Abstract
Rhino-orbito-cerebral mucormycosis (ROCM) has regained significance following its resurgence in the second wave of the COVID-19 pandemic in India. Rapid and progressive intracranial spread occurs either by direct extension across the neural foraminae, cribriform plate/ethmoid, walls of sinuses, or angioinvasion. Having known to have a high mortality rate, especially with intracranial extension of disease, it becomes imperative to familiarise oneself with its imaging features. MRI is the imaging modality of choice. This pictorial essay aims to depict and detail the various intracranial complications of mucormycosis and to serve as a broad checklist of structures and pathologies that must be looked for in a known or suspected case of ROCM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Mucormycosis is an invasive, potentially fatal, opportunistic fungal infection, caused by saprophytic fungi of the order Mucorales. A rapid surge in its incidence has been witnessed in the second wave of the COVID-19 pandemic in India. The proposed predisposing factors include hypoxia, uncontrolled blood sugars either steroid induced or due to diabetes mellitus, and prolonged multipronged immunosuppression [1]. Though it can involve different organ systems, the most common type is rhino-orbitocerebral mucormycosis (ROCM) [2].
The route of infection is usually by inhalation of the spores which invade the mucosa of nasal cavity and sinuses causing rhinosinusitis. Further rapid and progressive intracranial spread of the fungus occurs either by direct extension across the neural foraminae, cribriform plate/ethmoid, walls of the frontal and sphenoid sinuses, or angioinvasion of the walls of the arteries and veins, causing vascular thrombosis, occlusion, and infarction [3,4,5].
Symptoms of intracranial extension include altered sensorium, diplopia, ophthalmoplegia, cranial nerve deficits, and focal neurological deficits based on the region of spread. With the intracranial extension of disease, mortality is greater than 80% [3].
Early imaging is crucial and aids in assessing the extent of involvement of disease and assists in prompt initiation aggressive antifungal treatment and surgical debridement. Magnetic resonance imaging is the imaging modality of choice. Restricted diffusion in ROCM indicates tissue ischaemia and necrosis resulting from the angioinvasive property of the fungus and thereby the path of spread of fungal infection [6].
This pictorial review will showcase the MR imaging features of intracranial complications of ROCM. The commonest intracranial manifestations are cavernous sinus extension, narrowing or occlusion of the internal carotid artery (ICA), and infarction [7].
Extension into cavernous sinus
Cavernous sinus involvement can occur through the ethmoidal and orbital veins that drain into it, through direct extension from sphenoid sinus, or from perineural spread along neural foraminae [3,4,5]. Bulging of the lateral walls of the sinus, abnormal dural enhancement of the lateral wall, abnormal signal intensity of the sinus (iso-hypointensity on T1 and hyperintensity on T2), and presence of filling defects on post contrast scans (Fig. 1) indicate cavernous sinus thrombosis [8, 9]. Paracavernous soft tissue extension can also be seen (Figs. 1 and 2). Associated dilatation and thrombosis of the superior ophthalmic vein may also be visualised.
Cavernous sinus thrombosis: A Axial post contrast T1-FS-weighted image of the brain reveals non enhancement of the left cavernous sinus (white arrow), indicating cavernous sinus thrombosis. Normal enhancement of the right cavernous sinus is seen. Bilateral ICA are patent. B Axial T2-weighted image of the brain reveals T2 heterogeneously hyperintense left paracavernous soft tissue (white arrow) with convex bulge of the wall of the cavernous sinus on the left
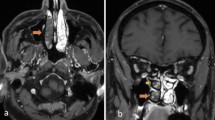
Cavernous sinus extension and right ICA thrombosis: Axial T2-weighted images of the brain reveal A convex bulge of the wall of the cavernous sinus on the right (white arrow), as opposed to a normal convex wall as seen on the left; A T2 hyperintense soft tissue in the right cavernous sinus (white asterisk), B, C extending into the right paracavernous region (white asterisk). A–F Invasion of the right ICA with resultant thrombosis is seen as loss of flow void in the petrocavernous right ICA (yellow arrows). Left intracranial ICA shows normal flow void (yellow asterisk)
ICA involvement
Angioinvasion of internal carotid artery across the sphenoid sinus or cavernous sinus and retrograde extension of mucor thrombus from the smaller ophthalmic arteries are the mechanisms involved. It can be evaluated on CT but is best evaluated on MRI. It presents as abnormal T2 signal intensity and post contrast enhancement in the wall (vasculitis) (Fig. 3), progressing to infiltration causing irregular luminal narrowing and ultimately complete thrombosis (Fig. 2) which is seen as loss of flow void on T2-weighted images [4].
