Abstract
In this study, we evaluated a consortium of probiotic bacteria as an environmentally-friendly strategy for controlling pathogenic Vibrio species during the brine shrimp incubation period. Probiotic strains were initially selected on basis of (i) their ability to colonize the cyst surfaces, (ii) their absence of cross-inhibitory effects, and (iii) no detrimental effect on cyst hatching. The cysts and nauplius surfaces were immediately colonized after the application of selected probiotic strains, without detrimental effects on survival. Ten probiotic strains were mixed at similar proportions (probiotic consortium) and evaluated at different concentrations into brine shrimp cultures during incubation and early stages of development. Subsequently, these cultures were challenged with Vibrio parahaemolyticus and Vibrio harveyi. The probiotic consortium was effective to reduce the abundance of pathogenic Vibrio species and to prevent the mortality during Vibrio challenges; however, its effect was concentration-dependent and was successful at a starting concentration of 1.8 × 106 CFU/ml. Our results suggest that this probiotic consortium offers an alternative to antimicrobial agents routinely used to reduce the incidence and prevalence of pathogenic Vibrio species in brine shrimp production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although brine shrimp (Artemia spp.) are an important feed resource for fish and shellfish production, their cysts are natural vectors of microorganisms, making them a risk factor for the emergence and spread of diseases in aquaculture. The cyst surface usually contains a variety of microorganisms, including pathogenic bacteria that eventually proliferate during incubation, colonize the nauplius surface, and reach farmed organisms when they are used as food. During the production of nauplii, chlorine is routinely used at theoretically appropriate concentrations to achieve complete disinfection (Sorgeloos and Persoone 1972; Austin and Allen 1982; Dehasque et al. 1991); however, a rapid re-colonization takes place during incubation and hatching periods (Douillet 1995; Griffith 1995; Quiroz-Guzmán et al. 2013). The final microbiota composition of brine shrimp nauplii is therefore directly dependent on microbial composition in the hatching water (Igarashi et al. 1989). This microbiota is particularly dominated by members of the Vibrio genus that proliferate and colonize the nauplii (Austin and Allen 1982; Dehasque et al. 1991; López Torres and Lizárraga-Partida 2001). In fact, it has been reported that Vibrio parahaemolyticus and Vibrio harveyi colonize the cyst surfaces during the first 3 h of exposure, affecting the hatching success and quality of hatched nauplii (Quiroz-Guzmán et al. 2013). Some cyst-associated bacteria are also acquired at the harvest sites and can proliferate in the hatching systems, which could affect the sanitary quality of brine shrimp (Austin and Allen 1982; Høj et al. 2009; Quiroz-Guzmán et al. 2013).
Given this, the use of probiotic bacteria has been proposed as a viable strategy for controlling pathogens in aquaculture. These microorganisms usually exhibit an antimicrobial activity which enables them to compete for available nutrients and adhesion sites with opportunistic or pathogenic microorganisms (Moriarty 1997; Balcázar et al. 2006; Pérez-Sánchez et al. 2014). Probiotic bacteria may also provide additional beneficial effects such as an increased performance of farmed organisms and improved water quality (Qi et al. 2009; Newaj-Fyzul et al. 2014).
Because brine shrimp have been suggested as a suitable way to deliver probiotics to fish and shellfish cultures (Verschuere et al. 1999), some probiotic strains may also confer beneficial effects on their growth, survival, and resistance to diseases (Hameed and Balasubramanian 2000; Patra and Mohamed 2003). However, to the best of our knowledge, previous studies mainly focused on the use of single probiotics, and it is speculative whether mixtures of probiotic strains would be beneficial for brine shrimp production. The aims of this study were therefore to evaluate a consortium of probiotic bacteria as well as evaluate their ability to reduce the abundance of pathogenic Vibrio species and their negative effects during the brine shrimp incubation period.
Material and Methods
Bacterial Strains
Probiotic bacteria used in this study were previously isolated and identified from white shrimp cultures (García-Rodríguez 2003; Balcázar and Rojas-Luna 2007) and the digestive tract of Nile tilapia, Oreochromis niloticus (Apún-Molina et al. 2009). Bacillus subtilis (ATCC 6051) was also included in this study, which was obtained from the American Type Culture Collection.
