Abstract
Monosex culture, common in animal husbandry, enables gender-specific management. Here, production of all-female prawns (Macrobrachium rosenbergii) was achieved by a novel biotechnology comprising three steps: (a) A single injection of suspended hypertrophied androgenic gland cells caused fully functional sex reversal of females into “neo-males” bearing the WZ genotype; (b) crossing neo-males with normal females (WZ) yielded genomically validated WW females; and (c) WW females crossed with normal males (ZZ) yielded all-female progeny. This is the first sustainable biotechnology for large-scale all-female crustacean aquaculture. The approach is particularly suited to species in which females are superior to males and offers seedstock protection, thereby ensuring a quality seed supply. Our technology will thus revolutionize not only the structure of the crustacean aquaculture industry but can also be applied to other sectors. Finally, the production of viable and reproducible females lacking the Z chromosome questions its role, with respect to sexuality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The availability of monosex crustacean populations offers advantages over mixed cultures since males and females of most species can be distinguished according to various parameters, including behavior, specific growth rate, final harvest size (Sagi and Aflalo 2005), and food conversion ratios (Moss et al. 2002; Moss and Moss 2006). As such, monosex populations are subject to ever-increasing demand due to commercial considerations (Hansford and Hewitt 1994; Ventura and Sagi 2012), such as yield improvement (Sagi et al. 1986), as well as for ecological purposes, such as sustainable biological control over pests, like human parasite-containing snails (Alkalay-Savaya et al. 2014). All-male populations of the giant freshwater prawn Macrobrachium rosenbergii are desired since males grow larger and hence yield better economic return (Nair et al. 2006). Generation of such populations require relatively low animal stocking densities (Malecha 2012) and may involve periodic selective harvesting (Malecha 1986) due to wide size variation, territoriality, and aggressiveness (Malecha 1986, 2012; Ventura et al. 2011b). On the other hand, all-female populations were proposed to have high commercial potential where culture is intensified since females are much less aggressive and, therefore, can be cultured at higher densities than mixed or all-male populations (Malecha 2012). Moreover, females exhibit homogenous size distribution at harvest, such that selective harvest would not be required (Gopal et al. 2010; Malecha 2012; Otoshi et al. 2003; Sagi et al. 1986).
A first step in the production of a monosex crustacean population involves manipulation of the androgenic gland (AG). First described by Cronin (1947), the AG functions as a major endocrine switch since its presence induces development of the male reproductive system, while its absence permits feminization (Charniaux-Cotton 1954; Manor et al. 2007; Sagi et al. 1997), regardless of the composition of the sex chromosomes. The various roles of the AG are mediated by the insulin-like androgenic gland hormone (IAG) (Ventura et al. 2011a), including the development of appendix masculina (AM) on the second pleopods. Development of the AM is assumed to be correlated with AG function since AG-implanted females generate AMs (Nagamine et al. 1980), while silencing IAG prevented its regeneration (Ventura et al. 2009). Hence, following AM development offers a reliable tool for evaluating successful AG manipulation.
The AG is acting within an endocrine axis defined as the eyestalk-androgenic gland-testis axis. Specific eyestalk-derived neuropeptides produced at the X-organ and stored and secreted from the Sinus gland (Keller 1992; Khalaila et al. 2002) are thought to regulate AG activity, including IAG synthesis and secretion. This notion is supported by the fact that eyestalk ablation results in a hypertrophied and hyperplastic AG (Khalaila et al. 2002; Sroyraya et al. 2010) termed “hAG” as well as overexpression of AG-specific genes (Chung et al. 2011; Rosen et al. 2013).
In the following study, we demonstrate a novel biotechnology for the production of viable M. rosenbergii neo-males, produced by a single injection of suspended hAG cells, which gave rise to large-scale production of WW females. These Z-chromosome free homogametic females were shown to produce all-female progeny.
Materials and Methods
Animals
Macrobrachium rosenbergii blue claw males (40 ± 5 g) were reared in 600-L tanks at 28 ± 2 °C with constant aeration, a light regime of 14:10 (L/D) and were fed ad libitum (shrimp pellets comprising 30 % protein) at the R&D facilities of Enzootic Holdings, Ltd. Young post-metamorphosis M. rosenbergii individuals (i.e., post larvae (PL)) were reared in a 3.5 m3 U-shaped tank, and maintained as above.
