Abstract
Immune checkpoint inhibitor (ICI)-associated myocarditis is a rare but potentially fatal complication associated with development of other immune-related adverse events (irAEs). Troponin levels, ECG, echocardiography, and cardiac MR can assist with the diagnosis of this rare albeit serious adverse effect related to immunotherapy. In this case report, we present the clinical and radiological features of myocarditis in three patients presenting with acute symptoms while receiving therapy with ICIs. Blood troponin and ECG were abnormal in all three myocarditis cases. Initial echocardiography was abnormal in two patients with reduced left ventricular ejection fraction (LVEF). The third patient demonstrated an initially normal LVEF with subsequent transient decrease in LVEF on follow-up echocardiogram. Cardiac MR was abnormal in three cases with areas of mid-myocardial/epicardial delayed enhancement. All patients experienced additional irAEs. One patient died shortly after myocarditis diagnosis, one was made comfort care due to poor clinical status, and one improved with steroid treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Immune checkpoint inhibitors (ICIs) are a novel category of drugs that promote immune system-mediated recognition and targeting of cancer cells [1]. ICI-induced T cell activation not only targets cancer cells but also produces a range of autoimmune toxicities [2]. These immune-related adverse events (irAEs) have been described involving the neurological, endocrine, pulmonary, gastrointestinal, cardiovascular, and renal systems.
Myocarditis is an uncommon but potentially fatal complication of treatment with ICIs [1]. This clinically important side effect is likely the most important pathology in major adverse cardiac event (MACE) that has been reported in patients receiving ICIs [3]. Description of myocarditis as a side effect of ICIs is relatively new in the literature, with more reports being published recently [3,4,5].
This case report presents clinical and radiological data gathered from three patients diagnosed with myocarditis associated with ICI therapy (Table 1). The diagnosis of acute myocarditis for each patient was based on a guideline-recommended scoring system for clinically suspected myocarditis that incorporates several variables, including the clinical, electrocardiographic, biomarker, and imaging features [6].
All patients had a baseline transthoracic echocardiography (TTE) within 6 months prior to treatment which confirmed normal systolic function and left ventricular ejection fraction (LVEF). All patients underwent at least one transthoracic echocardiography (TTE) during the admission for myocarditis. Cardiac MR (CMR) and coronary catheterization each were performed in all three cases.
Treatment with ICI was stopped in all three cases without rechallenge. All patients were treated with high-dose corticosteroids (methylprednisolone, loading dose of 1 g followed by 1 mg/kg/day), accompanied by plasma exchange in one of the cases. Major cardiac events occurred in two of the three cases.
Case 1
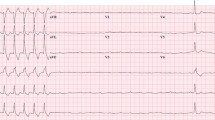
This 83-year-old male with a history of renal cell carcinoma presented with symptoms of abdominal pain, right-sided facial droop, and diffuse weakness shortly after receiving his second dose of nivolumab. ECG on arrival was remarkable for PVCs and ST elevation, with troponin elevated at 68. Initial echocardiogram in this patient with no prior cardiac history demonstrated a reduced left ventricular ejection fraction (LVEF) of 35% and small pericardial effusion without regional wall motion abnormalities. Coronary catheterization revealed no remarkable findings. Cardiac MRI was obtained and demonstrated a reduced LVEF of 30% and extensive mid-myocardial and epicardial delayed enhancement in the septum, inferior, and lateral walls (Fig. 1a and b). Significant myocardial edema was detected on T2 mapping images in the same area (Fig. 1c). Additional immune-related adverse events experienced by this patient included hepatitis, Bell’s palsy, myositis, and encephalitis. The patient received steroid therapy while hospitalized for 3 days and subsequently expired during the hospitalization secondary to cardiac arrest.