For the challenge tests, we used four pathogenic strains: Vibrio parahaemolyticus PS-017 (GenBank accession KC740491) and Vibrio harveyi EC11 (GenBank accession KC740490), which were isolated from shrimp larval cultures in Ecuador (Balcázar and Rojas-Luna 2007), and two reference strains, Vibrio parahaemolyticus (ATCC 17802) and Vibrio harveyi (ATCC 14126). All were grown on marine agar (MA) plates, which contained 1 L artificial seawater (Instant Ocean), 0.1%yeast extract, 0.5% meat peptone, and 1.7% agar.
Evaluation of Probiotic Strains for Brine Shrimp
A total of 10 different strains (two Bacillus subtilis UTM 126 and ATCC 6051 strains; two Lactobacillus sp. R18C and R12C strains; and six Lactococcus sp. B10C, Ba12, Be12, CB10Lta, CA10, and CbLt strains) were evaluated by determining (i) their ability to colonize the cyst surfaces, (ii) their absence of cross-inhibitory effects, and (iii) any detrimental effect on cyst hatching. All strains were initially characterized based on their morphological, Gram-staining, and biochemical properties as previously described (Wang et al. 2011).
Colonization of Brine Shrimp Cysts
The ability to colonize the cyst surface was evaluated as previously described (Quiroz-Guzmán et al. 2013). Briefly, bacteria-free cysts were dispensed into tubes with sterile artificial seawater (SASW), inoculated with different bacterial concentrations ranging from 2 × 105 (C1), 1.8 × 106 (C2), 9 × 106 (C3) to 9 × 107 CFU/ml (C4) and incubated at 28 °C for 15 h under continuous illumination in a tube rotor (Labquake, Thermo Fisher, Waltham, MA USA). Unhatched cysts and nauplii were harvested under aseptic conditions and rinsed thoroughly two times with SASW. Cysts and nauplii were re-suspended in 3 ml sterile seawater and homogenized using a cell disruptor (Biospec Products, Bartlesville, UK). Ten-fold serial dilutions were plated on marine agar (MA) and incubated at 30 °C for 24 h. Each strain and dose was tested in triplicate.
A parallel assay was performed using the above-mentioned conditions in order to obtain samples for electron microscopy. Samples were then collected every hour under sterile conditions and fixed in 5% glutaraldehyde, dehydrated with ethanol solutions (30, 50, 70, and 100%), and dried in a critical point drying system (Samdri-PVT-3B, Tousimis, Rockville, MD, USA). Each sample was placed in an aluminum sample holder with double-sided tape and twice coated with palladium ions for 35 s in a metal evaporator (Sputter Coater, Denton Vacuum Desk II, Moorestown, NJ, USA). The coated samples were observed under a scanning electron microscope (S-3000N, Hitachi High-Technologies, Tokyo, Japan) with a secondary electron detector.
Cross-Inhibitory Effects
The cross-inhibitory effects of potential probiotic strains were evaluated by the double-layer technique (Dopazo et al. 1988). The inhibitory effect of these probiotic strains was also evaluated against pathogenic Vibrio species. Briefly, MA plates carefully punctured were inoculated with each strain and incubated at 30 °C for 24 h. The resulting macro-colonies were killed by exposure to chloroform vapors during 20 min and poured with 10-ml soft media (MA at 50 °C) previously inoculated with 104 CFU/ml of each probiotic strain. After the plates were solidified, they were incubated at 30 °C for 24 h. The antagonistic effect was considered as the absence of growth around the punctures.
Probiotic Mixture
The probiotic mixture, composed of two Bacillus, two Lactobacillus, and six Lactococcus strains, was obtained from 24-h cultures on marine agar (MA) plates. Bacterial cells were resuspended in sterile seawater, and suspensions were then spectrophotometrically adjusted to OD585 = 1 in order to standardize the number of bacteria (~ 107 CFU/ml). Dilution plating was used to verify the relationship between optical density at 585 nm and CFU/ml.