Androgenic Gland Hypertrophy and Enzymatic Cell Dissociation
Hypertrophy and hyperplasia of M. rosenbergii blue claw male AGs was achieved by surgical removal of the neuroendocrine X organ-sinus gland complex, located in the eyestalk. Eight days post-endocrine manipulation, the induced males were anesthetized for 15 min in ice-cold water supplemented with 0.2 % hypochlorite for disinfection purposes. Thereafter, the animals were dissected and their hAGs were isolated under a dissecting microscope. Subsequently, hAG cells were separated by means of enzymatic dissociation. Briefly, all hAGs were pooled into a single tube and placed on ice. Thereafter, 1 mL of a specific enzyme mix containing antibiotics [Leibovitz L-15 medium with L-glutamine, 0.1 % (w/v) collagenase type I, 0.1 % (w/v) collagenase type IV, and penicillin-streptomycin solution] was added. The reaction tube was then centrifuged at a speed of 25 RPM for 40 min at room temperature (RT), and then at 2000 RPM for an additional 5 min. Following centrifugation, the tube was placed in a sterilized biological laminar flow hood. The upper phase was removed and the cell pellet was washed by re-suspension in 1 mL of feeding medium [Leibovitz L-15 medium with L-glutamine, 10 % (v/v) fetal bovine serum, and penicillin-streptomycin solution] and centrifuged at 2000 RPM for 5 min at RT. This washing procedure was thrice repeated. Finally, the hAG cells were re-suspended in 500 μL of feeding medium.
Cell Counting
To determine cell concentration and viability, an aliquot of hAG cells from the above preparation was stained with Trypan blue solution at a final concentration of 0.08 % and then loaded on a hemocytometer for examination in a light microscope at a magnification of ×100.
AG Primary Cell Culture
To evaluate cell viability after a passage through a capillary and throughout a primary culture period, 10-μL aliquots of suspended hAG cells in medium were either seeded in a 24-well plate coated with 20 μg/mL poly D-lysine (PDL) at a density of ∼1 × 104 cells per well or first loaded into a micro-injector apparatus, passed through the micro-injector glass capillary into a 1.5-mL tube and then seeded in a 24-well plate at a density of ∼1 × 104 cells per well. Of the collected cells, an aliquot was allocated for viability assessment and stained as described above. The hAG cells were grown in a CO2-free incubator at 27 °C. Twenty-four hours after seeding and thereafter, the growth medium was partially replaced daily. Overall, hAG cells were maintained for 21 days, during which time they were monitored under an inverted light microscope and their morphology, density, and interactions were documented.
Injection of hAG Cell Suspensions into PLs
The ability to isolate and grow hAG cells and then use them to induce sex reversal was examined. Mixed populations (males and females) of PL60 or earlier (n = 913) were injected with ∼2 × 103 hAG cells each. In general, each PL was restrained on a plasticine surface. Using a micro-injector apparatus, while observing through a dissecting microscope, hAG cells from the primary culture were suspended and administrated via a single injection into the muscular tissue of the first abdominal segment. Thereafter, the injected PLs were divided into two groups: the first, comprising the majority of PLs (n = 883), were kept in a rectangular earthen pond (∼250 m2 with a water depth of 1 m) at the Ministry of Agriculture Aquaculture research facilities at Dor, Israel, for grow-out. The second group, comprising representative PLs (n = 30), were kept in a 3.5-m3 volume U-shaped tank. These animals were examined every 2 months and followed up for sex reversal into suspected neo-males.
Appendix Masculina and Male Gonopore Examination
To evaluate masculine features, the second pleopod (swimming leg) was removed using fine tweezers and the presence or lack of the AM was confirmed under a light microscope. In mature neo-males (1 year of age) with confirmed AM, a regeneration assay was performed in which the animals were allowed to molt and AM regeneration was examined. To determine the presence of male gonopores at the base of the fifth pereiopods (walking leg), a PL was placed on its dorsal side and the gonopores were sought under a dissecting microscope.
Determining Sex Genotype Using Specific DNA Markers
Genomic DNA (gDNA), extracted from dissected pleopods with a REDExtract-N-Amp Tissue PCR Kit (Sigma, Rehovot, Israel), was used as a template for PCR amplification with either W- or Z-specific DNA sex primers to determine genotype (Ventura et al. 2011a). PCR products were separated on a 2 % agarose gel, stained with ethidium bromide and visualized on a UV table.
Histology and Immunohistochemistry
Testes, together with the proximal sperm duct (vas deferens) and fifth pereiopods, were dissected from neo-males (1 year of age), as were the fifth pereiopods from an adult eyestalk-ablated normal male presenting hAG as control. Testes tissue samples were fixed in modified Carnoy’s II solution [60 % (v/v) ethanol, 30 % (v/v) chloroform, 10 % (v/v) acetic acid, and 2 % (v/v) formaldehyde] for 24 h. Tissue samples from fifth pereiopods were fixed in 4 % buffered formalin for 48 h. Samples were gradually dehydrated through a series of increasing alcohol concentrations, incubated with xylene and embedded in Paraplast (Kendall, Mansfield, MA) according to conventional procedures. Five-micrometer-thick sections were cut and laid onto silane-coated slides (Menzel-Gläser, Braunschweig, Germany). Consecutive sections were stained with hematoxylin and eosin for morphological observations. In two of the neo-males tested, only one of five consecutive slides were hematoxylin and eosin-stained as described above while other selected slides, along with sections from a normal male’s fifth pereiopod serving as reference, were analyzed by immunohistochemistry using rabbit α-Mr-IAG antibodies, as well as DAPI for nuclear counter-staining, as previously described (Ventura et al. 2011b).
