuclear lamina. Genes, RNA pol I, RNA pol II, rRNA, and mRNA are present in the nucleus and assist the splicing speckle and transcription factory to work properly around the nucleolus. The genes are expressed in the form of clusters around the nucleolus. C According to its magnified 3D genomic features, the clustered chromatin forms several folds inside TADs. These TADs overlying the early replicating region, which assists in loop extrusion, emerge due to the interaction between CTCF and the cohesin ring. D In the early replicating region, the gene interacts with its cis-regulatory components (enhancer and promoter). The enhancer attached to the RNA pol II and the gene attached to the mRNA form a transcriptionally active region. This enhancer–promoter interaction assists the transcription modulation of the chromatin loop (created with BioRender.com)
Chromatin compartmentalization
TADs serve as the foundation for the A and B compartments, which are high-level structures. In most cases, the A compartment is active and the B compartment is inactive (Stevens et al. 2017). Gene expression is linked to compartment stability. Hence, the swapping between B and A compartment levels throughout embryonic development was examined. For instance, transcriptional active areas come in contact with certain other active domains on a regular basis and have great chromosomal convenience, active histone protein reformations, and gene diversity. By contrast, inactive areas commonly contain heterochromatin and repressed genes and mostly prefer contact with neighboring inactive domains (Padeken and Heun 2014). Such chromatin compartments, named active “A compartment” and inactive “B compartment” (Fig. 1), were observed using Hi-C, a genomic sequence technology, to assess the strength of these connections among certain ensembles of particular regions within chromatin (Battulin et al. 2015). Genes shifting their position from the A compartment to the B compartment have a low expression, and those shifting their position from the B compartment to the A compartment have a high expression (Osborne et al. 2004). Many genes have stage-specific A/B compartments and are specialized for reproduction. Male sex traits are enhanced in E7.5 fetuses with gene activity in sperm that necessitates switching from the A compartment to the B compartment; an example is HOOK1, which is crucial for the production of morphologically normal sperms (Moreno et al. 2006). During embryo development, a number of genes playing a role in development change their A and B compartments. For instance, Foxd3, a pluripotency factor, is found within the B compartment in the sperm but switches to the A compartment in the fetus (Nora et al. 2012). Microscopy-based techniques verified the spatial segregation of these compartments. Chromatin spatial segmentation is frequently associated with different nuclear structures (Chen et al. 2018).
Nuclear proteins and compartments
A compartment is often located within the inner nuclear domain, whereas the B compartment is typically connected to the nucleolus (Steensel and Belmont 2017) or the nuclear membrane (lamina) that produces lamina-related domains (Bickmore and Steensel 2013). The nuclear lamina, a dense merger of intermediate filaments connected to the innermost membrane of the nucleus, is considered a basic chromatin organization activator that ties thick heterochromatin at particular sequences known as lamina-associated domains (LADs) (Fig. 3). Lamin proteins are linked to around 40% of the genome in human fibroblasts (Guelen et al. 2008). The lamin B binding site, lamin C, and lamin A proteins are required for the placement of the locus-poor regions within the B compartment toward the nucleus boundary; the lack of all three proteins induces the heterochromatin to relocalize to the nuclear interior (Solovei et al. 2013). Cell culture investigations of A lamin and B lamin using genomic sequenced DNA adenine methyltransferase identification had consistently recognized LADs as gene-poor and transcriptionally silent regions (Steensel and Belmont 2017). Changes in A/C lamin stages promote heterochromatin separation from the nuclear lamina through downregulation, upregulation, or as revealed in A/C lamin gene abnormalities associated with laminopathy (Briand and Collas 2020). These alterations within nuclear condensation are associated with variations in the activation or suppression of genes in Drosophila melanogaster (Ulianov et al. 2019) and human cells (Harr et al. 2020). Such results revealed that LADs are highly sensitive toward A/C lamin stages at the lamina of the nucleus (Cremer et al. 2015). Liquid–liquid phase separation was applied to produce chromatin compartments (Nuebler et al. 2018). In NIH3T3 cells, heterochromatin production was triggered by phase separation mediated by heterochromatin protein 1 (HP1) (Larson et al. 2017). Once the heterochromatin domain develops in early embryos in Drosophila, HP1 assembles in the nuclei as phase-separated “puncta”; however, no evidence was obtained to confirm that phase separation is responsible for the creation of A and B compartments (Strom et al. 2017). Based on recent evidence, according to low-resolution analyses, active and suppressed chromatin has been temporally separated into A and B chromatin compartments, which are divided into smaller compartmental domains. In the nucleus of the cell, liquid–liquid phase separation (LLPS) facilitates the functional and spatial isolation of various molecular processes. In addition, the crucial part that liquid–liquid phase separation plays within functional compartmentalization is specifically in the assembly of different nuclear bodies, such as the splicing cajal bodies, speckles, and nucleolus (Ulianov et al. 2021). There is growing evidence that the mechanisms underlying the establishment of compartments as well as loops and TADs are shown to be markedly different, although it is not antagonistic. Despite the loss of loops and TADs caused by cohesin and CTCF reduction, compartments are unchanged and even strengthened (Li et al. 2019a). The chromosome’s nuclear architecture is likely to be influenced by condensates on super-enhancer. Super enhancers are clusters of enhancers that work together to assemble a greater density of transcriptional machinery to promote strong gene expression (Larson et al. 2018). Super-enhancers have been employed to demonstrate the phase separation of actively transcribed regions. The uneven presence of TADs, loops, and compartments inside the maternal chromosome of mouse zygote and pachytene spermatocyte at early embryonic development suggested that the mechanism underlying the establishment of these chromatin architectures is also unique (Li et al. 2019a). Furthermore, TADs and chromatin loops have great structures known as chromosomal compartments and nuclear territories, as observed in interphase cells (Rao et al. 2014). The transcriptional activity might be correlated with the organization of chromosomal compartments and nuclear territories inside the nucleus (Lieberman-Aiden et al. 2009). Compartments are clusters of chromosomal areas with comparable transcriptional activity that cluster together in close nuclear proximity (Zhang et al. 2012). Much of the evidence suggests that phase separation may also affect the compartments of early embryos.
Model explaining compartmentalization inside the nucleus. This model is made to easily understand the regions in the nucleus that are active or inactive at different times. In this model, the nucleus has been divided into two compartments for ease of understanding the active and inactive regions in the nucleus: the A compartment (active) containing early replicating chromatins and the B compartment (inactive) containing late-replicating chromatin. The A compartment usually occupies the central region of the nucleus, and the B compartment occupies the region around the nucleolus and the nuclear periphery touching the nuclear lamina. The A compartment is characterized as the area of early replication because it contains the factors associated with early replication within the nucleus. Together with these early replication-associated factors, the histone protein (H3K27Ac) creates a distinctive region within the A compartment containing early replication control elements (ERCEs). This region becomes rich in the factors crucial for DNA replication during the S-phase. The B compartment contains the factors associated with late replication, lacks histone protein and ERCEs, and is incapable of assisting replication during the S-phase (Created with BioRender.com)
Cell differentiation
TADs are more persistent than the regions formed during intercellular differentiation (Dixon et al. 2015). Despite the structural variations of chromatin occurring inside TAD throughout cell differentiation, a significant portion of the border surrounding TAD is retained across cell types. Compared with human embryonic stem cells (ESCs), differentiating cells contain a large number of TADs and decreased/increased intra-domain linkages within the areas exhibiting dynamic epigenetic modifications (including CTCF binding) and repressive chromosomal changes (Xie et al. 2013). Throughout the differentiation of mouse ESCs to neural stem cells, the “meta-TAD tree” topologies, which reflect wide-ranging linkages among adjacent TADs, undergo about 20% of reorganization (Fraser et al. 2015). Alterations in gene expression are associated with such chromatin rearrangements. When mouse ESCs depart from naïve pluripotency, the strength of CTCF-anchored loop domains increases (Pękowska et al. 2018). A study related to the structure of chromatin inside 21 different types of human cells and tissues discovered that some areas within genomes have enormously high quantities of local chromatin connections, and these areas are often named interacting regions (Schmitt et al. 2016).
Genome architecture in MII oocytes
3D genomic configuration is extremely complex throughout animal maturation, including lineage commitment, gamete formation, and early embryogenesis. In contrast to mature sperms, mouse MII oocytes stopped at meiosis II metaphase until fertilization and lacked TADs in both compartments (Du et al. 2017). According to a Hi-C study, mouse MII oocytes have a topology of gene-independent chromatin associations resembling the cells at the metaphase level (Naumova et al. 2013; Gibcus et al. 2018). Recent work used a few cells to perform in situ Hi-C-based assays to analyze early embryos and the chromatin topology of gametes in mammals (Lieberman-Aiden et al. 2009). Specimens at two-cell, four-cell, eight-cell, and embryonic development stages (E-3.5 and E-7.5) were obtained to create a minimum of two copies of metaphase II oocytes, early embryos, mouse sperms, and PN 4-type zygotes (Fulka et al. 1998). MII oocytes have no interphase chromatin structures, implying that the topology of the mother’s genome at early oogenesis shows severe deconstruction before implantation (Ke et al. 2017). In mitosis, the interphase chromatin structure is completely deconstructed and reconstructed to form mitotic chromatin consisting of linearly compressed loop arrays (Gibcus et al. 2018). The oocyte begins meiosis throughout fetal development and is inhibited throughout the prophase I diplotene phase, also known as the germinal vesicle (GV) phase (Stetina and Orr-Weaver 2011). Investigation of chromatin (3D organization) in mouse GV oocytes using the single-nucleus Hi-C technique revealed that mouse GV comprises significantly great arrangements such as TADs, compartments, and loops. However, the frequency of TADs, compartments, and loops decreases intensely from the immature but transcriptionally active oocytes to the mature but transcriptionally inactive oocytes (Flyamer et al. 2017). Furthermore, the fully developed GV oocytes have two basic types: one having “ring-like” chromatin borders encircling the boundary of nucleolus named surrounded nucleolus (SN) oocytes and the other one which lacks such arrangements named non-surrounded nucleolus (NSN) oocytes (Zuccotti et al. 1995). The conversion of NSN oocytes into SN oocytes occurs with genomic-sequenced transcription silencing or considerable compaction within the chromatin (De La Fuente 2006). GV oocytes have typical chromatin arrangements including loops, TADs, and compartments but exhibit differences across individual cells (Flyamer et al. 2017).
