Abstract
Neonatal herpes simplex virus (HSV) infection of the central nervous system (CNS) is an emergency that can have devastating structural consequences and clinical outcomes. As it presents non-specifically in neonates, it is difficult to rapidly diagnose without neuroimaging. Although once thought to cause widespread parenchymal destruction, neonatal CNS HSV infection may present with more focal parenchymal injury on neuroimaging, not involving the medial temporal lobes as in adults. We report a case of a three-week-old girl with herpes simplex virus type 2 (HSV-2) encephalitis with exclusive bilateral corticospinal and frontal opercular involvement, which remained undiagnosed and untreated until three months of age. Neuroimaging upon presentation to the emergency room demonstrates a highly suggestive pattern of severe neonatal CNS HSV-2 infection which followed the natural history on subsequent imaging, highlighting the importance of emergency neuroimaging as well as having a high index of suspicion for making the diagnosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Herpes simplex virus (HSV) central nervous system (CNS) infection is difficult to clinically diagnose in neonates, due to a low index of suspicion (prevalence of 10 in 100,000), lack of clear etiology (it is acquired perinatally, usually by exposure to herpetic genital lesions during delivery) and non-specific presentation (lethargy, fever, seizures) [1]. HSV can cause mucocutaneous vesicles at the skin, mouth, or eyes, but these lesions are not always seen in isolated CNS HSV infections [2]. Differential diagnoses include bacterial septic or CNS infection and other viral neonatal CNS infections such as enterovirus, rotavirus, and human parechovirus [3]. A full septic workup including blood, urine, and cerebrospinal fluid (CSF) cultures can rule out bacterial sepsis, and polymerase chain reaction (PCR) of CSF and vesicle fluid can instead identify HSV [2]. However, CSF samples may yield inconclusive results during the early stages of the disease or because of contamination with blood. If left untreated, however, CNS HSV infection can cause encephalitis and parenchymal necrosis, leading to significant neurological deficits or death. Neuroimaging in the emergency setting is therefore vital for rapid, accurate diagnosis, and prompt initial treatment to limit or prevent neural damage.
A three-week-old infant, born via spontaneous vaginal delivery at term in a remote community in northern Canada, presented to her local hospital with seizures affecting her left arm and left leg. A septic workup was negative and the presence of blood in the CSF sample following lumbar puncture precluded PCR testing.
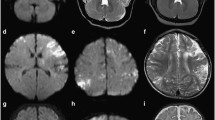
As part of the workup in the emergency setting, brain CT was performed and showed patchy hypoattenuation in the deep and periventricular cerebral white matter bilaterally, most prominent in the perirolandic region (Fig. 1a). On brain MRI, there was diffusion restriction with associated hyperintensity on T2-weighted images in the perirolandic region bilaterally, as well as along the corticospinal tracts in the corona radiata, posterior limb of internal capsule, and brainstem (Fig. 1b–f). There was no associated hemorrhage. MR angiogram and MR venogram were normal. Findings were interpreted as acute ischemic infarction.
Brain CT at initial presentation at three weeks of age shows patchy hypoattenuation in the bilateral cerebral white matter, most conspicuous in the perirolandic region (1a). Brain MRI at initial presentation shows diffusion restriction bilaterally along the precentral gyrus (1b), and along the corticospinal tracts, including the corona radiata (1c) and posterior limb of the internal capsule, most evident on the left (1d). T2-weighted images demonstrate loss of grey-white differentiation in the perirolandic region bilaterally (1e–1f)
The patient’s seizures were treated with phenobarbital and the patient was discharged.