Male-Related Gene Expression in Neo-Males
The testes, fifth walking legs, hepatopancreas, muscle, and cuticle were dissected from a representative neo-male of each of the known M. rosenbergii morphotypes described by Kuris et al. (1987), namely blue claw males (BC), orange claw males (OC), and small males (SM). RNA was extracted from each tissue using an EZ-RNA Total RNA Isolation Kit (Biological Industries, Beit Ha’emek, Israel) according to the manufacturer’s instructions. Complementary DNA (cDNA) was synthesized by reverse transcription using a qScript cDNA Kit (Quanta BioSciences, Gaithersburg, MD) according to the manufacturer’s instructions with 1 μg of total RNA. The cDNA was amplified by PCR (94 °C for 3 min, followed by 40 cycles of 94 °C for 30 s, 57 °C for 30 s, and 72 °C for 45 s, and then by a final elongation step of 72 °C for 10 min) with 1 μL of forward primer, 1 μL of reverse primer, 12.5 μL of Ready Mix REDTaq (Sigma) and water to a final volume of 25 μL. Spatial expression of Mr-IAG (FJ409645) was performed with the following specific primers: Mr-IAG-F: 5′-ATGGGATACTGGAATGCCGAG-3′ and Mr-IAG-R: 5′-CTGGAACTGCAGGTGTTAACG-3′, while the expression of Mar-mrr (DQ066890), M. rosenbergii male reproduction-related gene (Cao et al. 2006), was performed with the following specific primers: Mar-mrr-F: 5′-TCTCTGAAGCTGCAAGTGATTTAC-3′ and Mar-mrr-R: 5′-AATCTGGGTCATTCTCCTGATTGG-3′). Expression of Mr-actin as a positive control (AF221096) was performed using the following specific primers: Mr-actin-F: 5′-GAGACCTTCAACACCCCAGC-3′ and Mr-actin-R: 5′-TAGGTGGTCTCGTGAATGCC-3′. PCR products were separated on 1.8 % agarose gels, stained with ethidium bromide, and visualized on a UV table.
Crossing Neo-males with Normal Females
Macrobrachium rosenbergii neo-males were stocked with normal females in communal tanks (3.5–10 m3). Once a week, the females were collected from the tank after which time, only egg-bearing females were removed into individual glass tanks. The females were monitored daily until egg color had changed from orange to gray, at which point they were transferred to spawning tanks of saline water (12–15 ppt). After hatching, the females were removed and larvae culture was initiated (Ventura et al. 2011a). To determine whether a female was indeed fertilized by a neo-male, each progeny was kept separately, and immediately upon metamorphosis, the PLs were genetically characterized using the genomic sex marker described above to verify that WW PLs were indeed present and to exclude the possibility of the female being fertilized by a normal ZZ male. A population of 600 animals from one of the above-mentioned progenies (“population 1”) was grown in an earthen pond (at Dor station, as described above) for a period of 3 months (August to October). Upon harvest, 378 were phenotypically sorted into males and females according to gonopore identification, under a laboratory dissecting microscope. The remaining progenies (”population 2,” “population 3,” etc.) were grown in 600-L tanks in the R&D facilities of Enzootic Holdings, Ltd. Samples from four populations (n 1 = 174, n 2 = 40, n 3 = 40, n 4 = 20) were collected and genetically identified according to the genomic sex markers described above. The observed sex phenotype ratios of population 1, (expected to be females (75 %) and males (25 %)), as well as sex genotype ratios of the samples from populations 1 to 4, (expected to be WZ (50 %), WW (25 %), and ZZ (25 %)), were statistically tested by a chi-square goodness of fit test, relative to the expected ratios, using Statistica v9.0 (StatSoft, Tulsa, OK).
Crossing WW Females with Normal Males to Produce an All-Female Population
WW females were grown to sexual maturity and crossed with normal ZZ males, in a 3.5-m3 U-shaped tank to obtain 100 % all-female WZ progeny. WW-fertilized females were separated into different tanks and after hatching, a sample of the larval population from the progeny of each female was genotypically examined using the genomic sex marker as mentioned above.
Fecundity Measurements
Successfully fertilized WW females and fertilized WZ normal females were tested for fecundity by weighing the animals before and after the eggs were either removed manually or the animals released their larvae, so as to calculate the ratio between the egg mass and body weight, termed “brood somatic index” (BSI) (Lezer et al. 2015). Since the data were not normally distributed according to the Shapiro-Wilk test, any difference between the BSI measurements in the WW and WZ females was tested by the non-parametric Mann-Whitney test using Statistica v9.0 (StatSoft, Tulsa, OK).
