Abstract
Take-all (caused by the fungal pathogen Gaeumannomyces graminis var. tritici, Ggt) and common root rot (caused by Bipolaris sorokiniana) are devastating root diseases of wheat (Triticum aestivum L.). Development of resistant wheat cultivars has been a challenge since no resistant wheat accession is available. GmPGIP3, one member of polygalacturonase-inhibiting protein (PGIP) family in soybean (Glycine max), exhibited inhibition activity against fungal endopolygalacturonases (PGs) in vitro. In this study, the GmPGIP3 transgenic wheat plants were generated and used to assess the effectiveness of GmPGIP3 in protecting wheat from the infection of Ggt and B. sorokiniana. Four independent transgenic lines were identified by genomic PCR, Southern blot, and reverse transcription PCR (RT-PCR). The introduced GmPGIP3 was integrated into the genomes of these transgenic lines and could be expressed. The expressing GmPGIP3 protein in these transgenic wheat lines could inhibit the PGs produced by Ggt and B. sorokiniana. The disease response assessments postinoculation showed that the GmPGIP3-expressing transgenic wheat lines displayed significantly enhanced resistance to both take-all and common root rot diseases caused by the infection of Ggt and B. sorokiniana. These data suggested that GmPGIP3 is an attractive gene resource in improving resistance to both take-all and common root rot diseases in wheat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wheat (Triticum aestivum L.) is one of the most important food crops in the world. It provides ∼20 % of the calories consumed by humankind (Fu et al. 2009). Take-all disease, caused by the necrotrophic fungus Gaeumannomyces graminis var. tritici (Ggt), is one of the most destructive root diseases of wheat worldwide (Gutteridge et al. 2003; Daval et al. 2011). The symptoms of take-all include black lesions on the roots and based stems, plant stunting, premature ripening, and white heads even empty spikes, leading to severely yield losses of wheat (Gutteridge et al. 2003; Daval et al. 2011). Common root rot is primarily caused by the soil-borne fungus Bipolaris sorokiniana and impacts the wheat production in the many areas of the world (Kumar et al. 2002). Take-all and common root rot diseases occur simultaneously to the wheat plants in some fields (Shivanna et al. 1996).
In plant–pathogen interactions, plants have evolved a multilayered immunity system to counter infection of microbial pathogens. The plant cell wall represents the first line of defense for plant cells against pathogen infection. To gain access to the plant tissue, most of fungal pathogens secret a variety of enzymes that degrade the plant cell wall. Necrotrophic pathogens kill host plant cells via secreting abundant hydrolytic enzymes and feed on the dead tissues (Laluk and Mengiste 2010). Endopolygalacturonase (PG; EC 3.2.1.15) is one of secreted enzymes, which cleave the α-1,4 linkages between d-galacturonic acid residues in homogalacturonan and cause cell separation and maceration of host plant tissues (Cantu et al. 2008). The significance of PG in pathogen infection and colonization has been demonstrated for many fungal pathogens, such as B. sorokiniana (Clay et al. 1997), Botrytis cenerea (ten Have et al. 1998), Alternaria citri (Isshiki et al. 2001), Sclerotinia sclerotiorum (Zuppini et al. 2005), and Claviceps purpurea (Oeser et al. 2002), and for bacteria pathogens including Agrobacterium tumefaciens (Rodriguez-Palenzuela et al. 1991) and Ralstonia solanacearum (Huang and Allen 2000).
Many plants possess the polygalacturonase-inhibiting proteins (PGIPs) with extracellular leucine-rich repeat motif, belonging to the cell wall glycoproteins (Jones and Jones 1997; D’Ovidio et al. 2006). Some PGIPs are capable of inhibiting the pathogen PG activity and protecting the host tissues from degradation. Moreover, the interaction between plant PGIPs and pathogen PGs favors accumulation of oligogalacturonides, which elicit a wide range of defense responses (Cervone et al. 1997; Ridley et al. 2001; D’Ovidio et al. 2006). In the defense responses, plants produce various isoforms of PGIPs that display differential recognition specificity and inhibition efficiency to PGs (De Lorenzo et al. 2001). Many Pgip genes have been cloned and characterized from various plants. The effectiveness of some PGIPs in limiting pathogens infection has been verified in Arabidopsis (Ferrari et al. 2003), tomato (Powell et al. 2000), tobacco (Joubert et al. 2006), and grape (Aguero et al. 2005). The ectopic expression of bean PvPGIP2 improves resistance to B. sorokiniana and Fusarium graminearum in transgenic wheat (Janni et al. 2008; Ferrari et al. 2012). In the recent study, four soybean Pgip genes were cloned (D’Ovidio et al. 2006). Only GmPGIP3 showed broad inhibition spectrum to PGs from eight different fungi in vitro, with the highest inhibition activity against PGs from F. graminearum (D’Ovidio et al. 2006). However, it is not clear whether the expression of GmPGIP3 in plants can enhance resistance to fungal pathogens.