Effects of Probiotic Consortium During Cyst Incubation
The effects of probiotics on hatching and early survival of brine shrimp were evaluated under monoxenic conditions. One hundred bacteria-free cysts were placed in 10-ml tubes with sterile seawater. They were then inoculated with probiotic strains, previously mixed (probiotic consortium), at different concentrations ranging from 2 × 105 (C1), 1.8 × 106 (C2), 9 × 106 (C3) to 9 × 107 CFU/ml (C4). All assays were performed in quadruplicate. The inoculums were obtained from 24-h cultures on MA plates, which were harvested in sterile seawater and adjusted to OD585 = 1. The inoculated cysts were incubated at 28 °C in a rotary shaker under continuous illumination (at 1500 lx). The hatching rate and survival were recorded for 24 h, and negative controls were simultaneously evaluated.
Effect of Probiotic Consortium During Cyst Incubation and Exposed to Vibrio Species
To determine the capacity of probiotic consortium for colonizing and competing against opportunistic pathogens during cyst incubation, bacteria-free cysts were placed in tubes (100 cysts per tube) with 10 ml of sterile seawater and were inoculated with 100 μl of a suspension containing Vibrio species (1 × 107 CFU/ml). Different concentrations ranging from 2 × 105 (C1), 1.8 × 106 (C2), 9 × 106 (C3) to 9 × 107 CFU/ml (C4) of probiotic consortium were also included in the tubes, and each assay was carried out in triplicate for each Vibrio strain. The tubes were kept during 20 h in a rotary shaker at 28 °C under continuous light. The hatching rate and larvae survival were recorded (at 5 h post-hatching), and negative controls were also included during each assay.
Effect of Probiotic Consortium During Nauplius Stages and Exposed to Vibrio Species
To determine the capacity of probiotic consortium for colonizing and competing against opportunistic pathogens during the hatching, gnotobiotic nauplii were obtained from axenic cysts, which were exposed to a suspension of probiotics at different concentrations ranging from 2 × 105 (C1), 1.8 × 106 (C2), 9 × 106 (C3) to 9 × 107 CFU/ml (C4). The incubation was done in a sterile glass chamber provided with continuous illumination and aeration (air was filtered through a 0.2 μm membrane). The chamber was maintained in a water bath at 28 °C until hatching (20 h). The nauplii were then aseptically transferred to sterile polycarbonate culture vessels with 100 ml of seawater at a density of 1 nauplius per milliliter, each vessel was inoculated with 100 μl of a suspension of V. parahaemolyticus or V. harveyi (OD585 = 1) and maintained at 28 °C. The number of surviving nauplii was recorded at 48 h. Each Vibrio strain was assayed in triplicate, and positive controls (infected with Vibrio species) and blanks (axenic) were included.
Statistical Analysis
Normality and homoscedasticity were evaluated using the Kolmogrov-Smirnov and Bartlett test, respectively. Data were then analyzed using one-way ANOVA and Tukey’s multiple comparison test implemented in the Statistica 7.0 software (Statsoft Inc. USA).
Results
Characterization of Probiotic Strains
All strains were Gram-positive, facultative anaerobic, and round- or rod-shaped bacteria. Moreover, all strains formed cream-colored, circular, smooth, and convex colonies on MA plates. The physiological and biochemical features confirmed the affiliation of UTM 126 to the genus Bacillus, R18C and R12C strains to the genus Lactobacillus, and B10C, Ba12, Be12, CB10Lta, CA10, and CbLt strains to the genus Lactococcus.
Colonization of Brine Shrimp Cysts
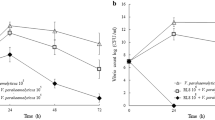
Probiotic strains were evaluated to determine their colonization capacity, which apparently began in the bursting area (Fig. 1). Cysts and nauplii were immediately colonized by probiotic bacteria ranging from 106 to 108 CFU/cyst-nauplius (Fig. 2), whose numbers directly correlated (p < 0.05) with the number of inoculated bacteria (R = 0.99).