Results
Characterization of Neo-males
Animals injected with hAG cells, reared in earthen ponds, were examined upon final harvest, 9 months after manipulation. In this population of 100 animals, some females had completely sex-reversed into neo-males, as revealed by the development of an AM and two visible male gonopores at the base of the 5th pereiopods. Moreover, the neo-male group exhibited the three well-known M. rosenbergii male morphotypes described by Kuris et al. (1987): BC, OC and SM (Fig. 1a). Of the neo-male population, 12 animals were BC neo-males, 32 were OC neo-males, and the rest were either small males or unsuccessfully manipulated females. The WZ genotype of the three neo-male morphotype groups was confirmed using genomic sex markers (Fig. 1b).
Phenotypic and genotypic characterization of M. rosenbergii neo-males. a Representative male morphotypes from an adult neo-male population at the end of a 4.5-month grow-out period in an earthen pond. BC (blue claw), OC (orange claw), and SM (small male). b Representation of genomic sex markers in three different neo-male morphotypes (WZ): offspring of a neo-male (WW), normal males (ZZ), and normal females (WZ). Mr-actin served as a positive control. c External sexual characteristics in male, neo-male, and female juvenile prawns and in a manipulated juvenile population. The second pleopod (top) and the base of the fifth pereiopod (bottom) are depicted in a juvenile male, neo-male and female. Appendix interna (black arrows) are present in the second pleopod of all phenotypes, while appendix masculina is present only in the male and neo-male (white arrows). The presence of masculine gonopores at the base of the fifth pereiopods in an intact male (white arrows) and a neo-male (black arrows) is indicated. Bar = 500 μm
In order to follow the manipulation process before the end of the grow-out season, a group comprising 16 manipulated females and 14 intact males were held separately. The intact males were validated by genetic sex markers as well as the presence of AM and male gonopores, Fig. 2. The rest of the manipulated animals were immediately stocked for grow-out in earthen ponds. Approximately 50 days post-manipulation, all 16 hAG cell-injected females had developed AMs, while 13 also developed male gonopores (∼81 %) (Fig. 1c, center column), and could thus be considered ‘neo-males’. The 14 intact males of the same age as the manipulated females, serving as references, presented both AMs and male gonopores at the same evaluation point (Fig. 1c, left column and Fig. 2). Moreover, representative neo-males were histologically shown to possess sperm-filled sperm ducts (Fig. 3a, c), and had developed functional testes with regions either containing dividing spermatogonium or mature spermatozoa (Fig. 3b, d).
Appendix masculina development, male gonopores, and a female-specific sex marker in a hAG-cell-injected population of juvenile prawns. Sixteen manipulated individuals were found to be genetically female (columns 1, 4, 6, 7, 9–12, 17–19, 22, 25, and 28–30) and 14 were found to be intact males (columns 2, 3, 5, 8, 13–16, 20, 21, 23, 24, 26, and 27). Genotypic analysis was performed using a female-specific genomic sex marker and Mr-actin as a positive control. The negative control is represented by NC, while one intact female served as a reference for the sex marker
Histological cross sections stained with hematoxylin and eosin. a Sperm-filled sperm duct along with b highly active testicular lobules reflected by either highly (“dividing”) or lightly (“mature”) dense regions. c Sperm within the sperm duct exhibited the “inverted umbrella” morphological characteristic of M. rosenbergii mature sperm. d Round large spermatogonium (Sg) cells were heavily stained and were located in the periphery of a lobule as opposed to spermatozoa (Sz), which were lightly stained and accounted for the majority of the lobule volume. Bar = 250 μm (a, b); 50 μm (c, d)
Spatial Expression of Male-Related Genes in Neo-male Morphotypes
Macrobrachium rosenbergii (Mr)-IAG was only detected at the base of the right fifth pereiopod in the single BC neo-male tested but not in the left fifth pereiopod of the same individual, nor in samples taken from hepatopancreas, muscle, or cuticle. At the same time, Mr-IAG was not detected in the fifth pereiopods, hepatopancreas, muscle, or cuticle of other neo-male animals tested (Fig. 4, upper panel). Mar-mrr was, however, detected at the base of both fifth pereiopods in each neo-male morphotype but not in the testes, hepatopancreas, muscle, or cuticle (Fig. 4, middle panel).
