Abstract
Acute invasive fungal sinusitis (AIFS) is a fungal infection of the nasal cavity and paranasal sinuses with associated invasion of adjacent vessels and soft/hard tissues. It usually occurs in immunocompromised patients and may follow a rapid course of less than four weeks with high mortality rate. We report a 39-year-old male with relapse of acute myelogenous leukemia (AML) who was under evaluation for neutropenic fever. On his sinus CT, there was loss of calcification of his nasal septum when compared to a prior head CT, a sign indicative of an aggressive infectious process. He was diagnosed with AIFS and underwent emergent surgical debridement and systemic antifungal therapy, leading to a positive outcome. The sign described on CT (“Vanishing Nasal Septum” sign) may provide an additional, reliable tool to prospectively identify locally aggressive cases of invasive fungal infections of the nasal cavity at an earlier stage and improve patient outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fungal sinusitis (FS) encompasses a wide disease spectrum of the nasal cavity and paranasal sinuses. In 1965, there was an initial attempt to classify FS into noninvasive and invasive subtypes, but it was not until 2008 that the International Society for Human and Animal Mycology Group officially categorized FS as such [1]. The noninvasive subtypes encompass allergic FS and mycetoma, while the invasive subtypes consist of acute, chronic, and granulomatous invasive FS [1]. AIFS is defined by its rapid, progressive course of less than 4 weeks with associated invasion into adjacent vessels and soft/hard tissues [1]. Patients most affected by this disease are those with compromised immunities either primarily related to illness, treatment of the illness, or genetic inheritance predisposing to immunodeficiency [1,2,3]. Examples include those with malignancies (namely hematologic malignancy), those undergoing immunosuppressive treatments (such as chemotherapy or systemic steroids), and those with conditions including aplastic anemia, immunodeficiency syndromes, organ transplantation, and other deficient cell-mediated immunity conditions [1]. In comparison to other forms of FS, AIFS is the deadliest, with mortality rates ranging from 50–80% [1]. This factor, in combination with its rapidly destructive course, makes it paramount that clinicians and radiologists maintain high levels of suspicion for early signs of disease. The workup usually includes a non-contrast computed tomography (CT), typically the most appropriate initial study [4], along with nasal endoscopy (NE) with biopsy [1]. Maxillary and ethmoid sinuses are the most commonly affected paranasal sinuses. Common CT imaging findings are non-specific including mucosal thickening, along with partial or complete opacification of the affected sinus [5, 6]. Ulceration of mucosa and invasion of adjacent retroantral, premaxillary, and orbital fat planes are other more-specific and ominous signs [7]. Furthermore, the presence of hyperattenuating areas within the opacified sinuses should raise suspicion for AIFS, especially in immunocompromised patients. In some cases, areas of bone dehiscence and erosion can also be seen on CT [8]. In this case report, we discuss a novel sign of calcium resorption of the cartilaginous nasal septum in a patient with AIFS.
Case presentation
The patient is a 39-year-old male who presented to the hospital with new fatigue, fever, body aches, and malaise. He had a notable history of AML with allogeneic stem cell transplant in 2020 and was undergoing chemotherapy after relapsing. Initial physical exam was unremarkable, and laboratory values were notable for severe neutropenia (0.01 WBC). Given concern for neutropenic fever, he was admitted for further workup. His chest radiograph and urine/blood/cerebrospinal cultures were negative. Given his history of prior AML with central nervous system involvement, a CT head was performed on hospital day 2 to rule out abscess or other intracranial pathology as possible etiology, which was also negative. Broad-spectrum antibiotics were initiated given his continued fevers with notable improvement of symptoms over the next several days.