In this study, we generated GmPGIP3 transgenic wheat lines and further investigated if the ectopic expression of GmPGIP3 improves resistance to the infection of Ggt and B. sorokiniana in wheat. The results showed that GmPGIP3-expressing transgenic wheat lines displayed significantly enhanced resistance to the infection of the two pathogens.
Materials and methods
Plant and fungal materials
The wheat cultivar (cv.) Yangmai 18 was used as the recipient of GmPGIP3 transformation. Yangmai 18, provided by Professor Boqiao Zhang in Yangzhou Agricultural Institute, is a Chinese spring wheat variety with susceptibility to both Ggt and B. sorokiniana. Soybean cv. Zhonghuang 13, provided by Prof. Lianzheng Wang in Institute of Crop Science, CAAS, was used to clone GmPGIP3 sequence.
The fungal pathogen Ggt XNQS-2 was isolated, identified, and provided by Dr. Yang Wang, College of Plant Protection, Northwest A&F University, China. The fungal pathogen B. sorokiniana ACC30209 was purchased from the Agricultural Microbial Culture Collection, CAAS, China.
DNA and RNA extraction and the first-strand cDNA synthesis
The genomic DNA was extracted from leaves of wheat plants using the CTAB method (Saghai-Maroof et al. 1984). Total RNA was extracted from wheat roots or soybean leaves using TRIzol reagents (Invitrogen) and subjected to the RNase-free DNase I for removing the genomic DNA. Two micrograms of total RNA was used to synthesize the first-strand complementary DNA (cDNA) for each sample using M-MLV reverse transcriptase (TaKaRa, Dalian, China).
Construction GmPGIP3-expressing transformation vector
The GmPGIP3 sequence containing complete open reading frame (ORF) was cloned from cDNA of soybean Zhonghuang 13 using primers GmPGIP3-OF (5′- CATTCATTCAAGATAGATGTCAAAGTT-3′) and GmPGIP3-OR (5′-TTAAGTGCATGGT GGAAGAGGAG-3′), which were designed based on the GmPGIP3 sequence (GenBank accession no. AJ972662). The ORF was subcloned into Sma I and Sac I sites of the monocot expression vector pAHC25 (Christensen and Quail 1996), resulting in the transformation vector pA25-GmPGIP3. In the GmPGIP3 expressing cassette of pA25-GmPGIP3 vector, GmPGIP3 transcription was controlled by the maize ubiquitin promoter and the terminator of nopaline synthase (Tnos) gene (Fig. 1a).