Spatial expression of Mr-IAG and Mar-mrr in blue claw (BC), orange claw (OC), and small male (SM) neo-males. Expression was tested in the testis (Tes), base of right and left fifth walking legs (R-5th, L-5th), hepatopancreas (Hep), muscle (Mus), and cuticle (Cut). A negative control is represented as Neg, while expression of Mr-actin served as a positive control
The AG in Neo-males
Examination of ∼3000 histological sections prepared from the dissected fifth pereiopods of four randomly selected neo-males (an SM, an OC and two BCs) provided no evidence for the presence of an AG adjacent to the sperm duct (Fig. 5a, left and center panels). In contrast, the AG is readily detected in the same section obtained from a normal male (Fig. 5a, right panel). While the results obtained were consistent, one of the tested neo-males (a BC morphotype), nonetheless, presented a tissue with typical AG-like appearance, albeit only in the left fifth pereiopod (Fig. 5b, neo-male, H&E). The suspected region, which was examined in an immunohistochemistry assay using anti-Mr-IAG antibodies and DAPI for nuclear counter-staining (Fig. 5b, top panels), has confirmed to produce Mr-IAG and was, therefore, AG tissue, similar to that recognized in the normal male which served as a positive control (Fig. 5b, bottom panels).
Histological sections and immunohistochemistry of fifth pereiopods. a Randomly selected hematoxylin and eosin (H&E)-stained sections from two neo-males (one SM (left) and one BC (center)) shows the expected location of the AG, with the section from a BC normal male (right) shows the AG (black arrow) at this location. b staining with H&E, with FITC-conjugated anti-Mr.-IAG antibodies (green), and with DAPI (blue) for nuclei counter-staining of neo-male BC (top row) and normal male BC (bottom row). A negative control (without the use of rabbit α-Mr-IAG antibodies) is represented as NC. The sperm duct (SD) and the androgenic glands (AG), along with size bars are indicated
Progeny of the Neo-males
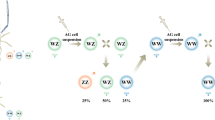
A breeding scheme that began with the production of neo-males who were crossed with normal females was devised (Fig. 6a). Distribution of the offspring population (defined as population 1) did not vary significantly from the classic Mendelian model of inheritance (Sorsby 1965). Specifically, population 1 displayed the expected 1:3 (male/female) ratio. When samples from population 1 and three additional populations (2, 3, and 4) were genotypically characterized, the expected 1:2:1 (WW/WZ/ZZ) ratio (P value >0.15) was obtained. Chi-square goodness of fit tests confirmed the significance of these observations (Fig. 6b). All crosses between WW females and ZZ males gave rise to 100 % WZ all-female progeny, as expected (Fig. 6c).
Breeding schemes of expected and observed sex ratio of progeny. a Breeding scheme and expected progeny upon cross-breeding neo-males (WZ) with normal females (WZ) and WW genotype females with normal males (ZZ). b Table showing the sex and genotype ratios of four representative offspring populations from the above crosses between neo-males and normal females. The observed and expected results, along with chi-square goodness of fit test results (chi-square statist, degrees of freedom and P value) for each tested population, are shown. c Sex ratios in samples of four representative offspring populations from crosses between WW genotype females and normal males (ZZ)
Fecundity and Progeny of WW Genotype Females
The mean ratio between the egg mass and body weight (BSI (%)) was 16.22 (SE = 3.11) for WW females and 15.33 (SE = 1.97) for normal WZ females. According to Mann-Whitney test results, there was no significant difference in BSI measurements between WW females and normal WZ females (Pexact = 0.72).
Discussion
In this study, we report the first complete sex reversal of M. rosenbergii females into neo-males following a single injection of suspended hAG cells. Partial sex reversal in crustaceans by AG implantation had been previously reported (Barki et al. 2003; Karplus et al. 2003; Khalaila et al. 2001; Manor et al. 2004; Nagamine et al. 1980; Taketomi and Nishikawa 1996). Moreover, the production of functional neo-males had also been achieved, although survival and success rates were as low as ∼10 % (Malecha et al. 1992). In contrast, our novel technology involving a single injection of hAG cells allowed for much higher survival rates (∼70 %). This allows, for the first time, extensive production of fully functional neo-males in numbers that can be upscaled to industrial levels.
Our histological findings indicate that the neo-males produced, representing the three known M. rosenbergii male morphotypes (Kuris et al. 1987), developed functional testes and sperm-filled sperm ducts. These typical male features support the masculine viability of these neo-males since they not only survived manipulation but also grew to sexual maturity and established a male hierarchy characteristic of this species (Ra’anan and Cohen 1985).
Two masculine-related genes were used to molecularly evaluate the extent of sex reversal, specifically, the AG location/formation, in the present study. Mar-mrr, a male reproduction-related gene was detected at the base of the fifth pereiopods of all tested neo-males. This gene is specifically expressed in the male M. rosenbergii reproductive tract, especially during AG development and thus assumed to play a crucial role during male reproduction (Cao et al. 2006). This confirms that these animals achieved masculine sexual maturity. The other gene considered, Mr-IAG, is typically expressed at the base of male’s fifth pereiopods (Ventura et al. 2011b), also the site of the AG (Sharabi et al. 2016; Ventura et al. 2011b). Mr-IAG expression was detected in only one fifth pereiopod of a single neo-male, who also presented the AG in its usual location. This scenario raises questions regarding AG formation and functionality in neo-males.