Electron micrographs of cysts and nauplii colonized by probiotic bacteria: a cysts axenic, b no axenic cysts, c cysts colonized by probiotic bacteria at 2 h of inoculation in the bursting area. d Metanauplius cyst colonized during hatching. e, f Enlargements of the corresponding areas. Scale (1 μm); working distance (14.5 mm)
Cross-Inhibitory Effects
Because an antagonistic effect was not detected among probiotic strains, they were mixed (probiotic consortium) for further analysis. However, none of the probiotic strains had an antagonistic effect against V. parahaemolyticus and Vibrio harveyi strains under in vitro conditions.
Effect of Probiotic Consortium During Cyst Incubation
Administration of probiotic consortium showed a high survival of brine shrimp at two stages of development: (i) cysts and (ii) post-hatching. A 90% survival was observed at 1.8 × 106 CFU/ml, without significant differences (p > 0.05) with respect to the control; however, significant differences (p < 0.05) were obtained at higher concentrations (9 × 106 and 9 × 107 CFU/ml) with respect to the control, which yielded a hatching and survival rate of 93%. Moreover, administration of probiotic consortium also resulted in better hatching of nauplii, because they reached the nauplius II stage whereas most hatched organisms remained in the umbrella or metanauplius I stages in the control (Fig. 3).
Percentage of cyst hatching (white bars) and nauplius survival (gray bars) under treatment with a probiotic consortium at different concentrations ranging from 0 (C0), 2 × 105 (C1), 1.8 × 106 (C2), 9 × 106 (C3) to 9 × 107 CFU/ml (C4). Data are based on the means ± standard deviations from four independent assays (*p < 0.05)
Effects of Probiotic Consortium on Cysts Exposed to Vibrio Species
Administration of probiotic consortium revealed a concentration-dependent effect to reduce the mortality caused by V. parahaemolyticus strains (Fig. 4) and V. harveyi strains (Fig. 5) in cysts. In general terms, survival of treated cysts at 2 × 105 was not significantly different (p > 0.05) from the control; however, significant differences (p < 0.05) were obtained at higher concentrations (particularly at 9 × 106 and 9 × 107 CFU/ml) with respect to the control, which completely avoided the adverse effects of Vibrio species.
Percentage of hatching and survival of brine shrimp cysts experimentally infected with 5.5 × 106 CFU/ml of Vibrio parahaemolyticus PS-017 (a, b) and ATCC 17802 (c, d) strains under treatment with a probiotic consortium at different concentrations ranging from 0 (C0), 2 × 105 (C1), 1.8 × 106 (C2), 9 × 106 (C3) to 9 × 107 CFU/ml (C4). NC, negative control. Data are based on the means ± standard deviations from three independent assays. Asterisks denote statistical differences between control (C0) and treated groups (*p < 0.05)
Percentage of hatching and survival of brine shrimp cysts experimentally infected with 5.5 × 106 CFU/ml of Vibrio harveyi EC11 (a, b) and ATCC 14126 (c, d) strains under treatment with a probiotic consortium at different concentrations ranging from 0 (C0), 2 × 105 (C1), 1.8 × 106 (C2), 9 × 106 (C3) to 9 × 107 CFU/ml (C4). NC, negative control. Data are based on the means ± standard deviations from three independent assays. Asterisks denote statistical differences between control (C0) and treated groups (*p < 0.05)
Effects of Probiotic Consortium on Nauplii Exposed to Vibrio Species
Although the results showed that administration of probiotic consortium increases the survival of nauplii exposed to Vibrio species, the effect was concentration-dependent. Survival of treated nauplii at 2 × 105 and 1.8 × 106 CFU/ml was not significantly different (p > 0.05) from the control; however, significant differences (p < 0.05) were obtained at higher concentrations (9 × 106 and 9 × 107 CFU/ml) with respect to the control (Fig. 6).