Genomic architecture in spermatogenesis
The sperm genome is largely composed of protamine. Histones cover 1–15% of the DNA in mammalian cells (Akama et al. 1996). Large, compact protamine toroids are formed from sperm DNA (Hud et al. 1993). Hi-C analysis indicated that long-range chromatin is more abundant in sperm contacts than in fibroblasts and ESCs, thus presumably implying sperm chromatin condensation (Battulin et al. 2015; Du et al. 2017). During spermatogenesis, chromosome architecture undergoes significant reprogramming (Battulin et al. 2015). In the male meiotic cell cycle, a variety of chromatin-organizing processes occur, including homologous chromosomal pairing, meiotic recombination, and de-synapsis synaptonemal complex formation (Cloutier and Turner 2010). A recent study in mice and rhesus monkeys focused on 3D genome organization during spermatogenesis (Wang et al. 2019) and found that during the pachytene stage of prophase in meiosis, TADs are significantly reduced in both animals. The synaptonemal complex is formed when two homologous chromosomes bind together, giving the active transcription of pachytene chromatin (Turner 2007). These findings suggest that during some embryonic stages, transcription can function independently of TADs. Furthermore, interphase chromosomes are divided into megabase-sized TADs, through which loci tend to interact. In sperms, genomic regions > 2 Mb separate a large fraction of contacts, indicating that contacts across TADs are frequent. TAD interactions account for 30% of overall interactions in sperms (Dixon et al. 2012). Moreover, TAD is predicted to be used as the component inside the sperm genome and in the majority of interactions beyond TADs. Interactions between TADs (inter-TAD) continuously increase (Franke et al. 2016) and account for the vast majority of sperm interactions and the largest proportion of all phases. Researchers analyzed the prevalence of TAD interactions isolated by chromosomal distances. TADs are constant in sperm, E3.5, E7.5, and eight-cell embryos. In sperms between two TADs separated by around 2 Mb of sequence, the overall interaction is smaller than that in E3.5 and E7.5 embryos. The interaction ratio in sperms is higher than that in early embryos when the distance is more than 4 Mb; as the distance increases, the difference rises. Owing to the dense shape of sperm chromosomes, sperms have a high ratio of inter-chromosomal interactions and an extra-long range of intra-chromosomal interactions (Lieberman-Aiden et al. 2009).
Mechanisms of 3D genome formation in early embryonic development
Loop extrusion is hypothesized to produce TADs and loops in mammals (Sanborn et al. 2015). Two cohesin-based loop extruding aspects migrate in opposite ways and consequently produce progressive folds until they are blocked by convergent-oriented CTCF proteins (Weintraub et al. 2017). Loop extrusion describes the preferred positioning of CTCF domains, the abundance of cohesin and CTCF near TAD borders, the motifs merging after the loss of borders, and the degradation of loops and TADs in architect protein-regulated demise (Ye et al. 2016). The role of architecture proteins in 3D genome remodeling during embryonic development is being explored (Ye et al. 2016). Although chromosomes are compressed in mouse sperms, CTCF and cohesin binding are conserved. Responsive sites corresponding to the CTCF motif may also be found in GV and MII oocytes (Jung et al. 2017). This genetically inherited architecture protein binding may promote the formation of embryonic chromatin structure through loop extrusion. Exclusive loss of cohesin in the mouse zygote’s maternal allele removes poor folds and TADs, but the deletion of the cohesin-releasing factor named wings apart-like protein homolog (WAPL) results in a strong architecture (Gassler et al. 2017). A TAD domain study on open chromatin regions revealed the abundance of structural proteins GAF and BEAF-32 throughout the accessible chromatin areas in the TAD borders of Drosophila embryos, indicating their functions in developing insulators across TAD boundaries. The chromatin domains in the early stages of embryogenesis are weaker than those in later stages, and somatic cells remain a mystery. Therefore, researchers used somatic cell nuclear transfer to inject mRNA encoding the H3K9 me3 demethylase lysine-specific demethylase 4D (KDM4D), KDM4B, or the histone deacetylase inhibitor trichostatin A into mouse and monkey embryos (Matoba et al. 2014). Histone acetylation was found to increase chromatin accessibility (Görisch et al. 2005), and H3K9 me3 is crucial in the development of heterochromatin (Becker et al. 2016). The combination of these two factors may aid in the regulation of chromatin relaxation and efficient remodeling during early development. Further research is required to confirm these assumptions (Hug et al. 2017).
In briefly expressed genes, the absence of transcription results in the loss of a boundary-like structure. This type of synchronization indicates that transcription can aid in the formation of 3D genomes (Fuente and Rabindranath. 2006). By contrast, transcription inhibition prevents the development of high-order genomic architectures in mice and Drosophila (Ke et al. 2017). ZGA is inhibited in the presence of transcription inhibitors, although TADs remain firm in both species. Before ZGA, compartments and TADs may develop throughout zebrafish development without transcription, revealing that transcription is not essential to chromatin structure. Furthermore, the absence of transcription in Drosophila decreases the level of inter-TAD insulation. Such findings showed that in early embryonic development, high-order chromatin structures are formed regardless of transcription. Nevertheless, transcription may be crucial in improving and preserving chromatin stability (Kaaij et al. 2018). A study that employed nuclease-deactivated Cas9 to stimulate indigenous genes revealed that transcription is crucial but not required for local chromatin insulation inside TAD borders (Bonev et al. 2017). Researchers then explored the influence of ZGA in the development of a high chromatin structure and found that alpha-amanitin does not cause DNA replication in two-cell embryos but may delay ZGA (Bolton et al. 1984). According to heat maps, high-order genomic architectures are more prevalent in ZGA-blocked two-cell embryos than in untreated two-cell embryos. This hypothesis is supported by the larger comparative fluctuations of the TAD signal in ZGA-blocked embryos than in untreated two-cell embryos. According to these findings, ZGA is still not required for the development of high-order chromatin architecture in developing mouse embryos. These results are supported by prior Drosophila studies. By contrast, aphidicolin suppresses the formation of TAD structures by inhibiting DNA replication. In summary, such findings show that the formation of a great chromatin architecture in two-cell embryos is not dependent on ZGA but rather requires DNA replication (Harrison and Eisen 2015).
Loop extrusion
TADs were first detected in 2D chromatin interaction maps using 5C and Hi-C statistics from packed cells showing engagements along square diagonals, which are indicative of native interactions (Nora et al. 2012). The basic chromatin organizational proteins include cohesin and CTCF, both of which have been used to explain TAD borders in mammals (Rudan et al. 2015; Ganji et al. 2018). Histone alterations linked to active gene areas, including the trimethylation of the histone proteins, include H3K36 me3 and H3K4 me3 (H3 Lys4 histone protein) and the abundance of the constitutive locus (Rao et al. 2014). This loop extrusion organizes genomes into loop regions, or TADS (Alipour and Marko 2012). The multisubunit cohesin ring complex (Yatskevich et al. 2019) is predicted to bidirectionally eject DNA at a speed of 0.5–1.0 kb/s throughout interphase (Riggs 1990; Kim et al. 2019) until it is stopped by a barrier (Davidson et al. 2019). Nevertheless, the insulator CTCF protein appears under the global Hi-C method when loops and TADs are visible and is designated as the major periphery aspect in many animals, especially mammals (Hansen et al. 2018). Loop extrusion could be enhanced by cohesin and CTCF, which promotes the development of TADs (Matoba et al. 2014). In this scenario, cohesin extrudes chromatin forward until it encounters chromatin boundaries, which are frequently produced through CTCF (Fudenberg et al. 2016). This phenomenon leads to the creation of fold-like patterns that promote connection within TADs while isolating the regions around TAD boundaries (Ganji et al. 2018). Such chromatin folds arise as the “apexes” of the elevated rate of interaction in the Hi-C study. A group of CTCF-binding regions on loop anchors mainly appears inside a convergent positioning with asymmetry domains “facing” each other (Guo et al. 2015). Variations in CTCF domain positioning can destabilize chromatin TADs and folds (Görisch et al. 2005; Li et al. 2020). Such data significantly support the idea because the sets of CTCFs promote the formation of chromatin folds. Furthermore, removing the putative loop extruding factors cohesin and its loading factor NIPBL reduces or eliminates chromatin domains (Fudenberg et al. 2017; Schwarzer et al. 2017). A recent study using 4C analysis (4C-seq) found that knocking down one CTCF-associated TAD border has no effect on the local chromatin patterns of interaction (Sima et al. 2019). An important finding is that the CTCF establishes a “permeable barrier,” indicating that all but the most powerful binding sites in the CTCF are only engaged for some time (on average, 68% in HeLa and 50% in mESCs) (Holzmann et al. 2019). As a result, extruding cohesin would frequently bypass a convergent CTCF-binding site. This phenomenon explains the similar or different loops arising in cells at various times (Hansen et al. 2017) and the establishment of regions and folds in Hi-C mapping (Beagan and Phillips-Cremins 2020). Although the chromatin structure within this malfunction is yet to be analyzed (for example, using Hi-C or 5C), these processes, excluding cohesin and CTCF, possibly play a substantial role in TAD creation.
Architecture proteins
Mediator
The mediator’s development into eukaryotes corresponds with the evolution of other fundamental transcription factors, such as TFIIH and the TAF components of TFIID, which is compatible with the mediator’s role as a key transcription factor (Takagi and Kornberg 2006). Nevertheless, the monomer proportion, sequences (Bourbon 2008), and role of the mediator all change during eukaryotic evolution (Poss et al. 2013). The low sequence retention across yeast and human mediators could be partially attributed to the high number of anticipated intrinsically disordered regions inside their component sequences (Tóth-Petróczy et al. 2008) that change relatively quickly across time (Lee et al. 2014b). In eukaryotes, a mediator complex similar to a huge multi-protein complex (1 MDa) plays multiple roles in gene expression (Kornberg 2005). Through the transmission of information between enhancer-bound activator factors and RNA polymerase II (RNAP II) transcription factory, these mediators act as a major factor of transcription, a co-suppressor, or a co-regulator (Takagi and Kornberg 2006; Kornberg 2005). The mediator’s primary role is to transfer the regulating information of DNA-bound TFs toward the RNA pol II enzyme. Although the precise methods through which the mediator regulates pol II activity are unknown, they certainly include complex protein–protein interactions among the mediator, pol II, and other broad and gene-specific transcription regulatory factors. A mediator is required for the reorganization of genomic DNA into topological domains, such as gene loops, which are essential architectures that permit the coordinated control of cellular transcription (Plank and Dean 2014). A mediator is also engaged in at least some aspects of several key transcriptional processes, such as chromosomal structure, transcriptional extension, promoter–enhancer gene folding, and transcriptional initiation, thereby indicating its functional flexibility (Plank and Dean 2014; Ansari and Morse 2013). In the promoters and enhancers of strongly transcribed loci, mediators are present (Kagey et al. 2010) and help induce transcriptional processes in genes by extending RNA pol II and recruiting the pre-initiation complex (Allen and Taatjes 2015). From the perspective of 3D chromatin structure, cohesin possibly acts with promoters and enhancers to make them physically interact with each other. The RNAi knockdown of the mediator reduces the efficiency of chromatin looping (Phillips-Cremins et al. 2013) and therefore is required in some portions of the interactions. Given that mediator plays a vital role in gene transcription, differentiating its role in RNA pol II-associated gene transcription and chromatin folding is perhaps challenging and critical (Lai et al. 2013).