Given our molecular and histological findings, it would appear that the presence of an AG at the base of the fifth pereiopod is not mandatory for the induction of masculinization. Crustaceans possess an open circulatory system (Maynard 1960), such that injected hAG cells could travel throughout the organism till they come to land in a random spot, other than the base of the fifth pereiopod, from where they would release their propagating agents (e.g., IAG hormone). The injected hAG cells could not serve as a temporary slow release apparatus of AG factors only during the differentiation window of opportunity. This is supported by the fact that in later ages, the injected animals exhibited the three known morphotypes (Kuris et al. 1987). Maintenance of male sexual characters including morphotypic differentiation was suggested to be dependent on active AGs (Sagi et al. 1990). IAG expression, serving as an indicator for AG localization, was not detected in our sporadic investigation of several neo-male tissues. However, it is important to note that despite the fact we rarely found the AG at the base of the fifth pereiopod, AM regeneration was confirmed even in mature neo-males; thus, the possibility of AG formation at a different site remains. The latter is supported by the fact that AM regeneration is correlated with AG factors (Ventura et al. 2009). A wider screening of neo-male tissues will help clarify this issue.
The genotype ratio obtained upon crossing neo-males with normal females, yielded a progeny of WW (25 %), WZ (50 %), and ZZ (25 %), as validated by our genomic sex marker assessment. This result strongly supports the well-accepted assumption that M. rosenbergii relies on a WZ/ZZ sex determination system (Sagi and Cohen 1990). Moreover, our study also represents the first mass production of all-female progeny in M. rosenbergii by crossing WW females with normal ZZ males. The fact the fecundity of WW females did not significantly deviate from that of normal heterogametic WZ females not only makes our technology a promising industrial tool for large-scale production of all-female populations but also suggests that the gene content of the Z chromosome is not crucial for normal female reproductive output. The physical existence of the Z chromosome (could be proven by karyotyping and/or genome mapping), like its genetic content, has yet to be revealed. The notion that the content of a sex chromosome is of importance when sexual determination is transpiring but is less relevant later on was recently demonstrated in mammals. In this study, assisted reproduction which bypassed the Y chromosome was achievable when homologs of only two genes, usually located on the Y chromosome, were instead overexpressed from the X chromosome (Yamauchi et al. 2016).
In conclusion, a single injection of suspended hAG was found to be an efficient, easy to perform, relatively non-expensive and reproducible technology for inducing fully functional sex reversal (including development of the AM, male gonopores and gonadogenesis, morphotypic differentiation, and reproductive output). The sex-reversed M. rosenbergii neo-males, in turn, produce genomically validated WW females in their progeny that could be used as dams in subsequent crosses, resulting in the production of all-female populations. Still, although our novel technology can be used to produce all-female populations, such populations have never been cultured under commercial conditions. Further studies should investigate various traits of such populations, such as size variation as a function of culture densities, as well as the point of reproductive maturity.
Since the discovery of the first decapod IAG in our laboratory (Manor et al. 2007), IAG-producing cells have been found in all major groups of cultured crustaceans used in the aquaculture industry (i.e., crabs (Huang et al. 2014), lobsters (Ventura et al. 2015), crayfish (Manor et al. 2007), prawns (Alkalay-Savaya et al. 2014), and shrimp (Li et al. 2012)). This suggests the technology described in this study could be tailored to any desired species in the industry. Moreover, monosex culture is sustainable and our biotechnology is fitted also to species in which females grow faster than males, as is the case in the shrimp industry, which dominates global crustacean aquaculture with a staggering production portion of ∼67 % (FAO 2015). Additionally, the technology offers seedstock protection that will ensure quality seed supply, an important consideration for large breeding companies, as opposed to the inferior inbreeding procedures commonly occurring in the shrimp industry nowadays. In summary, the novel biotechnology described here will revolutionize the way seed-producing parties and growers interact in the crustacean aquaculture industry, and could also have wider applications in other sectors.
References
Alkalay-Savaya A et al. (2014) The prawn Macrobrachium vollenhovenii in the Senegal River basin: towards sustainable restocking of all-male populations for biological control of schistosomiasis. PLoS Negl Trop Dis 8:e3060.
Barki A, Karplus I, Khalaila I, Manor R, Sagi A (2003) Male-like behavioral patterns and physiological alterations induced by androgenic gland implantation in female crayfish. J Exp Biol 206:1791–1797.
Cao JX, Yin GL, Yang WJ (2006) Identificaflon of a novel male reproduction-related gene and its regulated expression patterns in the prawn, Macrobrachium rosenbergii. Peptides 27:728–735.