Survival of brine shrimp nauplii experimentally infected with V. parahaemolyticus PS-017 (a) and ATCC 17802 (b) strains or V. harveyi EC11 (c) and ATCC 14126 (d) strains under treatment with a probiotic consortium at different concentrations ranging from 0 (C0), 2 × 105 (C1), 1.8 × 106 (C2), 9 × 106 (C3) to 9 × 107 CFU/ml (C4). NC, negative control. Data are based on the means ± standard deviations from four independent assays. Asterisks denote statistical differences between control (C0) and treated groups (*p < 0.05)
Discussion
The present study suggests that the manipulation of microbial communities through the use of a probiotic consortium may be beneficial during and after the hatching of brine shrimp, as probiotic bacteria may provide protection by competitive exclusion, thereby blocking adhesion and spread of opportunistic pathogens (e.g., Vibrio species). Moreover, previous studies have suggested that various factors can influence the growth of brine shrimp particularly their associated microbial communities (Douillet 1995; Intriago and Jones 1993; Rico-Mora and Voltolina 1995; Gorospe et al. 1996; Verschuere et al. 1999), which support our results.
Because pathogenic and opportunistic bacteria may colonize the brine shrimp cysts and nauplius surfaces, the sanitary quality of brine shrimp may be compromised, as they are widely used as feed for fish and shellfish production. Brine shrimp cysts naturally release nutrient compounds during hatching, which favor bacterial growth. It has been described that administration of bacterial strains with probiotic properties may promote the growth and survival of brine shrimp (Intriago and Jones 1993; Douillet and Langdon 1994). In the present study, a selective colonization was achieved with each of the tested strains, which was confirmed by the use of traditional microbiological methods and scanning electron microscopy, demonstrating that each strain and the mixture (probiotic consortium) adhere to the cysts during incubation (1 h after inoculation). These observations also demonstrated that the probiotic consortium has the ability to penetrate to the embryo and colonize its surface before hatching. This early colonization may favor competitive exclusion of opportunistic pathogens during the brine shrimp hatching.
In order to confirm these observations, gnotobiotic brine shrimp models at hatching and nauplius stages were applied to explore the beneficial properties of the probiotic consortium. We found that this consortium significantly improved survival of brine shrimp (at both stages) challenged with pathogenic V. parahaemolyticus and V. harveyi strains. Similar results were described by Mahdhi et al. (2011), who observed that a consortium of Bacillus strains was able to protect gnotobiotic brine shrimp against pathogenic Vibrio alginolyticus. Likewise, Avella et al. (2010) demonstrated that administration of a Bacillus mixture has beneficial effects on sea bream larvae in terms of growth and stress tolerance. Giarma et al. (2017) demonstrated that the administration of Bacillus subtilis, Lactobacillus plantarum, or Lactococcus lactis was able to protect brine shrimp nauplii against pathogenic Vibrio anguillarum by enhancing the activity of antioxidant enzymes superoxide dismutase, glutathione reductase, glutathione transferase, and phenoloxidase which contributed to reduce oxidative damage and increased survival.
Although previous studies have suggested that probiotics provide protection through the creation of a hostile environment for pathogens by the production of antimicrobial compounds or by competing for essential nutrients and adhesion sites (Lauzon et al. 2014; Pérez-Sánchez et al. 2014), none of the probiotic strains showed an inhibitory effect against pathogenic V. parahaemolyticus and Vibrio harveyi strains under in vitro conditions; however, an antagonistic effect in vivo was observed when they were mixed. Previous studies have suggested that in vitro activity cannot be used to predict a possible in vivo effect (Gram et al. 2001). Touraki et al. (2012) administered B. subtilis or Lb. plantarum to Artemia nauplii, which exhibited in vitro antagonism against V. anguillarum but only B. subtilis offered efficient in vivo protection. Because the inhibitory effect of the probiotic consortium was likely of little influence in mediating the improved disease resistance, competitive exclusion could be attributed to other mechanisms such as colonization capacity, thereby blocking adhesion and spread of pathogens or triggering cell-signaling events that deactivate the production of virulence factors (Balcázar et al. 2007).
Conclusions
The probiotic consortium was composed of bacteria with potential probiotic properties, whose application allowed the manipulation of associated microbiota in brine shrimp cultures. In fact, the probiotic consortium reduced the abundance and impact of pathogenic Vibrio species in brine shrimp at two stages of development (cysts and nauplii). These characteristics may be attributed to their ability to colonize mucosal surfaces as well as to compete for available nutrients. Moreover, administration of this probiotic consortium may provide beneficial effects on brine shrimp cultures, which would justify its extensive use.