Cohesin
When DNA replicates, the replicated DNA molecules remain physically linked to one another. Such cohesin between sister chromatids is essential for the bi-orientation of chromosomes on meiotic and mitotic spindles, allowing for symmetric distribution throughout cell division (Dewar et al. 2004). Cohesin is a protein complex related to the protein family and the structural maintenance of chromosomes (SMC). Among all living beings from prokaryotes to eukaryotes, these SMC protein complexes arrange chromosomal DNA architecture and have been considered the most primitive, though their evolution predated the formation of histones (Li et al. 2020). A recent study concentrated on mammalian cohesin, which is composed of two basic sets of proteins, SMC3 and SMC1, which are subdivisions of hinges (Gruber et al. 2003). NIPBL, PDS5A/B, and STAG1/2 (SA1/2) are the subunits belonging to the protein family, “HEAT-repeat containing proteins associated with lesions,” which can interact with RAD21 (Ouyang and Yu 2017; Wells et al. 2017). Hereinafter, STAG1 or STAG2 will be referred to as STAG, and PDS5A or PDS5B will be referred to as PDS5; however, STAG2–cohesins and STAG1–cohesins perform slightly distinct functions (Casa et al. 2020; Kojic et al. 2018). For NIPBL and PDS5, the STAG protein is almost usually found in cohesin. NIPBL controls cohesin loading on DNA (Higashi et al. 2020) and is often needed in vivo for loop extrusion (Kim et al. 2019; Davidson et al. 2019; Zhang et al. 2019). PDS5 controls cohesin release in DNA by WAPL (Fig. 4) (Gandhi et al. 2006; Ouyang et al. 2016). Furthermore, cohesin interacts with CTCF (Rubio et al. 2008) and mediators in the framework of chromatin structure and becomes a component of the loop extrusion complex in interphase cells. Considering the assumed role in chromatin looping (Hadjur et al. 2009), two different studies investigated the global chromatin architecture in cohesin-deficient postmitotic cells and yielded slightly disconcerting findings. TADs continue to remain mostly intact, inter-domain interactions are enhanced, and intra-domain cohesin- or CTCF-anchored loops are disrupted (Seitan et al. 2013). Although cohesin may topologically surround the DNA inside the ring it has formed, the loop extrusion seems to exhibit pseudo- or non-topological interactions within the DNA (Davidson et al. 2019; Higashi et al. 2020).
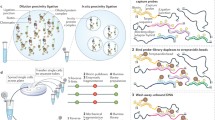
Extrusion of a chromatin loop. The presence/absence and increase/decrease of various factors affect the size of the extrusion of the chromatin loop. A The cohesin ring loading factor (NIPBL) assists in cohesin loading onto the chromatin to form a loop. CTCF (11-ZF CCCTC-binding component) recruits toward the cohesin ring. Cohesin bypasses the convergent CTCF-binding site to allow for strong compartmentalization and a perfectly shaped chromatin loop. B The absence of a cohesin unloading factor, WAPL protein, increases the residence time of the cohesin ring and the size of the chromatin loop and creates weak compartmentalization. C The lack of a cohesin ring decreases the loop size. D NIPBL unavailability hinders the loading of the cohesin ring and consequently reduces the size of the chromatin loop (created with BioRender.com)
CTCF-binding protein
CTCF is a DNA-binding protein with 11 zinc fingers (ZFs) that is found in most mammals but not in plants, C. elegans, or yeast (Harr et al. 2020; Ong and Corces 2014). Within the genomes of mammals, this CTCF protein is able to attach to 40,000–90,000 genes with their 30–60% cell type specificity based mostly on antibody use or bioinformatic threshold; among which, nearly half are present on the introns, exons, or promoters, and the other half within inter-genic domains between the genes [143,-145]. CTCF is a ZF-binding protein that has already been connected with a variety of features for gene regulation, including protecting gene promoters against distal enhancers (Wendt and Peters 2009). CTCF co-localizes with more than 90% of almost every cohesin ChIP-Seq-peaks, indicating that this protein regulates the placement of cohesin on the chromatin loop but not its insertion inside the chromatin loop (Rubio et al. 2008; Ishihara et al. 2006; Parelho et al. 2008); however, CTCF reduction only influences chromatin location but has no effect on the quantity of cohesin insertion in the chromatin loop (Wendt et al. 2008). Early research suggested that blocking an enhancer at the H19 and IGF2 genes is crucial for imprinting an expression of the gene (Bell and Felsenfeld 2000) or by generating chromatin loops that are allele-specific, CTCF can also control this role (Kurukuti et al. 2006). Cohesin also plays a vital role in enhancer-blocking action by CTCF at the H19 and IGF2 genes and the chicken b-globin locus (Chung et al. 1993), including the regulation of other genes (Dorsett and Merkenschlager 2013). These parameters have been observed in G1-phase cells or postmitotic cells, both of which contain cohesin, implying that cohesin’s role in the cohesin between sister chromatids does not produce a secondary effect on them but rather specifies cohesin’s independent role (Pauli et al. 2008).
Convergent rule
Even when reaching a convergent CTCF site, cohesin might initially contact the N-terminus of CTCF. This notion was originally proposed in a molecular investigation as the convergent rule. Given that cohesin easily extrudes loops, a near and tight deterministic contact between the cohesin ring and the N-terminus of CTCF is necessary to confirm the above hypothesis (Wutz et al. 2017). Recent research suggested and validated a multistep method that includes halting and stabilization to describe the convergent rule (Nora et al. 2019). Although cohesin stabilization is presumably necessary for the creation of CTCF validation loops, which appear as “corner apexes” on the maps of Hi-C technology, stabilization and halting both assist in insulating TAD. Interfering with stabilization might have a greater impact on the “loops” in the Hi-C maps than in the TADs (Li et al. 2020).
CTCF-mediated halting of cohesin extrusion
Despite the overall variation between the sizes of cohesin “50 nm” and CTCF “3–5 nm,” how CTCF pauses and stops cohesin to extrude in an adjacent manner remains unclear. Meanwhile, CTCF is an uncommon DNA-binding protein that binds DNA for minutes (Agarwal et al. 2017; Kieffer-Kwon et al. 2017) rather than seconds (Teves et al. 2016) and may have the unique capacity to arrest cohesin (Davidson et al. 2016). CTCF has the exclusive capability to envelope its DNA-binding domains in 20 chromatin structures (nucleosomes) (Owens et al. 2019), suggesting that this binding in CTCF establishes a distinct micro-environment inside chromatin that might function similarly to a steric barrier to halt signal. When binding to DNA, CTCF consistently generates a distinctive structure (MacPherson and Sadowski 2010). Investigations on the CTCF hierarchy and CTCFL are useful in this context: CTCFL and CTCF have essentially similar 11ZF domain and motif preferences. CTCFL may also restore CTCF-mediated genome organization (Nishana et al. 2020). CTCF protein undergoes a number of post-translational changes, such as SUMOylation (MacPherson et al. 2009) and poly-ribosylation; some of these changes are of extreme importance (Zhang et al. 2013a). According to preliminary findings, poly-ADP-ribosylation aids in CTCF insulation (Farrar et al. 2010). As determined by ChIP-Seq, the alteration of N-termini-based 11 vital amino acids, which are poly-ADP-ribosylated, along with therapy with PARP inhibitors significantly reduced CTCF’s capacity to stabilize cohesin at its signaling pathways (Pugacheva et al. 2020). These findings support the idea that poly-ADP-ribosylation plays a minor role in CTCF’s capability of pausing the extrusion of cohesin. The clustering of CTCF, poly-ADP-ribosylation, and chromatin structure (positioning of the nucleosome) are not absolutely exclusive but could be beneficial. Further work is highly required for confirmation.
CTCF-mediated stabilization of cohesin
A short area in CTCF from C-terminus to the 11-ZF motif was initially thought to be essential and sufficient to bind to the STAG2 component of the cohesin complex and was assumed to be the only straight interaction of CTCF with the cohesin complex (Xiao et al. 2011). Nevertheless, later research discovered that RBRi-CTCF, whose deletion includes this area, co-immunoprecipitates cohesion with wild-type CTCF (Yin et al. 2017), indicating that such region is not necessary for biochemical cohesin–CTCF interaction but still contributes to the creation of the loop (Wit et al. 2015). Despite numerous investigational works, new studies came to the surprising consensus that CTCF-N-terminus and two initial ZFs play important roles within cohesin–CTCF interaction; nevertheless, none of these regions are independent (Li et al. 2019b). The most basic concept involves CTCF and cohesin interacting directly with one another, as confirmed by reports on CTCF–cohesin co-immunoprecipitation (Justice et al. 2020). This finding also recently solved why the short CTCF protein (222–231 N-terminus based amino acids) crystal shape connects with STAG2-RAD21 interaction. STAG2 and RAD21 contract with the N-terminus of CTCF with an estimate of 0.6 m. In vivo, a robust protein–protein (cohesin–CTCF) contact might occur (Li et al. 2020). However, CTCF may directly or indirectly affect cohesin ATPase activity through ESCO1/PDS5, the controlling proteins in cohesin. CTCF protein’s capability of turning off the “extrusion” might “freeze” cohesin protein at the position; studies on stabilizing a CTCF–cohesin loop support this hypothesis (Wutz et al. 2020). WAPL interacts with PDS5 and releases cohesin from chromatin. WAPL loss prolongs cohesin’s retention time on chromatin (Kueng et al. 2006), allowing cohesin to reorganize into structures known as vermicelli that are assumed to run the whole length of interphase chromosomal regions and condensation of DNA. Throughout the chromatin interphase, the vermicelli comprise cohesin complexes that are positioned at the bottoms of the loop. Although this structure becomes recognizable only after WAPL loss, increased holding time and cohesin quantity might result in the formation or stabilization of more chromatin loops than usual (Fig. 3b) (Tedeschi et al. 2013). NIPBL is continuously required during cohesin extrusion in vivo (Harrison and Eisen 2015) and sub-stoichiometry with cohesin (Rhodes et al. 2017). Nevertheless, PDS5 or the N-terminal of CTCF could swap NIPBL or inhibit the ATPase action of cohesin throughout the pause of cohesin extrusion mediated by CTCF; this phenomenon automatically describes the cohesin–CTCF-mediated stability and convergent rule (Skibbens 2019). Cohesin’s heterodimer version, which interacts with two NIPBLs that can be simultaneously disassembled through PDS5/CTCF, is also necessary for verifying this hypothesis to predict reversible extrusion because cohesin may be halted individually on the left and right (Zhang et al. 2008). In vivo single-molecule investigations also showed that WAPLPDS5 impedes cohesin translocation on DNA, thus providing evidence for PDS5’s capacity to prevent extrusion (Kanke et al. 2016). Cohesin–STAG1 shows greater acetylation in SMC3 and more stable residency duration in DNA than cohesin–STAG2, which shows a reduction in acetylation and vigorously occupies DNA. CTCF might stabilize cohesin by recruiting ESCO1 via CTCF-PDS5, which further settles down and then acetylates the cohesin in CTCF regions of convergence. WAPL and CTCF simultaneous depletion results in certain cohesin resident times compared with their individual WAPL depletion, which is compatible with ESCO1 and CTCF shielding cohesin protein against WAPL protein. Meanwhile, PDS5-CTCF regulates ESCO1, which is recruited to acetylate cohesin and thus stabilizes cohesin (Wutz et al. 2020). Although recent research has greatly improved our understanding of how cohesin and CTCF can produce stable loops and maintain complexes in direct and indirect ways and has identified the critical regions and required proteins, the molecular process remains unknown. Further investigations are required to understand such a mechanism.