Angioinvasion of wall of cavernous ICA: A Axial T2-weighted image reveals hyperintense thickening of the wall of the left cavernous ICA. Lumen is patent with maintained flow void. B Time of Flight intracranial MR Angiogram depicts the mild luminal narrowing of left cavernous ICA with maintained flow related enhancement. C Post contrast T1FS images show enhancement of the wall of the right cavernous ICA, which is patent but narrow calibred, as opposed to the normal left cavernous ICA
Infarction
Involvement of the internal carotid artery can result in cerebral infarcts in the watershed territory, embolic in nature (Fig. 4). Infarcts can also occur secondary to direct vessel invasion (Fig. 5), which then harbour the fungal elements, visualised as blooming within the infarct on susceptibility weighted images [4].
Cranial nerve involvement
Two unique characteristics of cranial nerve involvement by ROCM are described below:
-
Ischaemic neuropathy. The optic nerve and the trigeminal nerve are the most commonly involved cranial nerves. Optic nerve infarction results from angioinvasion and thrombotic occlusion of the central retinal artery or ophthalmic artery or by direct infiltration of the nerve by the fungus. Trigeminal nerve infarction can also occur in a similar fashion. It is visualised as restricted diffusion along the affected nerve (Figs. 6 and 7), usually without any enlargement, altered T2/STIR signal intensity or post contrast enhancement [10, 11].
-
Perineural spread. On MRI, direct findings suggestive of perineural spread are enlargement/thickening of the nerve, irregularity and excessive enhancement of the cranial nerve or its branch (within the cistern, canal, or foramen) (Fig. 8), loss of the normal fat pad adjacent to the foramen, widening or excessive enhancement within the pterygopalatine fossa, Meckel’s cave, or the cavernous sinus [12, 13]. Perineural spread serves as a conduit of spread — for instance, from the orbit intracranially along the optic nerve, and along the trigeminal nerve to the brainstem (Fig. 7).
Trigeminal nerve infarction and involvement of brainstem: A, B Restricted diffusion in the root entry zone, cisternal and gasserian segments of the left trigeminal nerve (white arrows). Right trigeminal nerve is normal. C Restricted diffusion in the right trigeminal nucleus. D Follow-up scan shows expansion of the FLAIR hyperintense infarct to involve the adjacent lateral surface of right hemipons
Septic emboli
Hyphal arterial invasion can result in septic emboli. They manifest as peripheral ring enhancing lesions, with central restricted diffusion and perilesional FLAIR hyperintense oedema, usually located at the grey matter — white matter junctions, predominantly in the middle cerebral artery territory (Fig. 9) [14]. Septic emboli may also be seen as multiple microhaemorrhages on Susceptibility weighted images [15].
Cerebritis
It presents on CT as ill-defined intraparenchymal hypodense lesions with scattered areas of increased attenuation due to either haemorrhage or increased concentration of metal ions. On MRI, early cerebritis appears as a cortical-subcortical T1 iso-hypointense, T2/FLAIR hyperintense area with patchy restricted diffusion, and no post contrast enhancement. Progressive peripheral enhancement may be seen in late cerebritis (Fig. 10) [16].