References
Apún-Molina JP, Santamaría-Miranda A, Luna-González A, Martínez-Díaz SF, Rojas-Contreras M (2009) Effect of potential probiotic bacteria on growth and survival of tilapia L., cultured in the laboratory under high density and suboptimum temperature. Aquacult Res 40(8):887–894
Austin B, Allen DA (1982) The microbiology of laboratory hatched brine shrimp Artemia. Aquaculture 26:369–383
Avella MA, Gioacchini G, Decamp O, Makridis P, Bracciatelli C, Carnevali O (2010) Application of multi–species of Bacillus in sea bream larviculture. Aquaculture 305:12–19
Balcázar JL, Rojas-Luna T (2007) Inhibitory activity of probiotic Bacillus subtilis UTM 126 against Vibrio species confers protection against vibriosis in juvenile shrimp (Litopenaeus vannamei). Curr Microbiol 55:409–412
Balcázar JL, Vendrell D, de Blas I, Ruiz-Zarzuela I, Gironés O, Múzquiz JL (2007) In vitro competitive adhesion and production of antagonistic compounds by lactic acid bacteria against fish pathogens. Vet Microbiol 122:373–380
Balcázar JL, de Blas I, Ruiz-Zarzuela I, Cunningham D, Vendrell D, Múzquiz JL (2006) The role of probiotics in aquaculture. Vet Microbiol 114:173–186
Dehasque M, Verdonck L, Sorgeloos P, Swings J, Léger Ph, Kersters K (1991) Determination of the bacterial contamination in live food production system and in marine fish hatcheries in Southern Europe. In: Lavens P, Sorgeloos P, Jaspers E, Ollevier F (eds) Larvi’91–Fish & Crustacean Larviculture Symposium. European Aquaculture Society, Special Publication No. 15, Ghent, Belgium, pp 399–402
Dopazo C, Lemos M, Lodeiros C, Bolinches J, Barja J, Toranzo A (1988) Inhibitory activity of antibiotic-producing marine bacteria against fish pathogens. J Appl Bacteriol 65:97–101
Douillet P (1995) Microbial management in marine fish larviculture. In: Lavens P, Jaspers E, Roelants I (eds) Larvi’95–Fish & Shellfish Symposium. European Aquaculture Society, Special Publication No. 24, Ghent, pp 477
Douillet PA, Langdon CJ (1994) Use of a probiotic for the culture of larvae of the pacific oyster (Crassostrea gigas Thunberg). Aquaculture 119:25–40
García-Rodríguez R (2003) Relevancia de las bacterias ácido lácticas en los diferentes estadios del cultivo del camarón. Thesis Fishery Engineering Studies, UABCS, La Paz, B.C.S., Mexico
Giarma E, Amanetidou E, Toufexi A, Touraki M (2017) Defense systems in developing Artemia franciscana nauplii and their modulation by probiotic bacteria offer protection against a Vibrio anguillarum challenge. Fish Shellfish Immunol 66:163–172
Gorospe JN, Nakamura K, Abe M, Higashi S (1996) Nutritional contribution of Pseudomonas sp. in Artemia culture. Fish Sci 62:914–918
Gram L, Løvold T, Nielsen J, Melchiorsen J, Spanggaard B (2001) In vitro antagonism of the probiont Pseudomonas fluorescens strain AH2 against Aeromonas salmonicida does not confer protection of salmon against furunculosis. Aquaculture 199:1–11
Griffith DRW (1995) Microbiology and the role of probiotics in Ecuadorian shrimp hatcheries. In: Lavens P, Jaspers E, Roelants I (eds) Larvi’95–Fish & Shellfish Symposium. European Aquaculture Society, Special Publication No. 24, Ghent, pp 478
Hameed AS, Balasubramanian G (2000) Antibiotic resistance in bacteria isolated from Artemia nauplii and efficacy of formaldehyde to control bacterial load. Aquaculture 183:195–205
Høj L, Bourne D, Hall MR (2009) Localization, abundance and community structure of bacteria associated with Artemia: effects of nauplii enrichment and antimicrobial treatment. Aquaculture 293:278–285