83-year-old man with metastatic renal cell carcinoma (patient 1), who is being treated with nivolumab and presenting with abdominal pain and diffuse weakness. Post-gadolinium delayed enhancement cardiac MR in 4-chamber (a) and short-axis (b) views shows large areas of mid-myocardial and epicardial delayed enhancement (DE) seen in the septum, lateral, and inferior walls (arrows) in keeping with areas of scarring. The sub-endocardium is spared. Short-axis T2 mapping image (c) shows extensive areas of increased intensity in the same areas (arrows), in keeping with myocardial edema and highly suggestive of myocarditis
Case 2
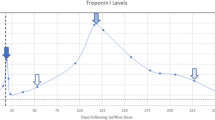
This 78-year-old female with metastatic melanoma presented 18 days after receiving an initial dose of nivolumab and ipilimumab with symptoms of malaise, blurry vision, and dysphagia. Initial echocardiogram demonstrated a normal LVEF of 65%. T-wave inversions were detected on ECG. Initial troponin was measured at 1.97, which ultimately increased to a peak value of 47.62 after 8 days. Cardiac MRI demonstrated delayed enhancement in the inferoseptal and midseptal wall (Fig. 2a and b), while LVEF was preserved. Follow-up CMRs showed no significant change in the distribution and severity of DE, but a decrease in LVEF to 40% was noted. Follow-up echocardiogram after 7 days revealed a transient decrease in the LVEF to 40%, which subsequently normalized on repeat echocardiogram after 17 days. In addition to myocarditis, the patient experienced additional immune-related adverse events, including myasthenia gravis, conjunctivitis/uveitis, hepatitis, and myositis. Coronary catheterization revealed no evidence of significant coronary artery stenosis. The patient was admitted for a 34-day hospital stay in which she received treatment with steroids and plasma exchange. The patient’s clinical course was complicated by an episode of pulseless electrical activity with subsequent resuscitation. Ultimately, the decision was made to pursue comfort care measures due to patient’s preference and progression of underlying malignancy.
78-year-old woman with metastatic melanoma (patient 2) who was being treated with nivolumab and ipilimumab presenting with malaise. Post-gadolinium delayed enhancement cardiac MR in 4-chamber (a) and short-axis (b) views shows areas of mid-myocardial and epicardial delayed enhancement (DE) seen in the septum (arrow in a) and inferoseptal wall (arrow in b) in keeping with areas of scarring. The sub-endocardium is spared. T2 mapping images were unremarkable (not shown)
Case 3
This 81-year-old male with a history of metastatic melanoma developed chest pain and watery diarrhea approximately 25 days after receiving initial doses of nivolumab and ipilimumab. Prior to this, the patient had received immunotherapy with pembrolizumab with no previous episodes of myocarditis. Following presentation, the patient was found to have a newly reduced LVEF of 45% without regional wall motion abnormalities. Initial CMR similarly demonstrated a reduced LVEF of 35% with a focal area of delayed enhancement in the base of the lateral wall (Fig. 3). Follow-up CMR showed no significant change in DE, and LVEF remained reduced. Follow-up TTE after 2 weeks showed improvement in LVEF (65%). The patient also experienced immune-related colitis during his hospital admission. The patient received high-dose steroids for treatment of immune-related myocarditis during the course of his 22-day hospital stay. The patient ultimately improved clinically and was discharged from the hospital. Subsequent follow-up imaging after 16 months demonstrated progression of metastatic disease but no clinical recurrence of myocarditis.
81-year-old man with metastatic melanoma (patient 3) who was being treated with nivolumab and ipilimumab presenting with chest pain and frequent premature ventricular contractions. Post-gadolinium delayed enhancement cardiac MR in 4-chamber view shows an area of mid-myocardial delayed enhancement (DE) in the lateral wall (arrow) in keeping with an area of scarring. The sub-endocardium is spared. T2 mapping images were unremarkable (not shown)
Discussion
Immune checkpoint inhibitors are being more frequently used in the treatment of malignancies, and the use of ICIs is expected to increase significantly in the coming years. Myocarditis is an uncommon complication of treatment with ICIs. Cardiac myocytes may share targeted antigens with tumors, thus resulting in targeting by activated T cells and subsequent lymphocytic infiltration and myocarditis [4].
The true incidence of ICI-associated myocarditis is not known, but it has been reported to be as low as 0.09% [4] to as high as 1.1% [1]. Due to the relatively low incidence of ICI-associated myocarditis, knowledge of the clinical and radiological features associated with this condition is quite limited.
The three patients included in this case series developed ICI-related myocarditis early in the course of treatment, which is in accordance with prior studies [1]. In two of the cases, myocarditis developed after the first dose of ICI, while in the other patient, myocarditis occurred after the completion of the second dose. All three patients also experienced other irAEs, including myositis, hepatitis, myasthenia gravis, conjunctivitis/uveitis, and colitis.
All three patients were treated with nivolumab either as a single agent or in combination with ipilimumab. Nivolumab has been previously described as an agent commonly triggering ICI-associated myocarditis [1]. In a case series by Mahmood et al., 30% of single-agent treatment ICI-induced myocarditis was caused by nivolumab, while in 75% of combination therapies, nivolumab was present as one of the treatment agents [1].
ECG is a useful inexpensive tool for initial assessment of suspected cases of myocarditis. All three cases showed ECG changes such as ST elevation, T-wave changes, and PVCs. This is in concordance with prior reports about the value of ECG in suggesting the presence of myocarditis [1, 4].