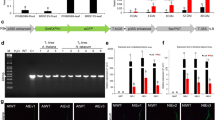
GmPGIP3 transformation vector and molecular characterization of transgenic wheat. a Schema of the transformation vector pA25-GmPGIP3. Ubi promoter, maize ubiquitin promoter; intron, the first intron from the ubiquitin promoter; and Tnos, terminator of the Agrobacterium tumefaciens nopaline synthase gene. The arrow indicates the region amplified in the PCR assays and used for the Southern blot probe. b PCR pattern of the GmPGIP3 transgenic lines in T3 generation using the primers specific to GmPGIP3-Tnos expressing cassette. Lane P, pA25-GmPGIP3 plasmid as the positive control of PCR assay; lane H 2 O, template is H2O; lane Y18, nontransformed Yangmai 18 as the negative control; and lanes G08, G09, G10, and G12, GmPGIP3 transgenic lines. c Southern blot assay. The Dra I-digested genomic DNAs from transgenic lines G08, G09, G10, G12, and nontransformed Yangmai 18 were hybridized with the fragment specific for GmPGIP3. Lane P, pA25-GmPGIP3 vector DNA; lane M, λDNA/Hind III markers. d RT-PCR analysis on transcription of GmPGIP3 in the four transgenic lines. Housekeeping gene TaActin in wheat as internal reference is used to normalize the initial DNA contents among samples. Lane Y18, nontransformed Yangmai 18; lanes G08, G09, G10, and G12, GmPGIP3 transgenic lines
Wheat transformation by biolistic bombardment
The transformation of pA25-GmPGIP3 into wheat cv. Yangmai 18 was performed using biolistic bombardment following the procedure described by Xu et al. (2001) and Chen et al. (2008). Here, a biolistic gun, PDS-1000 He (Bio-Rad, USA), was used. A total of 1200 immature embryos of Yangmai 18 were bombarded with 1-μm gold particles coated with pA25-GmPGIP3 DNA under a pressure of 1100 psi.
PCR and Southern blot analyses on GmPGIP3 transgenic wheat plants
The presence of GmPGIP3 in transgenic wheat plants in T0–T3 generations was detected by PCR analysis of leaf genomic DNA using primers GmPGIP3-TU (GGACGCTACCTCAGGGACTTAC, locating in GmPIGP3 sequence) and TnosL (ATGTATAATTGCGGGACTCTAAT, locating in the Tnos sequence of pA25-GmPGIP3). The PCR reaction was performed in a total volume of 20 μl containing 1× Taq buffer, 1.5 mM Mg2+, 0.05 mM each dNTP, 0.4 mM each primer, 1 unit Taq polymerase (TaKaRa, Dalian, China), and 50 ng template DNA. The PCR cycle was performed with an initial denaturation at 94 °C for 3 min, followed by 35 cycles of 94 °C for 45 s, 54 °C for 45 s, and 72 °C for 45 s and a final extension at 72 °C for 10 min. The amplified products were resolved on a 1.5 % agarose gel and visualized by ethidium bromide staining. The nontransformed Yangmai 18 and pA25-GmPGIP3 were separately used as negative control and positive control.
Southern blotting was performed following a modified protocols described by Sharp et al. (1989) and Zhang et al. (2012). Genomic DNAs (∼20 μg each) of four transgenic wheat lines and nontransformed Yangmai 18 were digested by the restriction enzyme Dra I and then separated on 0.8 % agarose gel, transferred to a Hybond N+ nylon membrane (GE Amersham). The amplification fragments specific to transgenic GmPGIP3 was labeled by α-32P-dCTP and then used as probe. The membrane containing the DNAs was hybridized with the probe at 65 °C for 20 h.
Transcription analysis of GmPGIP3 in transgenic wheat
RT-PCR was used to analyze GmPGIP3 transcription in transgenic wheat plants. The primers specific to GmPGIP3, GmPGIP3-QF (CCTAATCGGTCAAATCCCCT), and GmPGIP3-QR (GACAAGTCCACGAACGCCA) were used for the evaluation of transcriptional level of GmPGIP3 in transgenic wheat lines. The amplification of wheat Actin gene was used to normalize initial cDNA contents among samples using the specific primers including TaActin-F (CACTGGAATGGTCAAGGCTG) and TaActin-R (CTCCATGTCATCCCAGTTG).
Extraction of Ggt and B. sorokiniana PGs and expressing GmPGIP3 protein
The fungal mycelium culture and PG extraction of Ggt and B. sorokiniana were performed following the method described by Janni et al. (2008) for B. sorokiniana PGs. The crude protein extracts containing GmPGIP3 were extracted from leaves of GmPGIP3 transgenic plants as described by D’Ovidio et al. (2004a). Enzymatic activity of crude PGs from Ggt and B. sorokiniana was assayed following the protocol described by Taylor and Secor (1988) and Janni et al. (2008). The agarose plates containing PG reaction system were incubated at 30 °C for 18 h. Each reaction system contains 5 μl endo-PGs plus 25-μl 20-mM Na acetate solution (pH 4.7).