The patient presented to her local hospital again at three months of age after having several tonic–clonic seizures. After being stabilized, she was transferred to our centre. Brain MRI was performed and showed a similar pattern of brain involvement, however there was evolution of the findings that were acute at initial presentation — there was resolution of the previously seen diffusion restriction and no evidence of new injury outside of the originally involved areas. There was cortically based hyperintensity on T1-weighted images in the perirolandic region and along the corticospinal tracts (Fig. 2a–b), reflecting laminar necrosis. This hyperintensity extended inferolaterally from the perirolandic regions to the frontal opercula bilaterally, particularly on the right (Fig. 2c). T2-weighted images demonstrated encephalomalacia in the affected regions, with associated mild prominence of the adjacent CSF spaces (Fig. 2d). Susceptibility-weighted images demonstrated chronic blood products in the perirolandic region and along the corticospinal tracts, with a small focus of chronic intraventricular blood products in the occipital horn of the right lateral ventricle (Fig. 2e–f). MR spectroscopy was unremarkable. Following lumbar puncture, HSV-2 was found in the cerebrospinal fluid, confirming the diagnosis of neonatal HSV-2 encephalitis. Intravenous acyclovir was administered, as was antiepileptic therapy and erythromycin for concomitant ocular HSV-2 infection.
Brain MRI at three months of age shows cortically-based hyperintensity on T1-weighted images (reflecting laminar necrosis) in the perirolandic region bilaterally (2a) and the posterior limb of internal capsule bilaterally (2b) which extends into the opercular region (2c). T2-weighted MR images demonstrate encephalomalacia in the perirolandic regions bilaterally, more so on the right (2d). On susceptibility-weighted imaging, there is abnormal susceptibility effect in the perirolandic region, more so on the right (2e) and dependently in the occipital horn of the right lateral ventricle (2f), reflecting chronic blood products
At clinical follow-up, there was rigidity in the patient’s upper limbs and hypertonicity of the lower limbs, but she fed well and made no abnormal eye or body movements. The patient also experienced several infantile spasm-like episodes with hypertonia and breathing difficulties (breath stacking, increased work of breathing). The consulting neurologist has predicted cerebral palsy as a result of the identified brain injury.
Emergency neuroimaging can lead to rapid and accurate diagnosis, and expeditious treatment of neonatal CNS HSV infection during its initial stages, even in the absence of specific clinical signs or laboratory results. We highlight that CNS HSV infection can cause focal brain insult rather than the widespread parenchymal damage previously believed. Despite a clinical and laboratory workup, our patient’s infection remained undiagnosed and untreated for over two months. Her initial presentation of seizures was non-specific and blood in the CSF sample precluded PCR testing for HSV, making the diagnosis on these grounds challenging. Brain CT has low sensitivity and is insufficient for diagnosis — Vossough et al. found that in 5 out of 11 cases (45%) of neonatal HSV-2 encephalitis, CT findings were either negative or “questionable” for infection [4]. In the same study, 9 out of 11 cases (81%) showed abnormalities on conventional MRI, and diffusion-weighted MRI (DW-MRI) in particular revealed disease or showed a greater extent of affected tissue compared to CT or conventional MRI in 7 out of 10 cases (70%) [4]. Therefore, DW-MRI has been suggested as a diagnostic tool for neonatal HSV encephalitis [4,5,6], rather than relying on CSF PCR, which can only be performed if the lumbar puncture sample is not contaminated by blood. DW-MRI can identify early infectious changes, which appear as “stardust” hyperintense punctuate lesions, before cystic or signal abnormalities later appear on conventional MRI [3].
While once thought to cause widespread parenchymal damage in neonates, growing evidence has shown that there are distinct patterns of distribution in neonatal HSV encephalitis, including the watershed areas, the inferior frontal and temporal poles, and — as in this case — the corticospinal tracts [3]. Similarly, bilateral opercular lesions, as reported here, have also been linked to HSV encephalitis and related syndromes in children and adults: for example, corticobulbar paresis (known as Foix-Chavany-Marie syndrome) results in weakness of the voluntary masticatory, facial, pharyngeal, and tongue musculature [7, 8]. Our patient, however, did not show any weakness in these muscles or issues with feeding at clinical follow-up.