Charniaux-Cotton H (1954) Discovery in, an amphipod crustacean (Orchestia gammarella) of an endocrine gland responsible for the differentiation of primary and secondary male sex characteristics. C R Hebd Seances Acad Sci 239:780–782
Chung JS, Manor R, Sagi A (2011) Cloning of an insulin-like androgenic gland factor (IAG) from the blue crab, Callinectes sapidus: implications for eyestalk regulation of IAG expression. Gen Comp Endocrinol 173:4–10.
Cronin LE (1947) Anatomy and histology of the male reproductive system of Callinectes sapidus Rathbun. J Morphol 81:209–239
FAO (2015) Fisheries and aquaculture information and statistics service-aquaculture production 1950–2013. Food and Agriculture Organization of the United Nations. http://www.fao.org/fi/statist/FISOFT/FISHPLUS.asp. Accessed 17 March 2016
Gopal C et al. (2010) Weight and time of onset of female-superior sexual dimorphism in pond reared Penaeus monodon. Aquaculture 300:237–239.
Hansford SW, Hewitt DR (1994) Growth and nutrient digestibility by male and female Penaeus monodon—evidence of sexual dimorphism. Aquaculture 125:147–154.
Huang X, Ye H, Huang H, Yang Y, Gong J (2014) An insulin-like androgenic gland hormone gene in the mud crab, Scylla paramamosain, extensively expressed and involved in the processes of growth and female reproduction. Gen Comp Endocrinol 204:229–238.
Karplus I, Sagi A, Khalaila I, Barki A (2003) The influence of androgenic gland implantation on the agonistic behavior of female crayfish (Cherax quadricarinatus) in interactions with males. Behaviour 140:649–663.
Keller R (1992) Crustacean neuropeptides—structures, functions and comparative aspects. Experientia 48:439–448.
Khalaila I, Katz T, Abdu U, Yehezkel G, Sagi A (2001) Effects of implantation of hypertrophied androgenic glands on sexual characters and physiology of the reproductive system in the female red claw crayfish, Cherax quadricarinatus. Gen Comp Endocrinol 121:242–249.
Khalaila I, Manor R, Weil S, Granot Y, Keller R, Sagi A (2002) The eyestalk-androgenic gland-testis endocrine axis in the crayfish Cherax quadricarinatus. Gen Comp Endocrinol 127:147–156
Kuris AM, Raanan Z, Sagi A, Cohen D (1987) Morphotypic differentiation of male Malaysian giant prawns, Macrobrachium-rosenbergii. J Crustac Biol 7:219–237.
Lezer Y, Aflalo ED, Manor R, Sharabi O, Abilevich LK, Sagi A (2015) On the safety of RNAi usage in aquaculture: the case of all-male prawn stocks generated through manipulation of the insulin-like androgenic gland hormone. Aquaculture 435:157–166.
Li S, Li F, Sun Z, Xiang J (2012) Two spliced variants of insulin-like androgenic gland hormone gene in the Chinese shrimp, Fenneropenaeus chinensis. Gen Comp Endocrinol 177:246–255.
Malecha SR (1986) New techniques for the assessment and optimal management of growth and standing crop variation in the cultured fresh-water prawn, Macrobrachium rosenbergii. Aquac Eng 5:183–197.
Malecha S (2012) The case for all-female freshwater prawn, Macrobrachium rosenbergii (de man), culture. Aquac Res 43:1038–1048.
Malecha SR, Nevin PA, Ha P, Barck LE, Lamadridrose Y, Masuno S, Hedgecock D (1992) Sex-ratios and sex-determination in progeny from crosses of surgically sex-reversed freshwater prawns, Macrobrachium rosenbergii. Aquaculture 105:201–218.
Manor R, Aflalo ED, Segall C, Weil S, Azulay D, Ventura T, Sagi A (2004) Androgenic gland implantation promotes growth and inhibits vitellogenesis in Cherax quadricarinatus females held in individual compartments. Invertebr Reprod Dev 45:151–159.
Manor R et al. (2007) Insulin and gender: an insulin-like gene expressed exclusively in the androgenic gland of the male crayfish. Gen Comp Endocrinol 150:326–336.
Maynard DM (1960) Circulation and heart function. In: Waterman TH (ed) The physiology of Crustacea, vol 1. Academic Press, New York, pp 161–226
Moss DR, Moss SM (2006) Effects of gender and size on feed acquisition in the Pacific white shrimp Litopenaeus vannamei. J World Aquacult Soc 37:161–167
Moss DR, Hennig OL, Moss SM (2002) Sexual growth dimorphism in penaeid shrimp. Potential for all female culture? Global Aquac Advocate 5:60–61
Nagamine C, Knight AW, Maggenti A, Paxman G (1980) Masculinization of female Macrobrachium rosenbergii (de man) (Decapoda, Palaemonidae) by androgenic gland implantation. Gen Comp Endocrinol 41:442–457.