Elevated troponin was present in all three patients. Troponin as a marker of myocyte death, although not specific, is a very sensitive and inexpensive test which can be of great value in suggesting the presence of myocardial injury and myocarditis in the setting of ICI treatment [4]. In case series by Mahmood et al., 94% of cases with myocarditis had elevated troponin levels, and an increase in troponin level to levels > 1.5 ng/ml was associated with four-fold increased risk of myocarditis [1].
Treatment with ICI was discontinued in all three cases after the diagnosis of myocarditis. Resumption after initial discontinuation of ICIs is associated with high rate of recurrent or distinct irAEs and MACE [7]. Treatment with ICI was not rechallenged in any of the cases.
Serious adverse cardiac events occurred in two of our patients. All three patients were treated with high-dose corticosteroids, and in one case, plasma exchange was also performed. Prior studies have demonstrated high-dose corticosteroids and plasma exchange as effective treatment for myocarditis [1, 2, 4].
Echocardiography is typically the initial imaging modality in cases of suspected myocarditis and can provide useful information about myocardial function. While reduced LVEF is commonly seen in the setting of myocarditis, a depressed LVEF at the time of presentation is not a prerequisite for serious cardiac events.
Cardiac MR (CMR) is the imaging modality of choice for assessment of myocarditis [8]. The typical CMR findings in myocarditis include edema and myocardial DE sparing the subendocardial region in a nonischemic distribution [8]. In less severe cases of myocarditis, scarring and DE may be absent [8].
All three of our cases underwent evaluation with CMR during the course of treatment. Reduced LVEF was present in all three cases, but no regional motion abnormality was detected. One case showed myocardial edema on T2 mapping sequence. Delayed enhancement (DE) was abnormal in all CMRs showing areas of scarring in a mid-myocardial and epicardial distribution with sparing of the subendocardial region. Follow-up CMR performed 7 and 14 days after the first CMR in two of the cases did not show any change in the extent, pattern, distribution, and severity of delayed enhancement.
Conclusion
Myocarditis is an uncommon but potentially fatal complication of treatment with ICIs. Diagnostic tools such as blood troponin level, ECG changes, echocardiography, and cardiac MR can help in the diagnosis of ICI-induced myocarditis. Patients usually present with other immune-related adverse events and benefit from high-dose corticosteroid and plasma exchange.
References
Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, Sullivan RJ, Damrongwatanasuk R, Chen CL, Gupta D, Kirchberger MC, Awadalla M, Hassan MZO, Moslehi JJ, Shah SP, Ganatra S, Thavendiranathan P, Lawrence DP, Groarke JD, Neilan TG (2018) Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 71(16):1755–1764 Available from: http://www.sciencedirect.com/science/article/pii/S0735109718333680
Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob J-J, Cowey CL et al (2017) Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 377(14):1345–1356. Available from:. https://doi.org/10.1056/NEJMoa1709684
Awadalla M, Golden DLA, Mahmood SS, Alvi RM, Mercaldo ND, Hassan MZO, Banerji D, Rokicki A, Mulligan C, Murphy SPT, Jones-O'Connor M, Cohen JV, Heinzerling LM, Armanious M, Sullivan RJ, Damrongwatanasuk R, Chen CL, Gupta D, Kirchberger MC, Moslehi JJ, Shah SP, Ganatra S, Thavendiranathan P, Rizvi MA, Sahni G, Lyon AR, Tocchetti CG, Mercurio V, Thuny F, Ederhy S, Mahmoudi M, Lawrence DP, Groarke JD, Nohria A, Fradley MG, Reynolds KL, Neilan TG (2019) Influenza vaccination and myocarditis among patients receiving immune checkpoint inhibitors. J Immunother Cancer 7(1):53
Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y et al (2016) Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 375(18):1749–1755 Available from: https://pubmed.ncbi.nlm.nih.gov/27806233
Zimmer L, Goldinger SM, Hofmann L, Loquai C, Ugurel S, Thomas I et al (2016) Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur J Cancer 60:210–225
Caforio ALP, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB et al (2013) Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 34(33):2636–2648 2648a-2648d
Pollack MH, Betof A, Dearden H, Rapazzo K, Valentine I, Brohl AS et al (2018) Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann Oncol 29(1):250–255
Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT et al (2009) Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol 53(17):1475–1487 Available from: http://www.onlinejacc.org/content/53/17/1475
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
IRB statement
The institutional review board approved the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ansari-Gilani, K., Tirumani, S.H., Smith, D.A. et al. Myocarditis associated with immune checkpoint inhibitor therapy: a case report of three patients. Emerg Radiol 27, 455–460 (2020). https://doi.org/10.1007/s10140-020-01765-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10140-020-01765-6