Abstract
Fungal pneumonias are increasingly common in the population of immunosuppressed patients. The diagnosis of fungal pneumonias represents a challenge for clinicians, and the morbidity and mortality of these infections are high in immunocompromised patients. CT findings may be nonspecific; however, in the appropriate clinical setting, they may suggest and even help establish the specific diagnosis. This article provides an overview about the CT findings and possible differential diagnosis of the most common pulmonary fungal infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although fungal infection accounts for only a small portion of community-acquired and nosocomial pneumonias, it becomes of concern in the expanding population of immunosuppressed patients [1, 2]. Fungi typically enter the lung with inhalation of their spores though they can reach the lung through the bloodstream or can occur as a result of reactivation of a latent infection. Fungal pneumonia is caused by opportunistic or endemic fungi. Opportunistic fungal organisms (e.g., Candida, Aspergillus, Mucor species) tend to cause pneumonia in immunocompromised patients and endemic fungal pathogens cause infection in healthy and in immunocompromised individuals, in defined geographic locations of the Americas and around the world [1]. Histoplasmosis is the most common endemic mycosis in North America, and is followed by coccidioidomycosis, blastomycosis, and cryptococcosis [1]. Endemic fungi are prevalent in the Mississippi River Valley and the Ohio River Valley (e.g., Histoplasma capsulatum, Blastomyces dermatitidis), southwestern United States, and northwestern Mexico (e.g., Coccidioides immitis) [1].
Pulmonary aspergillosis can be subdivided into three categories: (a) noninvasive aspergillosis, occurring in patients with almost normal to normal immune status and includes aspergilloma and allergic bronchopulmonary aspergillosis (ABPA), (b) semi-invasive aspergillosis characterized by tissue necrosis and granulomatous inflammation and usually typically occurs in patients with moderate immunosuppression, and (c) invasive aspergillosis found almost exclusively in severely immunocompromised patients with airway invasion and invasion and occlusion of the pulmonary arteries, leading to the formation of necrotic hemorrhagic nodules or hemorrhagic infarcts [2, 3].
Clinical manifestations and prognosis of fungal pneumonia
In individuals who are neutropenic or immunocompromised, persistent fever may be an early sign of fungal infection [3]. Other manifestations of fungal pneumonia may include cough, chest pain, progressive dyspnea, and hemoptysis. The endemic fungal pneumonias are generally self-limited in healthy hosts and majority of patients recover without treatment. However, patients with fungal pneumonias may develop complications including disease dissemination to other sites, blood vessel invasion, and chronic pulmonary complications like cavitation, pleural effusions, bronchopleural and tracheoesophageal fistulas, and mediastinal fibromatosis. The leading cause of invasive pulmonary lung infection and death among patients who are neutropenic is aspergillosis with a mortality rate of 50–85% [3].
Chest CT findings of fungal infection
Aspergillomas (mycetomas) are manifested on CT by the presence of a solid, round, or oval fungal ball with soft tissue density within a preexistent lung cavity, usually related to tuberculosis and sarcoidosis [4]. The mass is mobile and is usually separated from the wall of the cavity by a gas density, resulting in the “air crescent” sign [5]. Air crescent sign is nonspecific and can be seen in a number conditions like angioinvasive aspergillosis, bacterial pneumonia, cavitating neoplasm, and vasculitis [5] (Fig. 1). Aspergillomas are often associated with wall thickening of the underlying cavity and adjacent pleura.
ABPA characteristic CT finding consists of predominantly upper lobes cystic or varicose bronchiectasis with mucoid impaction giving thick branching perihilar densities also known as glove finger appearance, most commonly in patients with long-standing bronchial asthma [4]. High attenuating or calcified mucus plug within the dilated bronchi can be present in 19–28% of cases and represents a very specific finding on CT that signifies more severe ABPA with associated increased recurrence rate [6, 7] (Fig. 2). Differential diagnosis also includes tuberculosis, actinomycosis, and histoplasmosis [6].
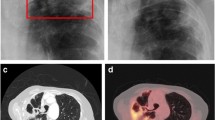
Semi-invasive aspergillosis typically manifests as upper lobe predominant opacities and pleural thickening unilateral or bilateral that progress over to the course of weeks, months, or years to cavitation. The central necrotic material separates away from the surrounding tissues and thus forms an air crescent sign, eventually leading to the formation of a thick-walled cavity/cavities with or without a central mycetoma. Appearances may then be the same as those of an aspergilloma. However, rather than Aspergillus colonizing a pre-existing cavity, in semi-invasive aspergillosis, a focally invasive aspergillosis occurs which undergoes central necrosis and cavitation forming its own cavity [8,9,10] (Fig. 3).
Airway-invasive aspergillosis can manifest clinically as acute tracheobronchitis with CT findings of tracheal or bronchial wall thickening, as bronchiolitis, seen on HRCT as centrilobular and tree-in-bud opacities, and as bronchopneumonia with predominantly perihilar consolidations [5] (Fig. 4).
Angioinvasive aspergillosis characteristic CT findings consist of nodules surrounded by a halo of ground-glass attenuation “halo sign” or pleura-based, wedge-shaped areas of consolidation which corresponds to areas of hemorrhagic infarcts [5] (Fig. 5). A halo sign has been described in other invasive infections including candidiasis, mucormycosis, herpes simplex, and cytomegalovirus as well as other diseases like Wegener granulomatosis, Kaposi sarcoma, and hemorrhagic metastases [6]. Crescentic and circular cavitations within a parenchymal consolidation or nodular opacity can occur 2–3 weeks after initiation of treatment as a result of retraction of the infarcted lung and can be appreciated on CT as “air crescent sign” [6] (Fig. 6). Although “halo sign” and “air crescent sign” are not exclusive to angioinvasive aspergillosis, in the setting of severe neutropenia, they are highly suggestive of the disease [6].