Our patient had corticospinal/perirolandic and frontal opercular involvement, both seen at initial presentation at three weeks of age and at three months of age. Vossough et al. reviewed 12 neonatal HSV-2 encephalitis cases, 11 of whom received noncontrast CT as initial imaging, and found that disease was multifocal in 8 (67%) patients and restricted in 4 patients (33%) [4]. There were 3 (25%) cases with disease restricted to the temporal and frontal lobes [4]. Similarly, Kidokoro et al. reviewed imaging for 13 neonatal HSV encephalitis cases, 12 of whom had frontal involvement (92%), and found that 5 cases had inferior frontal and temporal pole pathology (38%), whereas 4 had corticospinal tract patterns (30%) [3]. These two patterns correspond to our case, although our patient had exclusive frontal opercular involvement as opposed to inferior frontal and temporal pole involvement. Overall, out of the 25 combined cases of these two studies, 14 (56%) had identifiable patterns, with 4 (16%) having corticospinal involvement, as in the case we describe [3, 4].
In summary, we highlight the acute neuroimaging findings of neonatal HSV encephalitis with focal corticospinal tract and frontal opercular involvement, as well as the sequelae if left untreated. We strongly believe radiographic evidence be used as criteria for initiating treatment for suspected neonatal CNS HSV infection and strongly recommend that radiologists maintain a high index of suspicion when these findings are identified to avoid the devastating — and avoidable — consequences seen in this case.
Data availability
Not applicable.
Code availability
Not applicable.
References
Kleinschmidt-DeMasters BK, Keohane C, Gray F (2020) Herpes simplex virus infections of the CNS. In: Chrétien F, Wong KT, Sharer C, Keohane C, Gray F (eds) Infections of the central nervous system: pathology and genetics. John Wiley & Sons Ltd, Oxford, Chapter 5. https://doi.org/10.1002/9781119467748.ch5
Fernandes ND, Arya K, Ward R (updated 2021) Congenital herpes simplex. In: StatPearls. StatPearls Publishing, Treasure Island, Florida. https://www.ncbi.nlm.nih.gov/books/NBK507897/. Accessed 14 May 2021
Kidokoro H, de Vries LS, Ogawa C, Ito Y, Ohno A, Groenendaal F, Saitoh S, Okumura A, Ito Y, Natsume J (2002) Predominant area of brain lesions in neonates with herpes simplex encephalitis. J Perinatol 37:1210–1214. https://doi.org/10.1038/jp.2017.114
Vossough A, Zimmerman RA, Bilaniuk LT, Schwartz EM (2008) Imaging findings of neonatal herpes simplex virus type 2 encephalitis. Neuroradiology 50:355–366. https://doi.org/10.1007/s00234-007-0349-3
Bajaj M, Mody S, Natarajan G (2014) Clinical and neuroimaging findings in neonatal herpes simplex virus infection. J Pediatr 165(2):404-407.e1. https://doi.org/10.1016/j.jpeds.2014.04.046
Okanishi T, Yamamoto H, Hosokawa T, Ando N, Nagayama Y, Hashimoto Y, Maihara T, Goto T, Kubota T, Kawaguchi C, Yoshida H, Sugiura K, Itomi S, Ohno K, Takanashi J, Hayakawa M, Otsubo H, Okumura A (2015) Diffusion-weighted MRI for early diagnosis of neonatal herpes simplex encephalitis. Brain Dev 37(4):423–431. https://doi.org/10.1016/j.braindev.2014.07.006
McGrath NM, Anderson NE, Hope JK, Croxson MC, Powell KF (1997) Anterior opercular syndrome, caused by herpes simplex encephalitis. Neurology 49(2):494–497. https://doi.org/10.1212/WNL.49.2.494
De Kleermaeker FGCM, Bouwmans AEP, Nicolai J, Klinkenberg S (2014) Anterior opercular syndrome as a first presentation of herpes simplex encephalitis. J Child Neurol 29(4):560–563. https://doi.org/10.1177/0883073813482768
Author information
Authors and Affiliations
Contributions
IL and MTJ played equal roles in manuscript conception, composition, and revision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ladak, I., Jurkiewicz, M.T. Uncommon acute neuroimaging findings in severe neonatal herpes simplex virus 2 and consequences of delayed diagnosis. Emerg Radiol 28, 1225–1228 (2021). https://doi.org/10.1007/s10140-021-01962-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10140-021-01962-x