YY1
In multiple cell types among humans and mice, yin and yang 1 (YY1) linkage is firmly boosted by enhancers and promoters, with the exception of CTCF which binds primarily to insulator elements (Beagan et al. 2017). Investigations on YY1 ChIA-PET or HiChIP recognized that YY1 has other functions aside from being an applicant in assisting structural connections between enhancers and promoters, most probably through YY1 homodimerization. The elimination of binding region within YY1 (mediated by CRISPR) only at promoters (Etv4 and Raf1) resulted in diminished contacts with respective enhancers in mouse ESCs, which were restored to nearly average levels with Etv4 by intentionally attaching YY1 to the modified promoter similar to a recombinant protein using nuclease-dead-Cas9 (dCas9). Temporary YY1 protein removal drastically decreases the relationships among enhancers and promoters occupied by YY1; around 60% of genes within the YY1 promoter–enhancer interaction show substantial modifications in their expression (Weintraub et al. 2017). Approximately 40% of the genes in each of these categories are upregulated as a result of YY1 deficiency. Epigenetic changes throughout this grouping might be the result of indirect impacts that decrease the transcriptional suppressor mediated by YY1. Another possibility, which is not entirely incompatible, would be that YY1-mediated enhancer–promoter interactions are implicated during transcriptional repression at specific gene loci. YY1 is a polycomb suppressive complex of auxiliary proteins (Bracken and Helin 2009) that coordinate 3D genome organization and transcription repression in mESCs (Schoenfelder et al. 2015; Denholtz et al. 2013). YY1’s pleiotropic properties could be revealed by studying it as a protein complex that exhibits different activities to create suppressive and active genomic configurations. Additional research is needed to validate this concept and completely understand YY1’s “yin–yang” activities in gene regulation only at the mechanistic level.
Interactions of regulatory components
Compared with TADs, chromatin architecture may be rearranged more widely. The cell type-specific contacts taking place across cis-regulatory elements and genes as enhancers are an example of this rearrangement (Smallwood and Ren 2013). Protein- or region-centric chromatin contact observations, which include chromatin contact deep analysis through ChIA–PET (Fullwood et al. 2009), Hi-C (Mifsud et al. 2015), Hichip (Mumbach et al. 2016), and PLAC-seq (Table 1) (which stands for proximity ligation-assisted chromatin immunoprecipitation and sequencing) (Fang et al. 2016), are innovative assays that probe at the entire genome to achieve better resolution over Hi-C. By studying 17 human hematopoietic cell types (Javierre et al. 2016), research utilizing promoter capture Hi-C obtained high-resolution human aspect regarding promoter–enhancers.
cis-Regulatory elements (enhancer and promoter)
In eukaryotic genomes, coding and regulatory data are essentially detached. The mammalian genome consists of only 2% protein-coding sequences, with the remaining percentage comprising a variety of cis-regulatory DNA components. Such regulating components act in conjunction with trans-acting particles that hold the former and determine where or when genome protein-encoding data are generated to directly drive cell decisions throughout differentiation and growth. Enhancer–promoter (cis-regulatory) function, which is a component of DNA, comprises enhancers, promoters, insulators, and silencers; researchers focused on the function of promoters and enhancers throughout transcription regulation (Plank and Dean 2014). Transcriptional promoters attract RNA polymerase II (RNAPII), transcription factors directly control and precisely initiate transcription, and transcriptional enhancers stimulate the rates at which their target genes are activated (Furlong and Levine 2018). Enhancers are localized genomic sequences that are present in a wide range of cells and are involved in cofactor binding and histone changes. Putative enhancers can be found in mouse and human genomes (ENCODE Project Consortium 2012; Carter et al. 2002). Various studies were conducted to determine which promoter and enhancer contacts occur simultaneously with gene expression; whether these connections are the source or the result of gene activation remains unclear. Recent findings indicate that regulatory cues to drive transcription are transmitted by physical interaction across enhancers and promoters (Schagdarsurengin and Steger 2016). According to the findings from Blobel laboratory, a mediated interaction between the mouse-globin (Hbb) promoter and its gene control domain enhancer results in the strong transcriptional activation of the Hbb gene, even in the absence of the key transcriptional activator GATA1, implying that enhancer–promoter contacts may improve transcription (Deng et al. 2012). Forced chromatin looping eventually enables highly precise 3D genome reprogramming with therapeutic strategies, such as directing enhancers aside from disease illness alterations or redirecting regions to genes that might ameliorate manifestations, including globin in sickle cell anemia and thalassemia (Deng et al. 2014).
Promoter–enhancer loop interactions
The interaction between promoters and enhancers has been broadly studied. Enhancers activate promoters through a process known as looping (Visel and Rubin 2009). Such connecting loops occur as frequent local interactions, as opposed to CTCF regulating long-range chromatin loops (Phillips and Corces 2009). In this context, promoter–enhancer loops are referred to as “interaction loops,” indicating the enhanced interaction between such components. Meanwhile, CTCF-mediated loops can promote enhancer-promoter contacts by bringing together promoters and enhancers or by separating active and inactive chromatin regions (Hou et al. 2008). Hence, TADs account for a large proportion of enhancer-promoter interactions between regulatory components (Ji et al. 2016). Apart from the interaction loop, chromatin architectural “stripes” have recently been discovered and are linked to the discovery that one anchor area connects whole domains at a high frequency (Fudenberg et al. 2016, 2017). Although CTCF promotes the creation of insulating domains, which diminish enhancer activity, it does not mediate promoter–enhancer interactions on its own. Furthermore, transcription and mediators factors such as YY1 are thought to carry out such activities (Lai et al. 2013). Cohesin has various functions in different promoter–enhancer pairings. According to ChIP, NIPBL and cohesin preferentially bind to the chromatin inside cis-regulatory regions, where essential transcription factors are also represented (Kagey et al. 2010; Wendt et al. 2008).
Promoter–enhancer loops and transcription modulation
The genome involves several promoter–enhancer interactions that are particular to developmental stages and cell types (Freire-Pritchett et al. 2017) and are frequently created simultaneously with genetic alterations during mouse ESC differentiation from neural stem cells to cerebral cortex (Bonev et al. 2017). Genes that are frequently transcribed might bind to various ensembles of enhancers for different cell kinds, implying cell type-specific interactions (Kieffer-Kwon et al. 2013; Zhang et al. 2013b). Studies on the β-globin locus provided one of the greatest illustrations of how enhancer–promoter looping might stimulate transcription. The above locus comprises various globin-like genes, a substantial upstream gene promoter component known as the locus-binding domain, and several other regulatory elements (Noordermeer and Laat 2008). β-Globin and γ-globin genes appear particularly in adults and fetuses, respectively. When triggered, such genes exhibit phase-specific interactions with the locus-binding domain in humans. In mature human hematopoietic cells, artificially attaching the suppressed γ-globin genes to the locus regulatory region significantly increases their expression (Deng et al. 2014). Comparable results were obtained in mice once Ldb1 was directed to an adult erythroblast’s suppressed embryonic β-globin gene promoter (Deng et al. 2012). These findings revealed that inducing chromatin folding might overcome the transcriptional gene activation pattern, suggesting a potential therapeutic strategy for disorders such as sickle cell disease, which is triggered through alterations in β-globin loci. Meanwhile, interactions among cis-regulatory elements will not always result in transcription.
Enhancer–promoter interactions may exist prior to gene expression and might be linked to transcription halting. Most promoter–enhancer connections are formed before gene activation throughout fly embryogenesis (Ghavi-Helm et al. 2014). Prior to activation, several enhancers that react to transmission signals come into contact with their target genes to rapidly activate transcription (Jin et al. 2013). In several loci, the standard paradigm of stable promoter–enhancer looping has been invalidated (Gu et al. 2018). Live imaging of D. melanogaster embryos revealed that enhancers regulate a dynamic explosion of transcription (Fukaya et al. 2016); such burst incidence could be decreased by inserting insulators. These findings suggested active interactions across promoters and enhancers. Another study explored how the morphogenesis gene Shh interacts with brain-specific elements during ESC differentiation to neural progenitor cells (Benabdallah et al. 2017) and found that enhancers and Shh itself exhibit a wide range of interaction; however, persistent chromatin folds are not established after Shh activation. Activated cis-regulatory components such as enhancers and promoters in the same TAD can display great mobility; this increased movement allows these components to interact and communicate (Lee et al. 2015). Such findings reveal a “collision” paradigm that enables additional active promoter–enhancer interactions inside a de-compacted region. However, because this paradigm was only tested in a few places, its validity is unknown. Additional research is needed to determine the factors influencing enhancer decisions among collision and looping.
Polycomb group protein
Gene expression regulation is crucial for a range of biological processes, such as embryonic development, tissue homeostasis, and dosage compensation (Blackledge et al. 2015; Rhodes et al. 2020). Polycomb group proteins (PcGs) are crucial mediators in a wide range of epigenetic processes in vertebrates, including stem cell plasticity, genomic imprinting, X-chromosome inactivation and regeneration, cancer formation, and cell destiny selection (Mills 2010; Schuettengruber et al. 2007). PcG elements are important in the persistence of chromatin transcriptional memory modifications during cell proliferation and in active regulatory activities (Roure and Bantignies 2009). Therefore, studies of PcG processes are critical in understanding epigenetic remodeling that occurs throughout tumor formation, tissue regeneration, and the development of induced pluripotent stem cells. PcG proteins are found in eukaryotic organisms and interact by assembling multi-protein complexes. Five PcG complexes have already been found in D. melanogaster and named PcG repressor complexes 1 and 2 (PRC1 and PRC2), polycomb-repressive deubiquitinase complex, Pho-repressive complex, and dRing-associated factors (Beisel and Paro 2011). The first to be recognized are PRC1 and PRC2. In D. melanogaster, PRC1 contains an entire group of PcG proteins, including polyhomeotic (Ph), sex comb extra (Sce, also known as dRing), posterior sex comb (Psc), and polycomb (Pc). Meanwhile, PRC2 contains another basic group of PcG proteins, including ESC, and the histone methyltransferase enhancer of Z. Polycomb-like (Pcl) proteins might be found in PRC2 (Schmitt et al. 2005). PcG complexes, particularly PRC1 and PRC2, seem to have a role in both long-range chromatin architecture and local chromatin architecture. PcG proteins also promote the compacting of distal chromatin in vivo and in vitro (Eskeland et al. 2010; Margueron et al. 2008). 5C and 4C investigations of PcG-occupied loci showed that such regions establish discrete self-interacting domains that are shorter than TADs (Kundu et al. 2017; Li et al. 2018). Moreover, these regions interact with one another in D. melanogaster and human cells (Eagen et al. 2017; Wani et al. 2016) and might participate in the suppression of establishing genes (Isono et al. 2013). In mouse ESCs, four hox gene clusters and other genes encoding early embryonic transcriptional factors appear to be at the root of these repression clusters (Schoenfelder et al. 2015). PcG proteins are essential but are not sufficient for the development of PcG network linkages. Despite changing the whole genome organization (Joshi et al. 2015) or enhancer–promoter contacts inside this PcG network (Schoenfelder et al. 2015), PcG reduction leads to the loss of these links among PcG target genes. These findings suggested that polycomb-mediated interactions may be important in the control and regulation of developmental regions (genes).