Mucormycosis may be differentiated on CT from other infections, especially aspergillosis by several nonspecific findings including the presence of multiple nodules and pleural effusions and the development of a “reversed halo,” which is a ground-glass attenuation in the center of a nodule with a surrounding zone of consolidation [11] (Fig. 7). The reversed halo has been described in paracoccidioidomycosis, histoplasmosis, cryptococcosis, and sarcoidosis. However, when considered along with the appropriate clinical settings, it is very characteristic of mucormycosis [9].
Candida pulmonary infection may present with multiple nodules and areas of consolidation. These may have smooth or irregular margins and be surrounded by a halo of ground-glass opacity due to hemorrhage [12]. Cavitation and lymphadenopathy are uncommon and miliary pattern may occur [12] (Fig. 8).
Acute histoplasmosis often manifests with nonspecific multifocal areas of consolidation and lymphadenopathy [13] (Fig. 9). The disseminated form of histoplasmosis occurs mainly in immunocompromised patients and typically appears as tiny pulmonary nodules in a miliary distribution which may undergo calcification when healing occurs [14]. Histoplasmoma is a well-recognized pattern of disease. This usually manifests as a single, circumscribed nodule, measuring up to 3 cm, and often contains central “bull’s-eye” or “target” calcification. Chronic pulmonary histoplasmosis occurs almost exclusively in patients with COPD and may appear as upper lobe fibro cavitary consolidation that closely resembles TB [13]. Mediastinal node involvement with histoplasmosis may cause extensive necrosis and fibrosis that may result in fibrosing mediastinitis with narrowing or occlusion of the vessels and airways [13] (Fig. 10).
Coccidioides immitis primary pulmonary infection usually presents as unilateral air-space consolidation, often in the lower lobes that usually shows the tendency to resolve in one area and recur in another (phantom consolidation) [13] (Fig. 11). If the diseases progress, multifocal pneumonia or pulmonary nodules can be seen and may undergo progressive cavitation into a thin-walled cyst (“grape-skin”) [13] (Fig. 12). Disseminated coccidioidomycosis resembles disseminated histoplasmosis, with miliary nodules [14]. Chronic progressive coccidioidomycosis appears as upper lobe consolidation and cavitation that resemble TB and chronic histoplasmosis [14].
Cryptococcus infection in healthy patients usually manifests as one or more peripheral, circumscribed nodules and less commonly as air-space consolidation [13] (Fig. 13). Cavitation is uncommon. Diffuse interstitial pattern with reticulation and nodules may be seen in immunocompromised patients [13].
Blastomyces dermatitidis infection may present as consolidation that tends to be central and abuts the mediastinum [14]. The disease may progress rapidly to multifocal bilateral air-space disease or diffuse alveolar damage [14]. Cavitation may occur and could mimic tuberculosis. A military pattern has been documented [14].
Summary
Fungal infection is a serious disease that is frequently seen in immunocompromised patients. Although imaging findings in various types of fungal infection may be nonspecific, in the appropriate clinical setting, CT findings may suggest and even help establish the specific diagnosis.
References
Chen K, Ko S-C et al (2001) Pulmonary fungal infection: emphasis on microbiological spectra, patient outcome, and prognostic factors. Chest J 120:177–184
Zmeili OS, Soubani AO (2007) Pulmonary aspergillosis: a clinical update. QJM 100:317–334
Kousha M, Tadi R, Soubani AO (2011) Pulmonary aspergillosis: a clinical review. Eur Respir Rev 121:156–174
Dogra V, Sinha AK, Saxena R et al (2016) Aspergillus march: from ABPA to aspergilloma to subacute invasive aspergillosis. Allergy Asthma Clin Immunol 12:64
Franquet T, Müller N, Giménez A et al (2001) Spectrum of pulmonary aspergillosis: histologic, clinical, and radiologic findings. Radiographics 21:825–837
Prasad A,Agarwal K,Deepak D,et al (2016) Pulmonary aspergillosis: what CT can offer before it is too late! J Clin Diagn Res 10(4):TE01–TE05
Walker CM, Abbott GF, Greene RE et al (2014) Imaging pulmonary infection: classic signs and patterns. AJR 202:479–492
Saraceno JL, Phelps DT, Ferro TJ et al (1997) Chronic necrotizing pulmonary aspergillosis: approach to management. Chest 112:541–548
Yella LK, Krishnan P, Gillego V (2005) The air crescent sign: a clue to the etiology of chronic necrotizing pneumonia. Chest 127(1):395–397
Kato T, Usami I, Morita H et al (2002) Chronic necrotizing pulmonary aspergillosis in pneumoconiosis: clinical and radiologic findings in 10 patients. Chest 121(1):118–127
Jung J, Kim MY, HJ L et al (2015) Comparison of computed tomographic findings in pulmonary mucormycosis and invasive pulmonary aspergillosis. Clin Microbiol Infect 21(7):684.e11–684.e18
Choi MH, Jung JI, MD C, Do W et al (2014) Acute pulmonary complications in patients with hematologic malignancies. Radiographics 34:1755–1768
Chong S, Lee K, Yi C et al (2006) Pulmonary fungal infection: imaging findings in immunocompetent and immunocompromised patients. Eur J Radiol 59:371–383
Orlowski H, McWilliams S, Mellnick V et al (2017) Imaging spectrum of invasive fungal and fungal-like infections. Radiographics 37
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Hussien, A., Lin, C.T. CT findings of fungal pneumonia with emphasis on aspergillosis. Emerg Radiol 25, 685–689 (2018). https://doi.org/10.1007/s10140-018-1621-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10140-018-1621-z