Assay of inhibitory activity of expressing GmPGIP3 protein
Inhibitory activity of the expressing GmPGIP3 protein extracted from the transgenic wheat plants was tested using agarose diffusion assay according to the modified method described by Taylor and Secor (1988) and Janni et al. (2008). Total volume of each reaction system was adjusted to 30 μl using 20 mM Na acetate (pH 4.7). Each reaction system contains 5 μl endo-PGs and 20 μg crude protein extract from transgenic wheat plants or nontransformed Yangmai 18. The agarose plates containing inhibitory activity reactions were incubated at 30 °C for 18 h.
Responses of transgenic wheat plants to Ggt and B. sorokiniana
At 4 weeks postinoculation with Ggt (Liu et al. 2013), take-all responses of GmPGIP3 transgenic wheat and nontransformed Yangmai 18 plants were assessed. The average take-all index (TAI) for each line was calculated following Bithell et al. (2011). To further investigate the take-all resistance to these transgenic wheat lines, quantitative reverse transcription PCR (qRT-PCR) was used to assay the relative abundance of Ggt in transgenic wheat plants based on Ggt 18S ribosomal RNA (rRNA) in reference to wheat 18S rRNA as described by Liu et al. (2013).
Following inoculation with B. sorokiniana mycelia as described by Dong et al. (2010), common root rot responses of the transgenic and nontransformed wheat lines were evaluated. The average disease index of common root rot for each line was scored at plant maturity according to Dong et al. (2010). The disease assessments were performed in T1–T3 transgenic plants. In each disease assessment, at least 20 plants per line were tested.
Results
Generation and molecular characterization of GmPGIP3 transgenic wheat
To assess the effectiveness of GmPGIP3 in improving wheat resistance to the infection of Ggt and B. sorokiniana, transgenic wheat plants expressing GmPGIP3 were generated by bombarding pA25-GmPGIP3 into the immature embryos of wheat cv. Yangmai 18. The presence of GmPGIP3 in transgenic wheat plants was detected by the desired fragment (357 bp) of genomic PCR with GmPGIP3 transgene-specific primers. Genomic PCR assays showed that the expected band of GmPGIP3 transgene was present in four transgenic wheat lines (G08, G09, G10, and G12) from T0 to T3 generations, but not in nontransformed Yangmai 18 (Fig. 1b). The results indicated that the introduced GmPGIP3 was inheritable in these four transgenic lines and that the transformation efficiency was 0.33 %. Using the probe derived from the GmPGIP3 transgene-specific amplified fragment, Southern blot assay showed that the GmPGIP3 expressing cassette was integrated into the genomes of the four transgenic lines with one to two copies (Fig. 1c). The hybridization patterns in the four transgenic lines were different, indicating that these transgenic lines were derived from independent transformation events.
Expression and inhibitory activity of GmPGIP3 in transgenic wheat plants
RT-PCR assays were used to analyze the transcription of the introduced GmPGIP3 in these four transgenic wheat lines (G08, G09, G10, and G12). The results showed that the transcription of GmPGIP3 in these transgenic lines was detected with higher levels compared with nontransformed Yangmai 18 (Fig. 1d).
The inhibitory activity of transgenically expressing GmPGIP3 protein was detected by a semiquantitative agarose diffusion assay using the endo-PG activity produced in liquid culture from Ggt and B. sorokiniana. The results showed that endo-PGs from Ggt or B. sorokiniana had the PG enzymatic activity (Fig. 2a, b). The crude protein extracts from these four transgenic wheat lines (G08, G09, G10, and G12) significantly inhibited the activity of endo-PGs from Ggt and B. sorokiniana, as indicated by the lack of the halo. In contrast, crude protein extract from nontransformed Yangmai 18 did not have any effect on the activity of endo-PGs from Ggt and B. sorokiniana. The assays of the boiled extracts did not exhibit any activity, excluding the possibility of a nonproteinaceous inhibitor (Fig. 2a, b). These results revealed that the transformed GmPGIP3 proteins were expressed in these four transgenic wheat lines and possessed inhibitory activity against endo-PGs from Ggt and B. sorokiniana.