Late cerebritis/evolving abscess: A Axial plain CT reveals ill-defined hypodensity in the left temporal lobe. Axial plain and contrast MRI reveals ill-defined area of restricted diffusion (B, C), T2 hyperintensity (D), peripheral foci of blooming on SWI (E), and subtle thin enhancement in the lateral aspect of the lesion as seen on post contrast T1-weighted images (F). G Adjacent thin pachymeningeal enhancement along the left temporal convexity
Abscess
Cerebral parenchymal abscess due to Mucor are most often seen in the frontal lobe or temporal lobes. They appear as irregularly marginated T2 hyperintense lesions with T2 hypointense, irregular peripherally enhancing walls with intracavitary non-enhancing projections with characteristic restricted diffusion of the intracavitary projections and of the wall. Susceptibility artefacts may be seen in the periphery of the abscess indicating a fungal composition (Fig. 11) [10]. Ventriculitis (Fig. 12) and subdural/extradural abscesses are rare may be seen in advanced disease.
Fungal cerebral abscess: (A) DWI, (B) ADC, (C) Axial T2WI, (D) SWI, (E) Axial T1WI and (F) Post contrast axial T1FS MRI images reveal an irregularly marginated, peripherally enhancing, T2 heterogeneously hyperintense and T1 isointense lesion in the left frontal lobe, showing peripheral diffusion restriction in the wall and foci of SWI blooming mainly on the left lateral aspect of the lesion. Perilesional oedema, mass effect and subfalcine herniation to the right side noted. (G) Post contrast coronal T1FS images depict communication of the abscess with the left frontal, ethmoid, and maxillary sinuses
Leptomeningitis and ventriculitis in a case of mucormycosis post FESS: Axial post contrast FLAIR images reveal diffuse post contrast enhancement of bilateral hemispheric sulcal spaces (A, B) (white arrows), basal cisterns cisterns (C), and the cerebellar foliae (D). B Post contrast enhancement of the ependymal lining, ventricular walls (yellow arrows), hydrocephalus with dependent exudates in the occipital horns of bilateral lateral ventricles (arrow heads)
Leptomeningitis/pachymeningitis
MR images demonstrate thick, often nodular, meningeal enhancement on post contrast T1-weighted and FLAIR images, due to thick gelatinous exudates containing inflammatory cells, fibrin, and haemorrhage leading to arachnoiditis which in turn can lead to secondary hydrocephalus (Fig. 12) [16]. Dural thickening and enhancement (Figs. 13 and 14) is commonly in seen in the middle cranial fossa and paracavernous dura as well as along the frontal convexities and, rarely, can be diffuse.
Direct extension across ethmoid: In a case with known mucormycosis post FESS, A coronal post contrast T1 fat suppressed images show dural enhancement along the crista galli and base of anterior cranial fossa, indicating direct intracranial spread across the ethmoid sinus. B Coronal T2 fat suppressed image shows T2 hyperintense oedema in the right frontal lobe with no focal lesion
Skull base involvement
Being an angioinvasive fungus, bony involvement is not common and is usually seen only in chronic forms of the disease. However, early involvement of the bone marrow can be picked up on MRI, seen as patchy STIR hyperintense marrow oedema (Fig. 15) and heterogeneous post contrast enhancement [17]. Chronic forms can be visualised on CT as rarefaction, osteolysis, erosions, and sclerosis [18].
Conclusion
An exponential increase in the incidence of ROCM was witnessed in the second wave of the COVID-19 pandemic in India, reiterating the importance of familiarising oneself with its varied but crucial manifestations on imaging. MRI with contrast forms the crux of patient management and the brain has to be imaged in every case. It aids in diagnosis, assessment of extent of disease, and early identification of intracranial extension which may contribute to the reduction of mortality as it allows for timely initiation of aggressive treatment.
Data availability
Not applicable.
Code availability
Not applicable.