Alternation of the 3D genome in human diseases
Although appropriate chromatin folding is critical for gene regulation, a growing interest has been directed toward the association between chromatin structural changes and illnesses. Variations in the genes encoding cohesin and CTCF have already been linked to a variety of progressive abnormalities and human illnesses (Medrano-Fernández and Barco 2016). In particular, CTCF reduction might result in cardiac infarction due to abnormal chromatin structure and the misregulation of disease-causing genes in mice (Rosa-Garrido et al. 2017). Local chromatin domain alteration can potentially result in developmental problems (Kaiser and Semple 2017). In many circumstances, changed boundary elements can result in aberrant enhancer-promoter contacts, often called enhancer adoption or abduction. In humans, among the most well-reviewed incidences is limb malformation caused by chromatin structural changes in the EPHA4 locus. Limb syndromes, including polysyndactyly, F syndrome, and brachydactyly, occur as a result of inversions, duplications, or deletions within the borders of a single TAD overlapping locus (EPHA4). Researchers used CRISPR-Cas9 genome editing to create mouse simulations with chromosomal alternations similar to those described in humans having certain limb disorders (Lupiáñez et al. 2015). TAD boundary disruption is associated with autosomal dominant adult-onset demyelinating leukodystrophy, which results in abnormal interactions between the promoter of LMNB1 (encoding lumina B1) and the three enhancers (Giorgio et al. 2015). Another theory is that human sex reversal is generated by the duplication of an area upstream of the gene encoding, the RevSex region, which contains the transcription factor SOX9 (Benko et al. 2011). Beyond the TAD border of Sox9, a large structural variant including duplications such as the RevSex region was identified, resulting in the generation of additional chromatin motifs, the aberrant stimulation of the limb deformity, and the activation of neighboring genes (Franke et al. 2016). Aside from developmental problems, changes in chromatin structure can contribute to tumorigenesis (Valton and Dekker 2017). Moreover, research of 7416 cancer genomes from 26 different types of tumors identified that the deletion of a specific TAD border is linked to IRS4 dysfunction within squamous and sarcoma cancer (Gröschel et al. 2014). Throughout this work, genomic duplications were also shown to trigger the creation of a novel chromatin motif and the hyperexpression of IGF2, but only in colon tumors. Further research must explore how a modest inversion with the stimulator of the transcriptional gene GATA2 may aberrantly trigger EVI1 (also called ECOM) while providing GATA2 functional haploinsufficiency, both of which promote leukemia (Flavahan et al. 2016b). Epigenetic processes that do not disturb the basic CTCF patterns could also influence chromatin domains. For instance, acquired alterations in IDH1 or IDH2 cause numerous types of brain tumors. Hypermethylation around CTCF-binding sites is caused by mutant isocitrate dehydrogenase proteins interfering with the action of ten-eleven translocation methylcytosine dioxygenase proteins (Beliveau et al. 2012). Emerging chromosomal conformation data serve as a tremendous resource to revisit the etiology of several diseases (Lupiáñez, et al. 2016).
Future prospects
The eukaryotic genome has long been recognized as hierarchically organized inside the nucleus. Key concepts of chromatin folding and its activities have been gradually revealed over the last few decades. However, such studies have raised more concerns than they would have addressed, which is common in dynamically emerging areas. Modern Hi-C techniques need exceedingly deep sequencing to identify delicate chromatin structures. A restricted number of cells and a restricted laboratory budget further complicate the acquisition of this quality of data. Loop contacts among enhancers and promoters might be difficult to identify in several cases. The problem of low-resolution statistics is aggravated by the fact that the bulk of discovered chromatin connections may be essential, contributing to those linked with spatiotemporal gene regulation being scarce in such statistics. Moreover, developing methods to examine chromatin structure in great depth at high spatial and temporal resolution is a critical research topic. Alternative genome-wide approaches, including Capture-C, Hi-C, ChIA-PET, HiChIP, and PLAC-seq, can achieve high-resolution data with appropriate sequencing depth; however, these assays often need a huge number of cells at the moment. Advances in computational approaches are required to assist in filtering out background noise caused by innate chromatin contacts to precisely identify regulatory relationships.
Despite their considerable success, conclusions obtained with C technologies should be supplemented by orthogonal assay findings. Such different methodologies are critical for confirming C technology outcomes and for directly observing chromatin architecture within the nucleus. Nevertheless, excellent progress has been achieved in the area of FISH technologies. The use of markers produced from array-synthesized oligo libraries in DNA FISH analysis (Oligopaint FISH) permits the illumination of areas of approximately mega-bases throughout size (Chen et al. 2013). FISH, which is frequently integrated with super-resolution imaging, has been used to verify chromatin architecture including TADs or sections, which were first detected through Hi-C analysis. A recent study that used RNA FISH to probe introns from the nascent transcripts revealed that many active genes are predominantly located on the surface of chromosomal regions, and their nuclear structure also varies greatly across different cells. With the improvement of CRISPR-Cas technologies, the effective imaging of repeated components and nonrepetitive components of genomic areas in live cells is now possible (Ma et al. 2016), and many chromosomal loci can be tracked simultaneously [241].
Multiple experiments with fine resolution and broad genome covering are required to properly resolve chromatin organization. Despite the significant advances in determining the mechanics of chromatin structures, their roles remain unknown. Although cohesin-dependent TADs, CTCF, and loop motifs play critical parts in the representation, the significant instant influence of cohesin, inducible genes, and CTCF depletion on consistent transcription has never been observed. Ultimately, transcription involves the loss of robust TADs and CTCF-mediated chromatin loops at specific developmental stages. Learning how transcription is controlled at these distinct developmental phases is of great interest. The answers to these riddles might depend on the ongoing development of new technologies.
Data availability
All the relevant data and questions should be directed to the corresponding author.
References
Agarwal H, Reisser M, Wortmann C et al (2017) Direct observation of cell-cycle-dependent interactions between CTCF and chromatin. Biophys J 112:2051–2055
Akama K, Sato H, Furihata-Yamauchi M et al (1996) Interaction of nucleosome core DNA with transition proteins 1 and 3 from boar late spermatid nuclei. J Biochem 119(3):448–455
Alipour E, Marko JF (2012) Self-organization of domain structures by DNA-loop-extruding enzymes. Nucleic Acids Res 40:11202–11212
Allen BL, Taatjes DJ (2015) The mediator complex: a central integrator of transcription. Nat Rev Mol Cell Biol 16:155–166
Ansari SA, Morse RH (2013) Mechanisms of Mediator complex action in transcriptional activation. Cell Mol Life Sci 70:2743–2756
Arzate-Mejía RG, Recillas-Targa F et al (2018) Developing in 3D: the role of CTCF in cell differentiation. Development 145(6):dev137729
Bajpai G, Padinhateeri R (2020) Irregular chromatin: packing density, fiber width, and occurrence of heterogeneous clusters. Biophys J 118(1):207–218
Bannister AJ, Kouzarides T (2011) Regulation of chromatin by histone modifications. Cell Res 21(3):381–395
Battulin N, Fishman VS, Mazur AM et al (2015) Comparison of the three-dimensional organization of sperm and fibroblast genomes using the Hi-C approach. Genome Biol 16:77
Beagan JA, Phillips-Cremins JE (2020) On the existence and functionality of topologically associating domains. Nat Genet 52:8–16
Beagan JA, Duong MT, Titus KR et al (2017) YY1 and CTCF orchestrate a 3D chromatin looping switch during early neural lineage commitment. Genome Res 27:1139–1152
Beagrie RA, Scialdone A, Schueler M et al (2017) Complex multi-enhancer contacts captured by genome architecture mapping. Nature 543(7646):519–524
Becker JS, Nicetto D, Zaret KS (2016) H3K9 me3-dependent heterochromatin: barrier to cell fate changes. Trends Genet 32:29–41
Beisel C, Paro R (2011) Silencing chromatin: comparing modes and mechanisms. Nat Rev Genet 12:123–135
Beliveau BJ, Joyce EF, Apostolopoulos N et al (2012) Versatile design and synthesis platform for visualizing genomes with oligopaint FISH probes. Proc Natl Acad Sci U S A 109:21301–21306
Bell AC, Felsenfeld G (2000) Methylation of a CTCF-dependent boundary controls imprinted expression of the Igf2 gene. Nature 405:482–485
Benabdallah NS, Williamson I, Illingworth RS et al (2017) PARP mediated chromatin unfolding is coupled to long-range enhancer activation. Preprint at bioRxiv. https://www.biorxiv.orgcontent/10.1101/155325v1
Benko S, Gordon CT, Mallet D et al (2011) Disruption of a long distance regulatory region upstream of SOX9 in isolated disorders of sex development. J Med Genet 48:825–830
Bickmore WA, van Steensel B (2013) Genome architecture: domain organization of interphase chromosomes. Cell 152(6):1270–1284
Bilodeau S, Kagey MH, Frampton GM et al (2009) SetDB1 contributes to repression of genes encoding developmental regulators and maintenance of ES cell state. Genes Dev 23(21):2484–2489
Blackledge NP, Rose NR, Klose RJ (2015) Targeting Polycomb systems to regulate gene expression: modifications to a complex story. Nat Rev Mol Cell Biol 16:643–649
Bolton VN, Oades PJ, Johnson MH (1984) The relationship between cleavage, DNA replication, and gene expression in the mouse 2-cell embryo. J Embryol Exp Morphol 79:139–163
Bolzer A, Kreth G, Solovei I et al (2005) Three-dimensional maps of all chromosomes in human male fibroblast nuclei and prometaphase rosettes. PLoS Biol 3(5):e157
Bonev B, Cohen NM, Szabo Q et al (2017) Multiscale 3D genome rewiring during mouse neural development. Cell 171(3):557–572
Bourbon HM (2008) Comparative genomics supports a deep evolutionary origin for the large, four-module transcriptional mediator complex. Nucleic Acids Res 36:3993–4008