Agarose diffusion assay of crude protein extracts from leaves of GmPGIP3 transgenic wheat lines and nontransformed Yangmai 18. All incubation mixtures (except for 8) contain 5 μl endo-PGs produced by Gaeumannomyces graminis var. tritici, Ggt) (a) and Bipolaris sorokiniana (b) in liquid culture. 1, crude endo-PGs; 2–6, crude endo-PGs plus crude protein extract (20 μg) from transgenic wheat lines G08, G09, G10, G12, and nontransformed Yangmai 18, respectively; 7, crude endo-PGs plus boiled crude protein extract from the transgenic wheat line G08; 8, pectolyase Y-23 (0.5 μg); and 9, boiled crude endo-PGs
Expression of GmPGIP3 improved resistance to both take-all and common root rot in transgenic wheat
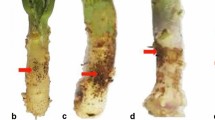
To investigate if expression of GmPGIP3 enhances resistance to both soil-borne diseases in transgenic wheat, the responses to take-all and common root rot were assessed in the four transgenic wheat lines and nontransformed Yangmai 18 following inoculation with the fungal pathogens Ggt and B. sorokiniana. The results showed that the GmPGIP3-expressing transgenic lines exhibited a significant reduction of take-all symptoms compared with nontransformed Yangmai 18 (Fig. 3a). The average TAIs of these four transgenic wheat lines were ranged from 11.44 to 35.56 depending on the different lines, whereas that of Yangmai 18 was 66.67 (Fig. 3b). Additionally, Ggt biomass was significantly decreased in these transgenic lines compared with that in nontransformed Yangmai 18, indicating that the expression of GmPGIP3 markedly improved wheat resistance to take-all caused by Ggt infection (Fig. 3c). Moreover, the assessment on common root rot responses showed that these four GmPGIP3-expressing transgenic lines exhibited a significant reduction of the disease symptoms compared with nontransformed Yangmai 18 (Fig. 4a). The average disease index in transgenic lines were ranged from 18.5 to 26.6, whereas that in nontransformed Yangmai 18 was 45.5, suggesting that the ectopic expression of GmPGIP3 in transgenic wheat enhanced resistance to common root rot caused by B. sorokiniana infection (Fig. 4b).
Take-all responses of GmPGIP3 transgenic and nontransformed wheat lines. a The typical take-all phenotypes on the roots of nontransformed Yangmai 18 (Y18) and four transgenic lines (G08, G09, G10, and G12). b Average disease index of take-all in nontransformed Yangmai 18 and these transgenic lines (asterisks indicate P < 0.05). c Ggt biomass in the roots of nontransformed Yangmai 18 and these transgenic lines were analyzed using quantitative RT-PCR. The relative transcript levels of Ggt 18S rRNA in transgenic lines were relative to those in nontransformed Yangmai 18. Three biological replicates of each line were averaged and statistically treated using t test (double asterisks indicate P < 0.01). Bars indicate the standard error of the mean
Common root rot responses of GmPGIP3 transgenic wheat and nontransformed Yangmai 18 lines. a The typical common root rot phenotypes on the roots and stem base of nontransformed Yangmai 18 (Y18) and the four transgenic lines (G08, G09, G10, and G12). b Average disease index of common root rot in nontransformed Yangmai 18 and these transgenic lines (asterisks indicate P < 0.05)
Discussion
More than 30 members of PGIP family were isolated from various plant species (D’Ovidio et al. 2004b; 2006; Ferrari et al. 2003; Janni et al. 2013; Li et al. 2003; Lu et al. 2012; Wang et al. 2013). The effectiveness of some members in increasing disease resistance has been verified in Arabidopsis, potato, tobacco, grape, and wheat (Aguero et al. 2005; Ferrari et al. 2003; Janni et al. 2008; 2013; Joubert et al. 2006; Powell et al. 2000). The bean PvPGIP2 is one of most effective inhibitors so far characterized (D’Ovidio et al. 2004a; 2006; Janni et al. 2008, 2011). Recently, GmPGIP3, one of PGIP family members in soybean, was isolated and studied (D’Ovidio et al. 2006). Sequence comparisons revealed that the deduced amino acid sequence of GmPGIP3 contains the key residues that are crucial for inhibition activity in PvPGIP2. Assays on inhibiting capacity to PGs revealed that the spectrum of inhibition of GmPGIP3 against eight different fungal pathogens was similar to that of bean PvPGIP2 (D’Ovidio et al. 2006). Interestingly, inhibitory activity of GmPGIP3 to PGs from fungal Botrytis aclada was three times more than that of PvPGIP2 (D’Ovidio et al. 2006). The accumulating knowledge on GmPGIP3 promotes us to investigate if ectopic expression of GmPGIP3 in wheat can improve resistance to the important fungal pathogens Ggt and B. sorokiniana.