However, on hospital day 7, he reported new onset sinus and maxillary/mandibular pain. CT sinus was acquired, where only mild paranasal sinus disease was reported initially. The team consulted otolaryngology (ENT) and infectious disease (ID) given their high degree of suspicion for AIFS. The physical exam (PE) findings conducted by ID described a dime-sized black eschar with surrounding pale, boggy mucosa of the left and right anterior nasal septum. There was bilateral decreased sensation within the left and right anterior nasal septum. Otorhinolaryngology (ENT) confirmed AIFS from Bipolaris species with angioinvasion through bedside nasal endoscopy (NE) biopsy and frozen section analysis. The patient underwent emergent surgical debridement and anterior septectomy, followed by posterior septectomy and left inferior turbinectomy two days afterwards. There were no complications, and the patient received systemic antifungal therapy. Follow-up imaging studies demonstrated no residual disease. The rest of the patient’s hospital course was uneventful, and the patient fully recovered.
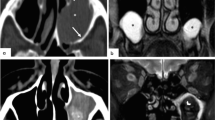
On retrospective review of the contrast-enhanced CT sinus, there were subtle signs of AIFS. These findings included necrotic/hypoattenuating nasal mucosa with aggressive resorption of the calcified component nasal septum, new from prior CT head without septal abscess or perforation, and no signs of sinus disease (Figs. 1 and 2). We describe this finding as the vanishing nasal septum sign.
Hospital day #7: Contrast-enhanced CT sinus—there is progressive soft tissue thickening of the nasal septum and aggressive loss of calcification associated with the nasal septum; we propose to call this calcification resorption loss the “vanishing nasal septum sign.” Furthermore, there is lack of contrast enhancement associated with the left nasal septal mucosa consistent with necrotic eschar (yellow arrow)
Discussion
Clinician awareness of invasive fungal infections has grown over recent years, highlighted by the COVID-19 pandemic. Studies have shown instances of acute immunocompromised states, such as with COVID-19 infection, that have significantly increased the mortality rates of AIFS patients [9]. On the initial CT head of hospital day 2, there was no septal thickening or opacification of the sinuses directly suggestive of sinus disease (Fig. 1). On hospital day 7, another CT sinus was ordered for clinical concern of AIFS, due to new onset sinus pain and interval development of left-sided nasal eschar discovered by ID. In this study, there was progressive demineralization of the nasal septum with increasing nasal septal edema and lack of left-sided nasal septal mucosal enhancement, corresponding to necrosis (Fig. 2). The rapid demineralization of the cartilaginous nasal septum is what we propose to call the vanishing nasal septum sign.
The identification of the vanishing nasal septum sign could aid in the early diagnosis of AIFS and, as such, improve patient outcomes of this aggressive disease process. Non-contrast CT sinus is the first imaging study used to assess for AIFS; therefore, a radiologic sign in this modality is needed in order to quickly and accurately describe this disease process early [4]. While there are a multitude of signs described, some are often subtle (e.g., mucosal ulceration and mucosal hypoenhancement), non-specific (e.g., mucosal thickening), or indicative of advanced stages of disease (e.g., invasion of adjacent fat planes about the paranasal sinuses or frank septal destruction with perforation). Dehiscence of the bony and cartilaginous nasal septum has been described in prior cases of AIFS, but progressive loss of calcification of the cartilaginous septum (a potential earlier finding) has not been formally described as a radiologic sign.
The classical sign described for AIFS has been the MR-specific black-turbinate sign which signifies tissue necrosis and is characterized by the absence of expected mucosal enhancement of nasal cavity structures, such as the nasal mucosa surrounding the turbinates [6, 8]. While MR provides more sensitivity and specificity in assessing for AIFS, MR imaging studies take much longer to perform in comparison to CT and are not always readily available. Hence, optimizing the ability to establish a diagnosis early and quickly is paramount to optimize outcome for the patient. Once the diagnosis is established, MR studies may be more valuable to define the extent of soft tissue fungal invasion and assess for complications in severe cases of AIFS, without delaying treatment. These signs and complications include the invasion of periantral fat, as well as adjacent bony edema/erosion, orbital inflammation, and pachymeningeal as well as leptomeningeal enhancement, the latter indicating frank intracranial extension [5,6,7]. Once AIFS has resolved, both CT and MRI can be used subsequently to assess for long-term complications in those who survive AIFS, such as neurological sequelae or chronic sinusitis [7].