Bracken AP, Helin K (2009) Polycomb group proteins: navigators of lineage pathways led astray in cancer. Nat Rev Cancer 9:773–784
Branco MR, Pombo A (2006) Intermingling of chromosome territories in interphase suggests role in translocations and transcription-dependent associations. PLoS Biol 4(5):e138
Briand N, Collas P (2020) Lamina-associated domains: peripheral matters and internal affairs. Genome Biol 21:85
Carter D, Chakalova L, Osborne CS et al (2002) Long-range chromatin regulatory interactions in vivo. Nat Genet 32:623–626
Casa V, Gines MM, Gusmao EG et al (2020) Redundant and specific roles of cohesin STAG subunits in chromatin looping and transcriptional control. Genome Res 30(4):515–527
Cech TR, Steitz JA (2014) The noncoding RNA revolution - trashing old rules to forge new ones. Cell 157(1):77–94
Chaffey N, Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2003) Molecular biology of the cell. 4th edn. 401–401
Chen B, Gilbert LA, Cimini BA et al (2013) Dynamic imaging of genomic loci in living human cells by an optimized CRISPR/Cas system. Cell 155:1479–1491
Chen Y, Zhang Y, Wang Y, Zhang L, Brinkman EK, Adam SA, Goldman R, van Steensel B, Ma J, Belmont AS (2018) TSA-Seq mapping of nuclear genome organization. bioRxiv, p 307892
Chung JH, Whiteley M, Felsenfeld G (1993) A 5′ element of the chicken beta-globin domain serves as an insulator in human erythroid cells and protects against position effect in Drosophila. Cell 74:505–514
Cloutier JM, Turner JMA (2010) Meiotic sex chromosome inactivation. Curr Biol 20:1823–1829
Cremer T, Cremer M (2010) Chromosome territories. Cold Spring Harb Perspect Biol 2(3):a003889
Cremer M, Grasser F, Lanctôt C et al (2008) Multicolor 3D fluorescence in situ hybridization for imaging interphase chromosomes. Methods Mol Biol 463:205–239
Cremer T, Cremer M, Hübner B et al (2015) The 4D nucleome: evidence for a dynamic nuclear landscape based on co-aligned active and inactive nuclear compartments. FEBS Lett 589(20):2931–2943
Croft JA, Bridger JM, Boyle S et al (1999) Differences in the localization and morphology of chromosomes in the human nucleus. J Cell Biol 145:1119–1131
Davidson IF, Goetz D, Zaczek MP et al (2016) Rapid movement and transcriptional re-localization of human cohesin on DNA. EMBO J 35:2671–2685
Davidson IF, Bauer B, Goetz D et al (2019) DNA loop extrusion by human cohesin. Science 366(6471):1338–1345
De La Fuente R (2006) Chromatin modifications in the germinal vesicle (GV) of mammalian oocytes. Dev Biol 292(1):1–12
de Wit E, Vos ES, Holwerda SJ et al (2015) CTCF binding polarity determines chromatin looping. Mol Cell 60:676–684
Dekker J, Heard E (2015) Structural and functional diversity of topologically associating domains. FEBS Lett 589(20):2877–2884
Deng W, Lee J, Wang H et al (2012) Controlling long-range genomic interactions at a native locus by targeted tethering of a looping factor. Cell 149:1233–1244 (This study is the first to demonstrate that an induced juxtaposition of an enhancer with its target promoter can induce gene transcription)
Deng W, Rupon JW, Krivega I et al (2014) Reactivation of developmentally silenced globin genes by forced chromatin looping. Cell 158:849–860
Denholtz M, Bonora G, Chronis C et al (2013) Long-range chromatin contacts in embryonic stem cells reveal a role for pluripotency factors and Polycomb proteins in genome organization. Cell Stem Cell 13:602–616
Dewar H, Tanaka K, Nasmyth K et al (2004) Tension between two kinetochores suffices for their bi-orientation on the mitotic spindle. Nature 428:93–97
Dixon JR, Selvaraj S, Yue F, Kim et al (2012) Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 485(7398):376–380
Dixon JR, Jung I, Selvaraj S et al (2015) Chromatin architecture reorganization during stem cell differentiation. Nature 518(7539):331–336
Dorsett D, Merkenschlager M (2013) Cohesin at active genes: a unifying theme for cohesin and gene expression from model organisms to humans. Curr Opin Cell Biol 25:327–333
Dostie J, Dekker J (2007) Mapping networks of physical interactions between genomic elements using 5C technology. Nat Protoc 2:988–1002
Dowen JM, Fan ZP, Hnisz D et al (2014) Control of cell identity genes occurs in insulated neighborhoods in mammalian chromosomes. Cell 159(2):374–387
Du Z, Zheng H, Huang B et al (2017) Allelic reprogramming of 3D chromatin architecture during early mammalian development. Nature 547(7662):232–235
Eagen KP, Aiden EL, Kornberg RD (2017) Polycombmediated chromatin loops revealed by a subkilobase-resolution chromatin interaction map. Proc Natl Acad Sci U S A 114:8764–8769
Eckersley-Maslin MA, Alda-Catalinas C, Reik W (2018) Dynamics of the epigenetic landscape during the maternal-to-zygotic transition. Nat Rev Mol Cell Biol 19(7):436–450
Edwards JR, Yarychkivska O, Boulard M et al (2017) DNA methylation and DNA methyltransferases. Epigenetics Chromatin 10(1):1–10
ENCODE Project Consortium (2012) An integrated encyclopedia of DNA elements in the human genome. Nature 489:57–74
Eskeland R, Leeb M, Grimes GR et al (2010) Ring1B compacts chromatin structure and represses gene expression independent of histone ubiquitination. Mol Cell 38:452–464
Fang R, Yu M, Li G et al (2016) Mapping of long-range chromatin interactions by proximity ligation-assisted ChIP-seq. Cell Res 26:1345–1348
Farrar D, Rai S, Chernukhin I et al (2010) Mutational analysis of the poly (ADP-ribosyl)ation sites of the transcription factor CTCF provides an insight into the mechanism of its regulation by poly (ADP-ribosyl)ation. Mol Cell Biol 30:1199–1216
Flavahan WA, Drier Y, Liau BB et al (2016) Insulator dysfunction and oncogene activation in IDH mutant gliomas. Nature 529(7584):110–114
Flavahan WA, Drier Y, Liau BB et al (2016) Insulator dysfunction and oncogene activation in IDH mutant gliomas. Nature 529:110–114
Flyamer IM, Gassler J, Imakaev M et al (2017) Single-nucleus Hi-C reveals unique chromatin reorganization at oocyte-to-zygote transition. Nature 544(7648):110–114
Franke M, Ibrahim DM, Andrey G et al (2016) Formation of new chromatin domains determines pathogenicity of genomic duplications. Nature 538(7624):265–269
Fraser J, Ferrai C, Chiariello AM et al (2015) Hierarchical folding and reorganization of chromosomes are linked to transcriptional changes in cellular differentiation. Mol Syst Biol 11(12):852
Freire-Pritchett P, Schoenfelder S, Varnai C et al (2017) Global reorganisation of cis-regulatory units upon lineage commitment of human embryonic stem cells. eLife 6:e21926
Fudenberg G, Imakaev M, Lu C et al (2016) Formation of chromosomal domains by loop extrusion. Cell Rep 15(9):2038–2049
Fudenberg G, Abdennur N, Imakaev M et al (2017) Emerging evidence of chromosome folding by loop extrusion. Cold Spring Harb Symp Quant Biol 82:45–55
Fukaya T, Lim B, Levine M (2016) Enhancer control of transcriptional bursting. Cell 166(2):358–368
Fulka J Jr, First NL, Moor RM (1998) Nuclear and cytoplasmic determinants involved in the regulation of mammalian oocyte maturation. Mol Hum Reprod 4:41–49
Fullwood MJ, Wei CL, Liu ET et al (2009) Next-generation DNA sequencing of paired-end tags (PET) for transcriptome and genome analyses. Genome Res 19:521–532
Furlong EE, Levine M (2018) Developmental enhancers and chromosome topology. Science 361:1341–1345
Gandhi R, Gillespie PJ, Hirano T (2006) Human wapl is a cohesin-binding protein that promotes sister-chromatid resolution in mitotic prophase. Curr Biol 16:2406–2417
Ganji M, Shaltiel IA, Bisht S et al (2018) Real-time imaging of DNA loop extrusion by condensin. Science 360(6384):102–105
Gassler J, Brandão HB, Imakaev M et al (2017) A mechanism of cohesin-dependent loop extrusion organizes zygotic genome architecture. EMBO J 36:3600–3618
Gatewood JM, Cook GR, Balhorn R et al (1990) Isolation of four core histones from human sperm chromatin representing a minor subset of somatic histones. J Biol Chem 265(33):20662–20666
Ghavi-Helm Y, Klein FA, Pakozdi T et al (2014) Enhancer loops appear stable during development and are associated with paused polymerase. Nature 512:96–100
Gibcus JH, Samejima K, Goloborodko A et al (2018) A pathway for mitotic chromosome formation. Science 359(6376):eaao6135
Giorgio E, Robyr D, Spielmann M et al (2015) A large genomic deletion leads to enhancer adoption by the lamin B1 gene: a second path to autosomal dominant adult-onset demyelinating leukodystrophy (ADLD). Hum Mol Genet 24:3143–3154
Görisch SM, Wachsmuth M, Tóth KF et al (2005) Histone acetylation increases chromatin accessibility. J Cell Sci 118:5825–5834
Gröschel S, Sanders MA, Hoogenboezem R et al (2014) A single oncogenic enhancer rearrangement causes concomitant EVI1 and GATA2 deregulation in leukemia. Cell 157:369–381
Gruber S, Haering CH, Nasmyth K (2003) Chromosomal cohesin forms a ring. Cell 112:765–777
Gu B, Swigut T, Spencley A et al (2018) Transcription-coupled changes in nuclear mobility of mammalian cis-regulatory elements. Science 359:1050–1055
Guelen L, Pagie L, Brasset E et al (2008) Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature 453(7197):948–951
Guo Y, Xu Q, Canzio D et al (2015) CRISPR inversion of CTCF sites alters genome topology and enhancer/promoter function. Cell 162(4):900–910
Hadjur S, Williams LM, Ryan NK et al (2009) Cohesins form chromosomal cis-interactions at the developmentally regulated IFNG locus. Nature 460(7253):410–413
Hagstrom KA, Meyer BJ (2003) Condensin and cohesin: more than chromosome compactor and glue. Nat Rev Genet 4(7):520–534
Hales BF, Grenier L, Lalancette C et al (2011) Epigenetic programming: from gametes to blastocyst. Birth Defects Res A Clin Mol Teratol 91(8):652–665
Hansen AS, Pustova I, Cattoglio C et al (2017) CTCF and cohesin regulate chromatin loop stability with distinct dynamics. eLife 6:e25776
Hansen AS, Cattoglio C, Darzacq X et al (2018) Recent evidence that TADs and chromatin loops are dynamic structures. Nucleus 9(1):20–32
Harr JC, Schmid CD, Muñoz-Jiménez C et al (2020) Loss of an H3K9me anchor rescues laminopathy-linked changes in nuclear organization and muscle function in an Emery-Dreifuss muscular dystrophy model. Genes Dev 34(7–8):560–579
Harrison MM, Eisen MB (2015) Transcriptional activation of the zygotic genome in Drosophila. Curr Top Dev Biol 113:85–112
Higashi TL, Eickhoff P, Sousa JS et al (2020) A structure-based mechanism for DNA entry into the cohesin ring. Mol Cell 79(6):917–933
Hilscher B, Hilscher W, Bülthoff-Ohnolz B et al (1974) Kinetics of gametogenesis: I. Comparative histological and autoradiographic studies of oocytes and transitional prospermatogonia during oogenesis and prespermatogenesis. Cell Tissue Res 154:443–470