In this study, we generated the GmPGIP3 transgenic wheat plants by biolistic bombardment. Genomic PCR and Southern blot analyses revealed that the introduced GmPGIP3 expressing cassette was integrated into the genomes of transgenic wheat lines and could be inherited from T0 to T3 generations. RT-PCR assay indicated that, compared with nontransformed Yangmai 18, GmPGIP3 was expressed with higher levels in four transgenic wheat lines, including G08, G09, G10, and G12. The resistance test of these transgenic lines showed that the ectopic expression of GmPGIP3 in transgenic wheat significantly improved resistance to both take-all and common root rot diseases. The inhibitory activity assays to endo-PGs showed that the transgenically expressing GmPGIP3 protein from the four transgenic wheat lines was capable of inhibiting the activity of endo-PGs from Ggt and B. sorokiniana. These data suggested that the expressing GmPGIP3 protein inhibited the activity of PGs produced by the fungal pathogens Ggt and B. sorokiniana and, in turn, might suppress the further infection and colonization of the two fungal pathogens, leading to the GmPGIP3-expressing transgenic lines that displayed significantly enhanced resistance to both take-all and common root rot diseases caused by the infection of the two pathogens. The transgenic wheat lines generated in this study may provide potential wheat germplasms for enhancing resistance to both take-all and common root rot diseases.
References
Aguero CB, Uratsu SL, Greve C, Powell AT, Labavitch JM, Meredith CP, Dandekar AM (2005) Evaluation of tolerance to Pierce’s disease and Botrytis in transgenic plants of Vitis vinifera L. expressing the pear PGIP gene. Mol Plant Pathol 6:43–51
Bithell SL, Butler RC, Harrow S, McKay A, Cromey MG (2011) Susceptibility to take-all of cereal and grass species, and their effects on pathogen inoculum. Ann Appl Biol 159:252–266
Cantu D, Vicente AR, Labavitch JM, Bennett AB, Powell AL (2008) Strangers in the matrix: plant cell walls and pathogen susceptibility. Trends Plant Sci 13:610–617
Cervone F, Castoria R, Leckie F, De Lorenzo G (1997) Perception of fungal elicitors and signal transduction. In: Aducci P (ed) Signal Transduction in plants. Birkhauser Verlag, Basel
Chen L, Zhang ZY, Liang HX, Liu HX, Du LP, Xu HJ, Xin ZY (2008) Overexpression of TiERF1 enhances resistance to sharp eyespot in transgenic wheat. J Exp Bot 59:4195–4204
Christensen AH, Quail PH (1996) Ubiquitin promoter-based vectors for high-level expression of selectable and/or screenable marker genes in monocotyledonous plants. Transgenic Res 5:213–218
Clay RP, Bergmann CW, Fuller MS (1997) Isolation and characterization of an endopolygalacturonase from Cochliobolus sativus and a cytological study of fungal penetration of barley. Phytopathology 87:1148–1159
D’Ovidio R, Mattei B, Roberti S, Bellincampi D (2004a) Polygalacturonase, poly- galacturonase-inhibiting proteins and pectic oligomers in plant-pathogen interactions. Biochim Biophys Acta 1696:237–244
D’Ovidio R, Raiola A, Capodicasa C, Devoto A, Pontiggia D, Roberti S, Galletti R, Conti E, O’Sullivan D, De Lorenzo G (2004b) Characterization of the complex locus of bean encoding polygalacturonase-inhibiting proteins (PGIPs) reveals sub-functionalization for defense against fungi and insects. Plant Physiol 135:2424–2435
D’Ovidio R, Roberti S, Di Giovanni M, Capodicasa C, Melaragni M, Sella L, Tosi P, Favaron F (2006) The characterization of the soybean polygalacturonase-inhibiting proteins (pgip) gene family reveals that a single member is responsible for the activity detected in soybean tissues. Planta 224:633–645