References
Singh A, Singh R, Joshi S, Misra A (2021) Mucormycosis in COVID-19: a systematic review of cases reported worldwide and in India. Diabetes Metab Syndr 15:102146. https://doi.org/10.1016/j.dsx.2021.05.019
Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL et al (2005) Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 41:634–653. https://doi.org/10.1086/432579
Herrera DA, Dublin AB, Ormsby EL, Aminpour S, Howell LP (2009) Imaging findings of rhinocerebral mucormycosis. Skull Base 19:117–125. https://doi.org/10.1055/s-0028-1096209
Patil A, Mohanty HS, Kumar S, Nandikoor S, Meganathan P (2016) Angioinvasive rhinocerebral mucormycosis with complete unilateral thrombosis of internal carotid artery-case report and review of literature. BJR Case Rep 2:20150448. https://doi.org/10.1259/bjrcr.20150448
Gamaletsou MN, Sipsas NV, Roilides E, Walsh TJ (2012) Rhino-orbital-cerebral mucormycosis. Curr Infect Dis Rep 14:423–434. https://doi.org/10.1007/s11908-012-0272-6
Gavito-Higuera J, Mullins CB, Ramos-Duran L, Olivas Chacon CI, Hakim N, Palacios E (2016) Fungal infections of the central nervous system: a pictorial review. J Clin Imaging Sci 6:24. https://doi.org/10.4103/2156-7514.184244
Mathur S, Karimi A, Mafee MF (2007) Acute optic nerve infarction demonstrated by diffusion-weighted imaging in a case of rhinocerebral mucormycosis. AJNR Am J Neuroradiol 28:489–490
Ghuman MS, Kaur S, Bhandal SK, Ahluwalia A, Saggar K (2015) Bilateral optic nerve infarction in rhino-cerebral mucormycosis: a rare magnetic resonance imaging finding. J Neurosci Rural Pract 6:403–404. https://doi.org/10.4103/0976-3147.158790
Lone PA, Wani NA, Jehangir M (2015) Rhino-orbito-cerebral mucormycosis: magnetic resonance imaging. Indian J Otol 21:215–218. https://doi.org/10.4103/0971-7749.159700
Chikley A, Ben-Ami R, Kontoyiannis DP (2019) Mucormycosis of the central nervous system. J Fungi (Basel) 5:59. https://doi.org/10.3390/jof5030059
Bhatia H, Kaur R, Bedi R (2020) MR imaging of cavernous sinus thrombosis. Eur J Radiol Open 7:100226. https://doi.org/10.1016/j.ejro.2020.100226
Koc Z, Koc F, Yerdelen D, Ozdogu H (2007) Rhino-orbital-cerebral mucormycosis with different cerebral involvements: infarct, hemorrhage, and ophthalmoplegia. Int J Neurosci 117:1677–1690. https://doi.org/10.1080/00207450601050238
Champey J, Pavese P, Bouvaist H, Kastler A, Krainik A, Francois P (2016) Value of brain MRI in infective endocarditis: a narrative literature review. Eur J Clin Microbiol Infect Dis 35:159–168. https://doi.org/10.1007/s10096-015-2523-6
Scully MA, Yeaney GA, Compton ML, Berg MJ (2012) SWAN MRI revealing multiple microhemorrhages secondary to septic emboli from mucormycosis. Neurology 79:1932–1933. https://doi.org/10.1212/wnl.0b013e318271f86c
Orguc S, Yücetürk AV, Demir MA, Goktan C (2005) Rhinocerebral mucormycosis: perineural spread via the trigeminal nerve. J Clin Neurosci 12:484–486. https://doi.org/10.1016/j.jocn.2004.07.015
Parsi K, Itgampalli RK, Vittal R, Kumar A (2013) Perineural spread of rhino-orbitocerebral mucormycosis caused by Apophysomyces elegans. Ann Indian Acad Neurol 16:414–417. https://doi.org/10.4103/0972-2327.116921
Chan LL, Singh S, Jones D, Diaz EM Jr, Ginsberg LE (2000) Imaging of mucormycosis skull base osteomyelitis. AJNR Am J Neuroradiol 21:828–831
Therakathu J, Prabhu S, Irodi A, Sudhakar SV, Yadav CK, Rupa V (2018) Imaging features of rhinocerebral mucormycosis: a study of 43 patients. Egypt J Radio Nucl Med 49:447–452. https://doi.org/10.1016/j.ejrnm.2018.01.001
Funding
This study was not supported by any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kaushik, K.S., Ananthasivan, R., Acharya, U.V. et al. Spectrum of intracranial complications of rhino-orbito-cerebral mucormycosis — resurgence in the era of COVID-19 pandemic: a pictorial essay. Emerg Radiol 28, 1097–1106 (2021). https://doi.org/10.1007/s10140-021-01987-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10140-021-01987-2