Holzmann J, Politi AZ, Nagasaka K et al (2019) Absolute quantification of cohesin, CTCF and their regulators in human cells. eLife 8:e46269
Hou C, Zhao H, Tanimoto K et al (2008) CTCF-dependent enhancer-blocking by alternative chromatin loop formation. Proc Natl Acad Sci U S A 105:20398–20403
Hud NV, Allen MJ, Downing KH et al (1993) Identification of the elemental packing unit of DNA in mammalian sperm cells by atomic force microscopy. Biochem Biophys Res Commun 193:1347–1354
Hug CB, Grimaldi AG, Kruse et al (2017) Chromatin architecture emerges during zygotic genome activation independent of transcription. Cell 169(2):216-228.19
Hug CB, Vaquerizas et al (2018) The birth of the 3D genome during early embryonic development. Trends Genet 34(12):903–914
Hughes JR, Roberts N, McGowan S et al (2014) Analysis of hundreds of cis-regulatory landscapes at high resolution in a single, high-throughput experiment. Nat Genet 46(2):205–212
Ishihara K, Oshimura M, Nakao M (2006) CTCF-dependent chromatin insulator is linked to epigenetic remodeling. Mol Cell 23:733–742
Isono K, Endo TA, Ku M et al (2013) SAM domain polymerization links subnuclear clustering of PRC1 to gene silencing. Dev Cell 26:565–577
Javierre BM, Burren OS, Wilder SP et al (2016) Lineage-specific genome architecture links enhancers and non-coding disease variants to target gene promoters. Cell 167:1369-1384.e19
Ji X, Dadon DB, Powell BE et al (2016) 3D chromosome regulatory landscape of human pluripotent cells. Cell Stem Cell 18:262–275
Jin F, Li Y, Dixon JR et al (2013) A high-resolution map of the three dimensional chromatin interactome in human cells. Nature 503:290–294
Joshi O, Wang SY, Kuznetsova T et al (2015) Dynamic reorganization of extremely long-range promoter-promoter interactions between two states of pluripotency. Cell Stem Cell 17:748–757
Jung YH, Sauria ME, Lyu X et al (2017) Chromatin states in mouse sperm correlate with embryonic and adult regulatory landscapes. Cell Rep 18:1366–1382
Justice M, Carico ZM, Stefan HC et al (2020) A WIZ/cohesin/CTCF complex anchors DNA loops to define gene expression and cell identity. Cell Rep 31:107503
Kaaij LJ, van der Weide RH, Ketting RF et al (2018) Systemic loss and gain of chromatin architecture throughout Zebrafifish development. Cell Rep 24:1–10
Kagey MH, Newman JJ, Bilodeau S et al (2010) Mediator and cohesin connect gene expression and chromatin architecture. Nature 467(7314):430–435
Kaiser VB, Semple CA (2017) When TADs go bad: chromatin structure and nuclear organisation in human disease. F1000Research 6
Kanke M, Tahara E, Huis in’t Veld PJ et al (2016) Cohesin acetylation and Wapl-Pds5 oppositely regulate translocation of cohesin along DNA. EMBO J. 35:2686–2698
Ke Y, Xu Y, Chen X et al (2017) 3D chromatin structures of mature gametes and structural reprogramming during mammalian embryogenesis. Cell 170(2):367–381
Kieffer-Kwon KR, Tang Z, Mathe E et al (2013) Interactome maps of mouse gene regulatory domains reveal basic principles of transcriptional regulation. Cell 155:1507–1520
Kieffer-Kwon KR, Nimura K, Rao SS et al (2017) Myc regulates chromatin decompaction and nuclear architecture during B cell activation. Mol Cell 67:566–578
Kim TH, Abdullaev ZK, Smith AD et al (2007) Analysis of the vertebrate insulator protein CTCF-binding sites in the human genome. Cell 128(6):1231–1245
Kim Y, Shi Z, Zhang H et al (2019) Human cohesin compacts DNA by loop extrusion. Science 366:1345–1349
Kojic A, Cuadrado A, De Koninck M et al (2018) Distinct roles of cohesin-SA1 and cohesin-SA2 in 3D chromosome organization. Nat Struct Mol Biol 25(6):496–504
Kornberg RD (2005) Mediator and the mechanism of transcriptional activation. Trends Biochem Sci 30:235–239
Kueng S, Hegemann B, Peters BH et al (2006) Wapl controls the dynamic association of cohesin with chromatin. Cell 127:955–967
Kundu S, Ji F, Sunwoo H et al (2017) Polycomb repressive complex 1 generates discrete compacted domains that change during differentiation. Mol Cell 65:432–446
Kurukuti S, Tiwari VK, Tavoosidana G et al (2006) CTCF binding at the H19 imprinting control region mediates maternally inherited higher-order chromatin conformation to restrict enhancer access to Igf2. Proc Natl Acad Sci U S A 103:10684–10689
Lai F, Orom UA, Cesaroni M et al (2013) Activating RNAs associate with mediator to enhance chromatin architecture and transcription. Nature 494(7438):497–501
Langley AR, Smith JC, Stemple DL et al (2014) New insights into the maternal to zygotic transition. Development 141(20):3834–3841
Larson AG, Elnatan D, Keenen MM et al (2017) Liquid droplet formation by HP1α suggests a role for phase separation in heterochromatin. Nature 547(7662):236–240
Larson AG, Elnatan D, Keenen MM et al (2018) Liquid droplet formation by HP1α suggests a role for phase separation in heterochromatin. Nature 547(7662):236–240
Lee MT, Bonneau AR, Giraldez AJ (2014) Zygotic genome activation during the maternal-to-zygotic transition. Annu Rev Cell Dev Biol 30:581–613
Lee K, Hsiung CCS, Huang P et al (2015) Dynamic enhancer-gene body contacts during transcription elongation. Genes Dev 29:1992–1997
Levsky JM, Singer RH (2003) Fluorescence in situ hybridization: past, present and future. J Cell Sci 116(14):2833–2838
Li X, Luo OJ, Wang P et al (2017) Long-read ChIA-PET for base-pair resolution mapping of haplotype-specific chromatin interactions. Nat Protoc 12:899–915
Li Y, Zheng H, Wang Q et al (2018) Genome-wide analyses reveal a role of polycomb in promoting hypomethylation of DNA methylation valleys. Genome Biol 19(1):1–16
Li F, An Z, Zhang Z (2019) The dynamic 3D genome in gametogenesis and early embryonic development. Cells 8(8):788
Li J, Huang K, Hu G et al (2019b) An alternative CTCF isoform antagonizes canonical CTCF occupancy and changes chromatin architecture to promote apoptosis. Nat Commun 10:1535
Li Y, Haarhuis JH, Sedeño Cacciatore Á et al (2020) The structural basis for cohesin–CTCF-anchored loops. Nature 578(7795):472–476
Lieberman-Aiden E, Van Berkum NL, Williams L et al (2009) Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science 326(5950):289–293
Lupiáñez DG, Kraft K, Heinrich V et al (2015) Disruptions of topological chromatin domains cause pathogenic rewiring of gene enhancer interactions. Cell 161:1012–1025
Lupiáñez DG, Spielmann M et al (2016) Breaking TADs: how alterations of chromatin domains result in disease. Trends Genet 32(4):225–237
Ma H, Tu LC, Naseri A et al (2016) Multiplexed labeling of genomic loci with dCas9 and engineered sgRNAs using CRISPRainbow. Nat Biotechnol 34:528–530
MacLennan M, Crichton JH, Playfoot CJ et al (2015) Oocyte development, meiosis and aneuploidy. Semin Cell Dev Biol 45:68–76
MacPherson MJ, Sadowski PD (2010) The CTCF insulator protein forms an unusual DNA structure. BMC Mol Biol 11(1):1–17
MacPherson MJ, Beatty LG, Zhou W et al (2009) The CTCF insulator protein is posttranslationally modified by SUMO. Mol Cell Biol 29:714–725
Margueron R, Li G, Sarma K et al (2008) Ezh1 and Ezh2 maintain repressive chromatin through different mechanisms. Mol Cell 32:503–518
Matoba S, Liu Y, Lu F et al (2014) Embryonic development following somatic cell nuclear transfer impeded by persisting histone methylation. Cell 159(4):884–895
Medrano-Fernández A, Barco A (2016) Nuclear organization and 3D chromatin architecture in cognition and neuropsychiatric disorders. Mol Brain 9(1):1–12
Mifsud B, Tavares-Cadete F, Young AN et al (2015) Mapping long-range promoter contacts in human cells with high-resolution capture Hi-C. Nat Genet 47:598–606
Mills AA (2010) Throwing the cancer switch: reciprocal roles of polycomb and trithorax proteins. Nat Rev Cancer 10:669–682
Misteli T (2007) Beyond the sequence: cellular organization of genome function. Cell 128:787–800
Moreno RD, Palomino J, Schatten G (2006) Assembly of spermatid acrosome depends on microtubule organization during mammalian spermiogenesis. Dev Biol 293:218–227
Mumbach MR, Rubin AJ, Flynn RA et al (2016) HiChIP: efficient and sensitive analysis of protein-directed genome architecture. Nat Methods 13:919–922
Nagano T, Lubling Y, Várnai C et al (2017) Cell-cycle dynamics of chromosomal organization at single-cell resolution. Nature 547(7661):61–67
Naumova N, Smith EM, Zhan Y et al (2012) Analysis of long-range chromatin interactions using chromosome conformation capture. Methods 58:192–203
Naumova N, Imakaev M, Fudenberg G et al (2013) Organization of the mitotic chromosome. Science 342(6161):948–953
Nishana M, Ha C, Rodriguez-Hernaez J et al (2020) Defining the relative and combined contribution of CTCF and CTCFL to genomic regulation. Genome Biol 21:1–34
Noordermeer D, de Laat W (2008) Joining the loops: beta-globin gene regulation. IUBMB Life 60:824–833
Nora EP, Lajoie BR, Schulz EG et al (2012) Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature 485(7398):381–385
Nora EP, Caccianini L, Fudenberg G et al (2019) Molecular basis of CTCF binding polarity in genome folding. bioRxiv 11(1):5612
Nuebler J, Fudenberg G, Imakaev M et al (2018) Chromatin organization by an interplay of loop extrusion and compartmental segregation. Biophys J 114(3):30a
Ong CT, Corces VG (2014) CTCF: an architectural protein bridging genome topology and function. Nat Rev Genet 15:234–246
Ooi SL, Henikoff S (2007) Germline histone dynamics and epigenetics. Curr Opin Cell Biol 19(3):257–265
Osborne CS, Chakalova L, Brown KE et al (2004) Active genes dynamically colocalize to shared sites of ongoing transcription. Nat Genet 36(10):1065–1071
Ouyang Z, Yu H (2017) Releasing the cohesin ring: a rigid scaffold model for opening the DNA exit gate by Pds5 and Wapl. BioEssays 39(4):1600207
Ouyang Z, Zheng G, Tomchick DR et al (2016) Structural basis and IP6 requirement for Pds5-dependent cohesin dynamics. Mol Cell 62(2):248–259
Owens N, Papadopoulou T, Festuccia N et al (2019) CTCF confers local nucleosome resiliency after DNA replication and during mitosis. eLife 8:e47898
Padeken J, Heun P (2014) Nucleolus and nuclear periphery: velcro for heterochromatin. Curr Opin Cell Biol 28:54–60
Parelho V, Hadjur S, Spivakov M et al (2008) Cohesins functionally associate with CTCF on mammalian chromosome arms. Cell 132(3):422–433
Pauli A, Althoff F, Oliveira RA et al (2008) Cell-type-specific TEV protease cleavage reveals cohesin functions in Drosophila neurons. Dev Cell 14:239–251
Pękowska A, Klaus B, Xiang W et al (2018) Gain of CTCF-anchored chromatin loops marks the exit from naive pluripotency. Cell Syst 7(5):482–495
Phillips JE, Corces VG (2009) CTCF: master weaver of the genome. Cell 137(7):1194–1211