Daval S, Lebreton L, Gazengel K, Boutin M, Guillerm-Erckelboudt AY, Sarniguet A (2011) The biocontrol bacterium Pseudomonas fluorescens Pf29Arp strain affects the pathogenesis-related gene expression of the take-all fungus Gaeumannomyces graminis var. tritici on wheat roots. Mol Plant Pathol 12:839–854
De Lorenzo G, D’Ovidio R, Cervone F (2001) The role of polygalacturonase- inhibiting proteins (PGIPs) in defense against pathogenic fungi. Annu Rev Phytopathol 39:313–335
Dong N, Liu X, Lu Y, Du LP, Xu HJ, Liu HX, Xin ZY, Zhang ZY (2010) Overexpression of TaPIEP1, a pathogen-induced ERF gene of wheat, confers host-enhanced resistance to fungal pathogen Bipolaris sorokiniana. Funct Integr Genomics 10:215–226
Ferrari S, Vairo D, Ausubel FM, Cervone F, de Lorenzo G (2003) Tandemly duplicated Arabidopsis genes that encode polygalacturonase- inhibiting proteins are regulated coordinately by different signal transduction pathways in response to fungal infection. Plant Cell 15:93–106
Ferrari S, Sella L, Janni M, De Lorenzo G, Favaron F, D’Ovidio R (2012) Transgenic expression of polygalacturonase-inhibiting proteins in Arabidopsis and wheat increases resistance to the flower pathogen Fusarium graminearum. Plant Biol 14:31–38
Fu D, Uauy C, Distelfeld A, Blechl A, Epstein L, Chen X, Sela H, Fahima T, Dubcovsky J (2009) A kinase-START gene confers temperature dependent resistance to wheat stripe rust. Science 323:1357–1360
Gutteridge RJ, Bateman GL, Todd AD (2003) Variation in the effects of take-all disease on grain yield and quality of winter cereals in field experiments. Pest Manag Sci 59:215–224
Huang Q, Allen C (2000) Polygalacturonases are required for rapid colonization and full virulence of Ralstonia solanacearum on tomato plants. Physiol Mol Plant Pathol 57:176–186
Isshiki A, Akimitsu K, Yamamoto M, Yamamoto H (2001) Endopolygalacturonase is essential for citrus black rot caused by Alternaria citri but not brown spot caused by Alternaria alternata. Mol Plant Microbe Interact 14:749–757
Janni M, Sella L, Favaron F, Blechl AE, De Lorenzo G, D’Ovidio R (2008) The expression of a bean PGIP in transgenic wheat confers increased resistance to the fungal pathogen Bipolaris sorokiniana. Mol Plant Microbe Interact 21:171–177
Janni M, Bozzini T, Moscetti I, Volpi C, D’Ovidio R (2013) Functional characterisation of wheat Pgip genes reveals their involvement in the local response to wounding. Plant Biol (Stuttg) 15:1019–1024
Jones DA, Jones JDG (1997) The roles of leucine rich repeats in plant defences. Adv Bot Res 24:90–167
Joubert DA, Slaughter AR, Kemp G, Becker VWJ, Krooshof GH, Bergmann C, Benen J, Pretorius IS, Vivier MA (2006) The grapevine polygalacturonase-inhibiting protein (VvPGIP1) reduces Botrytis cinerea susceptibility in transgenic tobacco and differentially inhibits fungal polygalacturonases. Transgenic Res 15:687–702
Kumar J, Schäfer P, Hückelhoven R, Langen G, Baltruschat H, Stein E, Nagarajan S, Kogel K (2002) Bipolaris sorokiniana, a cereal pathogen of global concern: cytological and molecular approaches towards better control. Mol Plant Pathol 3:185–195
Laluk K, Mengiste T (2010) Necrotroph attacks on plants: wanton destruction or covert extortion? Arabidopsis Book 8:e0136
Li R, Rimmer R, Yu M, Sharpe AG, Seguin-Swartz G, Lydiate D, Hegedus DD (2003) Two Brassica napus polygalacturonase inhibitory protein genes are expressed at different levels in response to biotic and abiotic stresses. Planta 217:299–308