Nair CM, Salin KR, Raju MS, Sebastian M (2006) Economic analysis of monosex culture of giant freshwater prawn (Macrobrachium rosenbergii de man): a case study. Aquac Res 37:949–954.
Otoshi CA, Arce SM, Moss SM (2003) Growth and reproductive performance of broodstock shrimp reared in a biosecure recirculating aquaculture system versus a flow-through pond. Aquac Eng 29:93–107.
Ra’anan Z, Cohen D (1985) Ontogeny of social structure and population dynamics in the giant freshwater prawn, Macrobrachium rosenbergii (de man). In: Wenner A, Schram FR (eds) Crustacean growth, vol 2. A. A. Balkema, Rotterdam, pp 277–311
Rosen O, Manor R, Weil S, Aflalo ED, Bakhrat A, Abdu U, Sagi A (2013) An androgenic gland membrane-anchored gene associated with the crustacean insulin-like androgenic gland hormone. J Exp Biol 216:2122–2128.
Sagi A, Aflalo ED (2005) The androgenic gland and monosex culture of freshwater prawn Macrobrachium rosenbergii (de man): a biotechnological perspective. Aquac Res 36:231–237.
Sagi A, Cohen D (1990) Growth, maturation and progeny of sex-reversed Macrobrachium rosenbergii males. World Aquacult 21:87–90
Sagi A, Raanan Z, Cohen D, Wax Y (1986) Production of Macrobrachium rosenbergii in monosex populations—yield characteristics under intensive monoculture conditions in cages. Aquaculture 51:265–275.
Sagi A, Cohen D, Milner Y (1990) Effect of androgenic gland ablation on morphotypic differentiation and sexual characteristics of male fresh-water prawns, Macrobrachium rosenbergii. Gen Comp Endocrinol 77:15–22.
Sagi A, Snir E, Khalaila I (1997) Sexual differentiation in decapod crustaceans: role of the androgenic gland. Invertebr Reprod Dev 31:55–61.
Sharabi O et al. (2016) Identification and characterization of an insulin-like receptor involved in crustacean reproduction. Endocrinology 157:928–941.
Sorsby A (1965) Gregor Mendel. Br Med J 1:333–338
Sroyraya M et al. (2010) Bilateral eyestalk ablation of the blue swimmer crab, Portunus pelagicus, produces hypertrophy of the androgenic gland and an increase of cells producing insulin-like androgenic gland hormone. Tissue Cell 42:293–300.
Taketomi Y, Nishikawa S (1996) Implantation of androgenic glands into immature female crayfish, Procambarus clarkii, with masculinization of sexual characteristics. J Crustac Biol 16:232–239.
Ventura T, Sagi A (2012) The insulin-like androgenic gland hormone in crustaceans: from a single gene silencing to a wide array of sexual manipulation-based biotechnologies. Biotechnol Adv 30:1543–1550.
Ventura T, Manor R, Aflalo ED, Weil S, Raviv S, Glazer L, Sagi A (2009) Temporal silencing of an androgenic gland-specific insulin-like gene affecting phenotypical gender differences and spermatogenesis. Endocrinology 150:1278–1286.
Ventura T, Aflalo ED, Weil S, Kashkush K, Sagi A (2011a) Isolation and characterization of a female-specific DNA marker in the giant freshwater prawn Macrobrachium rosenbergii. Heredity 107:456–461.
Ventura T, Manor R, Aflalo ED, Weil S, Khalaila I, Rosen O, Sagi A (2011b) Expression of an androgenic-gland-specific insulin-like peptide during the course of prawn sexual and morphotypic differentiation. ISRN Endocrinol 2011:476283
Ventura T, Fitzgibbon Q, Battaglene S, Sagi A, Elizur A (2015) Identification and characterization of androgenic gland specific insulin-like peptide-encoding transcripts in two spiny lobster species: Sagmariasus verreauxi and Jasus edwardsii. Gen Comp Endocrinol 214:126–133.
Yamauchi Y, Riel JM, Ruthig VA, Ortega EA, Mitchell MJ, Ward MA (2016) Two genes substitute for the mouse Y chromosome for spermatogenesis and reproduction. Science 351:514–516.
Acknowledgments
We thank Ms. Ayana Benet-Perlberg and her team at the Ministry of Agriculture - Aquaculture Research Station, Dor, Israel, for housing the prawns during parts of this study. Funding for this study was provided by Enzootic Holdings, Ltd. and partially by United States-Israel Binational Agricultural Research and Development Fund Grant IS-4493-12.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
A patent regarding functional sex reversal of decapod crustacean female is pending (PCT/IL2015/051,096).
Rights and permissions
About this article
Cite this article
Levy, T., Rosen, O., Eilam, B. et al. A Single Injection of Hypertrophied Androgenic Gland Cells Produces All-Female Aquaculture. Mar Biotechnol 18, 554–563 (2016). https://doi.org/10.1007/s10126-016-9717-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-016-9717-5