Igarashi MA, Segugita H, Deguchi Y (1989) Microflora associated with eggs and nauplii of Artemia salina. Nippon Suisan Gakkaishi 55:20–45
Intriago P, Jones DA (1993) Bacteria as food for Artemia. Aquaculture 113:115–127
Lauzon HL, Pérez-Sánchez T, Merrifield DL, Ringø E, Balcázar JL (2014) Probiotic applications in cold water fish species. In: Merrifield D, Ringø E (eds) Aquaculture nutrition: gut health, probiotics and prebiotics. Wiley, Chichester, pp 223–252
López Torres MA, Lizárraga-Partida ML (2001) Bacterial isolated on TCBS media associated with hatched Artemia cysts of commercial brands. Aquaculture 194:11–20
Mahdhi A, Hmila Z, Chaieb K, Kamoun F, Bakhrouf A (2011) Probiotic properties of halophilic Bacillus strains enhance protection of Artemia culture against pathogenic Vibrio. Aquat Biol 13:225–231
Moriarty DJW (1997) The role of microorganisms in aquaculture ponds. Aquaculture 151:333–349
Newaj-Fyzul A, Al-Harbi AH, Austin B (2014) Review: developments in the use of probiotics for disease control in aquaculture. Aquaculture 431:1–11
Patra SK, Mohamed KS (2003) Enrichment of Artemia nauplii with the probiotic yeast Saccharomyces boulardii and its resistance against a pathogenic Vibrio. Aquac Int 11:505–514
Pérez-Sánchez T, Ruiz-Zarzuela I, de Blas I, Balcázar JL (2014) Probiotics in aquaculture: a current assessment. Rev Aquac 6:133–146
Qi Z, Zhang XH, Boon N, Bossier P (2009) Probiotics in aquaculture of China – current state, problems and prospect. Aquaculture 290:15–21
Quiroz-Guzmán E, Balcázar JL, Vázquez- Juarez R, Cruz-Villacorta AA, Martínez-Díaz SF (2013) Proliferation, colonization and detrimental effects of Vibrio parahaemolyticus and Vibrio harveyi during brine shrimp hatching. Aquaculture 406–407:86–90
Rico-Mora R, Voltolina D (1995) Effects of bacterial isolates from Skeletonema costatum cultures on the survival of Artemia franciscana nauplii. J Invertebr Pathol 66:203–204
Sorgeloos P, Persoone G (1972) Three simple devices for aquatic invertebrates and fish larvae with continuous recirculation of the medium. Mar Biol 15:251–254
Touraki M, Karamanlidou G, Karavida P, Chrysi K (2012) Evaluation of the probiotics Bacillus subtilis and Lactobacillus plantarum bioencapsulated in Artemia nauplii against vibriosis in European sea bass larvae (Dicentrarchus labrax, L.). World J Microbiol Biotechnol 28:2425–2433
Verschuere L, Rombaut G, Huys G, Dhont J, Sorgeloos P, Verstraete W (1999) Microbial control of the culture of Artemia juveniles through preemptive colonization by selected bacterial strains. Appl Environ Microbiol 65:2527–2533
Wang J, Ji H, Zhang D, Liu H, Wang S, Shan D et al (2011) Assessment of probiotic properties of lactobacillus plantarum ZLP001 isolated from gastrointestinal tract of weaning pigs. Afr J Biotechnol 10(54):11303–11308
Acknowledgments
This research was supported by projects SIP 20100865 and CONACyT 085033. EQG thanks CONACyT for the support through grant number, 34984, and the Secretariat for Research and Graduate studies (SIP-IPN) for the support through PIFI grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Quiroz-Guzmán, E., Vázquez-Juárez, R., Luna-González, A. et al. Administration of Probiotics Improves the Brine Shrimp Production and Prevents Detrimental Effects of Pathogenic Vibrio Species. Mar Biotechnol 20, 512–519 (2018). https://doi.org/10.1007/s10126-018-9822-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-018-9822-8