Liu X, Yang L, Zhou X, Zhou M, Lu Y, Ma L, Ma H, Zhang Z (2013) Transgenic wheat expressing Thinopyrum intermedium MYB transcription factor TiMYB2R-1 shows enhanced resistance to the take-all disease. J Exp Bot 64:2243–2253
Lu L, Zhou F, Zhou Y, Fan X, Ye S, Wang L, Chen H, Lin Y (2012) Expression profile analysis of the polygalacturonase-inhibiting protein genes in rice and their responses to phytohormones and fungal infection. Plant Cell Rep 31:1173–1187
Oeser B, Heidrich PM, Muller U, Tudzynski P, Tenberge KB (2002) Polygalacturonase is a pathogenicity factor in the Claviceps purpurea/rye interaction. Fungal Genet Biol 36:176–186
Powell AL, Van Kan J, Ten Have A, Visser J, Greve LC, Bennett AB, Labavitch JM (2000) Transgenic expression of pear PGIP in tomato limits fungal colonization. Mol Plant Microbe Interact 13:942–950
Ridley BL, O’Neill MA, Mohnen D (2001) Pectins: structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 57:929–967
Rodriguez-Palenzuela P, Burr TJ, Collmer A (1991) Polygalacturonase is a virulence factor in Agrobacterium tumefaciens biovar 3. J Bacteriol 173:6547–6552
Saghai-Maroof MA, Soliman KM, Jorgensen RA, Allard RW (1984) Ribosomal DNA spacer-length polymorphisms in barley: mendelian inheritance, chromosomal location, and population dynamics. Proc Natl Acad Sci U S A 81:8014–8019
Sharp PJ, Chao S, Desai S, Gale MD (1989) The isolation, characterization and application in the Triticeae of a set of wheat RFLP probes identifying each homoeologous chromosome arm. Theor Appl Genet 78:342–348
Shivanna MB, Meera MS, Hyakumachi M (1996) Role of root colonization ability of plant growth promoting fungi in the suppression of take-all and common root rot of wheat. Crop Prot 15:497–504
Taylor J, Secor A (1988) An improved diffusion assay for quantifying the polygalacturonase content of Ervinia culture filtrates. Phytopathology 78:1101–1103
ten Have A, Mulder W, Visser J, van Kaan JAL (1998) The endopolygalacturonase gene Bcpg1 is required for full virulence of Botrytis cinerea. Mol Plant-Microbe Interact 11:1009–1016
Wang X, Zhu X, Tooley P, Zhang X (2013) Cloning and functional analysis of three genes encoding polygalacturonase-inhibiting proteins from Capsicum annuum and transgenic CaPGIP1 in tobacco in relation to increased resistance to two fungal pathogens. Plant Mol Biol 81:379–400
Xu HJ, Pang JL, Ye XG, Du LP, Li LC, Xin ZY, Ma YZ, Chen JP, Chen J, Chen SH, Wu HY (2001) Study on the gene transferring of Nib8 into wheat for its resistance to the yellow mosaic virus by bombardment. Acta Agron Sin 27:684–689 (in Chinese with English abstract)
Zhang Z, Liu X, Wang X, Zhou M, Zhou X, Ye X, Wei X (2012) An R2R3 MYB transcription factor in wheat, TaPIMP1, mediates host resistance to Bipolaris sorokiniana and drought stresses through regulation of defense- and stress- related genes. New Phytol 196:1155–1170
Zuppini A, Navazio L, Sella L, Castiglioni C, Favaron F, Mariani P (2005) An endopolygalacturonase from Sclerotinia sclerotiorum induces calcium-mediated signaling and programmed cell death in soybean cells. Mol Plant Microbe Interact 18:849–855
Acknowledgments
This study was supported by the National “Key Sci-Tech” program, China (Grant no.2013ZX08002001-004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Aiyun Wang and Xuening Wei contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, A., Wei, X., Rong, W. et al. GmPGIP3 enhanced resistance to both take-all and common root rot diseases in transgenic wheat. Funct Integr Genomics 15, 375–381 (2015). https://doi.org/10.1007/s10142-014-0428-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10142-014-0428-6