While MRI is fundamental in assessment of AIFS, CT is the initial lynchpin in imaging evaluation of AIFS due to the widespread availability of CT, its ability to accommodate patients regardless of support apparatuses or implanted devices, and the speed of imaging acquisition. CT can detect the subtle infiltration of adjacent sinus fat, bony erosion, abscess formation, and mucosal abnormalities indicative of AIFS [5,6,7]. Attention to reliable, reproducible imaging signs can further expedite definitive imaging and management of patients with AIFS. We propose that loss of calcification associated with the cartilaginous nasal septum (“the vanishing nasal septum sign”) is optimized for CT due to the ability of the radiologist to promptly discern loss of calcification on CT.
Conclusion
We present a case of AIFS in an immunocompromised male who was able to survive this highly fatal condition. In this patient, the resorption of the calcified component of the nasal septum (“the vanishing nasal septum sign.”) demonstrates a potentially reliable method to confirm an aggressive process such as AIFS. This straightforward sign could be used by radiologists to quickly and accurately diagnose AIFS early and, as a result, improve the overall prognosis for patients with this highly fatal disease.
References
Raz E, Win W, Hagiwara M, Lui YW, Cohen B, Fatterpekar GM (2015) Fungal sinusitis. Neuroimaging Clin N Am 25(4):569–576. https://doi.org/10.1016/j.nic.2015.07.004
Drakos PE, Nagler A, Or R, Naparstek E, Kapelushnik J, Engelhard D, Rahav G, Ne’emean D, Slavin S (1993) Invasive fungal sinusitis in patients undergoing bone marrow transplantation. Bone Marrow Transplant 12(3):203–208
Fung M, Babik J, Humphreys IM, Davis GE (2019) Diagnosis and treatment of acute invasive fungal sinusitis in cancer and transplant patients. Curr Infect Dis Rep 21(12):53. https://doi.org/10.1007/s11908-019-0707-4
Gavito-Higuera J, Mullins CB, Ramos-Duran L, Sandoval H, Akle N, Figueroa R (2016) Sinonasal fungal infections and complications: a pictorial review. J Clin Imaging Sci 6:23. https://doi.org/10.4103/2156-7514.184010
John DS, Shyam K, Andrew D, Cicilet S, Deepalam SR (2022) Utilizing CT soft-tissue markers as a screening tool for acute invasive fungal sinusitis. Br J Radiol 95(1132):20210749. https://doi.org/10.1259/bjr.20210749
Middlebrooks EH, Frost CJ, De Jesus RO, Massini TC, Schmalfuss IM, Mancuso AA (2015) Acute invasive fungal rhinosinusitis: a comprehensive update of CT findings and design of an effective diagnostic imaging model. AJNR Am J Neuroradiol 36(8):1529–1535. https://doi.org/10.3174/ajnr.A4298
Desai K, Nunez DB Jr, Potter CA (2017) Core curriculum illustration: invasive fungal sinusitis. Emerg Radiol 24(6):697–699. https://doi.org/10.1007/s10140-017-1481-y
Ni Mhurchu E, Ospina J, Janjua AS, Shewchuk JR, Vertinsky AT (2017) Fungal rhinosinusitis: a radiological review with intraoperative correlation. Can Assoc Radiol J = Journal l’Assoc Can Radiol 68(2):178–186. https://doi.org/10.1016/j.carj.2016.12.009
Borrelli M, Nasrollahi T, Ulloa R, Raskin J, Ference E, Tang DM (2022) Invasive fungal sinusitis during active COVID-19 infection. Ear Nose Throat J 101(10_suppl):12S–14S. https://doi.org/10.1177/01455613221112337
Funding
This case report was not supported by any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tine, A., Maldonado, S.M. & Cloran, F.J. The vanishing nasal septum sign: a case of severe fungal sinusitis. Emerg Radiol 30, 807–810 (2023). https://doi.org/10.1007/s10140-023-02176-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10140-023-02176-z