Phillips-Cremins JE, Sauria ME, Sanyal A et al (2013) Architectural protein subclasses shape 3D organization of genomes during lineage commitment. Cell 153(6):1281–1295
Plank JL, Dean A (2014) Enhancer function: mechanistic and genome-wide insights come together. Mol Cell 55:5–14
Poss ZC, Ebmeier CC, Taatjes DJ (2013) The Mediator complex and transcription regulation. Crit Rev Biochem Mol Biol 48:575–608
Pugacheva EM, Kubo N, Loukinov D et al (2020) CTCF mediates chromatin looping via N-terminal domain-dependent cohesin retention. Proc Natl Acad Sci 117(4):2020–2031
Rao SS, Huntley MH, Durand NC et al (2014) A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell 159(7):1665–1680
Rhodes J, Mazza D, Nasmyth K et al (2017) Scc2/Nipbl hops between chromosomal cohesin rings after loading. eLife 6:e30000
Rhodes JD, Feldmann A, Hernández-Rodríguez B et al (2020) Cohesin disrupts polycomb-dependent chromosome interactions in embryonic stem cells. Cell Rep 30:820-835.e10
Riggs AD (1990) DNA methylation and late replication probably aid cell memory, and type I DNA reeling could aid chromosome folding and enhancer function. Philos Trans R Soc Lond B Biol Sci 326:285–297
Rosa-Garrido M, Chapski DJ, Schmitt AD et al (2017) High-resolution mapping of chromatin conformation in cardiac myocytes reveals structural remodeling of the epigenome in heart failure. Circulation 136:1613–1625
Roure V, Bantignies F (2009) Polycomb group-mediated gene silencing mechanisms: stability versus flexibility. Epigenomics 1:301–318
Rubio ED, Reiss DJ, Welcsh PL et al (2008) CTCF physically links cohesin to chromatin. Proc Natl Acad Sci 105(24):8309–8314
Rudan MV, Barrington C, Henderson S et al (2015) Comparative Hi-C reveals that CTCF underlies evolution of chromosomal domain architecture. Cell Rep 10(8):1297–1309
Sanborn AL, Rao SS, Huang SC et al (2015) Chromatin extrusion explains key features of loop and domain formation in wild-type and engineered genomes. Proc Natl Acad Sci 112(47):E6456–E6465
Schagdarsurengin U, Steger K (2016) Epigenetics in male reproduction: effect of paternal diet on sperm quality and offspring health. Nat Rev Urol 13(10):584–595
Schlecht U, Demougin P, Koch R et al (2004) Expression profiling of mammalian male meiosis and gametogenesis identifies novel candidate genes for roles in the regulation of fertility. Mol Biol Cell 15(3):1031–1043
Schmitt S, Prestel M, Paro R (2005) Intergenic transcription through a polycomb group response element counteracts silencing. Genes Dev 19:697–708
Schmitt AD, Hu M, Jung I et al (2016) A compendium of chromatin contact maps reveals spatially active regions in the human genome. Cell Rep 17(8):2042–2059
Schoenfelder S, Sexton T, Chakalova L et al (2010) Preferential associations between co-regulated genes reveal a transcriptional interactome in erythroid cells. Nat Genet 42(1):53–61
Schoenfelder S, Sugar R, Dimond A et al (2015) Polycomb repressive complex PRC1 spatially constrains the mouse embryonic stem cell genome. Nat Genet 47:1179–1186
Schuettengruber B, Chourrout D, Vervoort M et al (2007) Genome regulation by polycomb and trithorax proteins. Cell 128:735–745
Schwarzer W, Abdennur N, Goloborodko A et al (2017) Two independent modes of chromatin organization revealed by cohesin removal. Nature 551(7678):51–56
Seitan VC, Faure AJ, Zhan Y et al (2013) Cohesin-based chromatin interactions enable regulated gene expression within preexisting architectural compartments. Genome Res 23(12):2066–2077
Sexton T, Yaffe E, Kenigsberg E et al (2012) Three-dimensional folding and functional organization principles of the Drosophila genome. Cell 148(3):458–472
Shah S, Takei Y, Zhou W et al (2018) Dynamics and spatial genomics of the nascent transcriptome by intron seqFISH. Cell 174(2):363–376
Sigal YM, Zhou R, Zhuang X (2018) Visualizing and discovering cellular structures with super-resolution microscopy. Science 361(6405):880–887
Sima J, Chakraborty A, Dileep V et al (2019) Identifying cis elements for spatiotemporal control of mammalian DNA replication. Cell 176(4):816–830
Skibbens RV (2019) Condensins and cohesins–one of these things is not like the other! J Cell Sci 132:jcs220491
Smallwood A, Ren B (2013) Genome organization and long-range regulation of gene expression by enhancers. Curr Opin Cell Biol 25:387–394
Solovei I, Wang AS, Thanisch K et al (2013) LBR and lamin A/C sequentially tether peripheral heterochromatin and inversely regulate differentiation. Cell 152(3):584–598
Stevens TJ, Lando D, Basu S et al (2017) 3D structures of individual mammalian genomes studied by single-cell Hi-C. Nature 544(7648):59–64
Strom AR, Emelyanov AV, Mir M et al (2017) Phase separation drives heterochromatin domain formation. Nature 547(7662):241–245
Szabo Q, Bantignies F, Cavalli G (2019) Principles of genome folding into topologically associating domains. Sci Adv 5(4):eaaw1668
Takagi Y, Kornberg RD (2006) Mediator as a general transcription factor. J Biol Chem 281:80–89
Tedeschi A, Wutz G, Huet S et al (2013) Wapl is an essential regulator of chromatin structure and chromosome segregation. Nature 501:564–568
Teves, S. S., An, L., Hansen, A. S., et al. A dynamic mode of mitotic bookmarking by transcription factors. eLife 5, e22280 (2016).
Tóth-Petróczy Á, Oldfield CJ, Simon I et al (2008) Malleable machines in transcription regulation: the mediator complex. PLoS Comput Biol 4(12):e1000243
Turner JMA (2007) Meiotic Sex Chromosome Inactivation. Development 134:1823–1831
Ulianov SV, Doronin SA, Khrameeva EE et al (2019) Nuclear lamina integrity is required for proper spatial organization of chromatin in Drosophila. Nat Commun 10(1):1176
Ulianov SV, Velichko AK, Magnitov MD et al (2021) Suppression of liquid–liquid phase separation by 1,6-hexanediol partially compromises the 3D genome organization in living cells. Nucleic Acids Res 49(18):10524–10541
Valton AL, Dekker J (2017) Pan-cancer analysis of somatic copy-number alterations implicates IRS4 and IGF2 in enhancer hijacking. Nat Genet 49:65–74
Van Bortle K, Corces VG (2012) Nuclear organization and genome function. Annu Rev Cell Dev Biol 28:163–187
Van Steensel B, Belmont AS (2017) Lamina-associated domains: links with chromosome architecture, heterochromatin, and gene repression. Cell 169:780–791
Van De Werken HJ, De Vree PJ, Splinter E et al (2012) 4C technology: protocols and data analysis. Methods Enzymol 513:89–112
Van Der Lee R, Buljan M, Lang B et al (2014) Classification of intrinsically disordered regions and proteins. Chem Rev 114(13):6589–6663
Visel A, Rubin EM et al (2009) Genomic views of distant-acting enhancers. Nature 461:199–205
Von Stetina JR, Orr-Weaver TL (2011) Developmental control of oocyte maturation and egg activation in metazoan models. Cold Spring Harb Perspect Biol 3:a005553
Wang Y, Wang H, Zhang Y et al (2019) Reprogramming of meiotic chromatin architecture during spermatogenesis. Mol Cell 73(3):547–561
Wani AH, Boettiger AN, Schorderet P et al (2016) Chromatin topology is coupled to polycomb group protein subnuclear organization. Nat Commun 7:10291
Weintraub AS, Li CH, Zamudio AV et al (2017) YY1 is a structural regulator of enhancer-promoter loops. Cell 171(7):1573–1588
Wells JN, Gligoris TG, Nasmyth KA et al (2017) Evolution of condensin and cohesin complexes driven by replacement of Kite by Hawk proteins. Curr Biol 27:R17–R18
Wendt KS, Peters JM (2009) How cohesin and CTCF cooperate in regulating gene expression. Chromosome Res 17:201–214
Wendt KS, Yoshida K, Itoh T et al (2008) Cohesin mediates transcriptional insulation by CCCTC-binding factor. Nature 451(7180):796–801
Wu X, Mao S, Ying Y et al (2019) Progress and challenges for live-cell imaging of genomic loci using CRISPR-based platforms. Genomics Proteomics Bioinforma 17(2):119–128
Wutz G, Várnai C, Nagasaka K et al (2017) Topologically associating domains and chromatin loops depend on cohesin and are regulated by CTCF, WAPL, and PDS5 proteins. EMBO J 36:3573–3599
Wutz G, Ladurner R, St Hilaire BG et al (2020) ESCO1 and CTCF enable formation of long chromatin loops by protecting cohesinSTAG1 from WAPL. eLife 9:e52091
Xiao T, Wallace J, Felsenfeld G (2011) Specific sites in the C terminus of CTCF interact with the SA2 subunit of the cohesin complex and are required for cohesin-dependent insulation activity. Mol Cell Biol 31:2174–2183
Xie W, Schultz MD, Lister R et al (2013) Epigenomic analysis of multilineage differentiation of human embryonic stem cells. Cell 153(5):1134–1148
Xu Q, Xie W (2018) Epigenome in early mammalian development: inheritance, reprogramming and establishment. Trends Cell Biol 28:237–253
Yatskevich S, Rhodes J, Nasmyth K (2019) Organization of chromosomal DNA by SMC complexes. Annu Rev Genet 53:445–482
Ye BY, Shen WL, Wang D et al (2016) ZNF143 is involved in CTCF-mediated chromatin interactions by cooperation with cohesin and other partners. Mol Biol 50:431–437
Yin M, Wang J, Wang M et al (2017) Molecular mechanism of directional CTCF recognition of a diverse range of genomic sites. Cell Res 27:1365–1377
Zhang N, Kuznetsov SG, Sharan SK et al (2008) A handcuff model for the cohesin complex. J Cell Biol 183:1019–1031
Zhang Y, McCord RP, Ho YJ et al (2012) Spatial organization of the mouse genome and its role in recurrent chromosomal translocations. Cell 148(5):908–921
Zhang Y, Wang J, Ding M, Yu Y (2013) Site-specific characterization of the Asp- and Glu-ADP-ribosylated proteome. Nat Methods 10:981–984
Zhang Y, Wong CH, Birnbaum RY et al (2013) Chromatin connectivity maps reveal dynamic promoter-enhancer long-range associations. Nature 504:306–310
Zhang Y, Zhang X, Ba Z et al (2019) The fundamental role of chromatin loop extrusion in physiological V (D) J recombination. Nature 573(7775):600–604
Zuccotti M, Piccinelli A, Rossi PG et al (1995) Chromatin organization during mouse oocyte growth. Mol Reprod Dev 41:479–485
Zuin J, Dixon JR, van der Reijden MI et al (2014) Cohesin and CTCF differentially affect chromatin architecture and gene expression in human cells. Proc Natl Acad Sci 111(3):996–1001
Funding
Self-funded.
Author information
Authors and Affiliations
Contributions
F.A.K, H.C, and N.S.P conceptualize the idea. F.A.K, H.C, N.S.P, A.A, M.R.Y, and F.S collect the data and wrote the article. F.S and A.A draw the figures. E.M.P, W.N, A.M, and B.W.H.E.P reviewed, revised, and improved the manuscript. L.K and D.S.W critically revised the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pandupuspitasari, N.S., Khan, F.A., Huang, C. et al. Recent advances in chromosome capture techniques unraveling 3D genome architecture in germ cells, health, and disease. Funct Integr Genomics 23, 214 (2023). https://doi.org/10.1007/s10142-023-01146-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10142-023-01146-5