Abstract
Dictyota menstrualis (Hoyt) Schnetter, Hörning & Weber-Peukert (Dictyotales, Phaeophyceae) was studied for the production of oil-based bioproducts and co-products. Experiments were performed to evaluate the effect of carbon dioxide (CO2) concentration, under nitrogen (NO3 −) limiting and saturation conditions, on growth rate (GR), photosynthesis, as well as nitrate reductase (NR), carbonic anhydrase (CA), and Rubisco activities. In addition, the biochemical composition of D. menstrualis under these conditions was estimated. GR, protein content, and N content in D. menstrualis were higher in treatments containing NO3 −, irrespective of CO2 addition. However, when CO2 was added to medium saturated with NO3 −, values of maximum photosynthesis, Rubisco, and NR activity, as well as total soluble carbohydrates and lipids, were increased. CA activity did not vary under the different treatments. The fatty acid profile of D. menstrualis was characterized by a high content of polyunsaturated fatty acids, especially the omega-3 fatty acids, making it a possible candidate for nutraceutical use. In addition, this species presented high GR, photosynthetic rate, and fatty acid content, highlighting its economic importance and the possibility of different biotechnological applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Based on their involvement in carbon and nitrogen assimilation, as well as O2 production, marine benthic algae are important photosynthetic organisms in coastal and estuarine ecosystems, playing a significant role in energy transformation and nutrient recycling from their environments (Hanisak 1983). In addition, they provide food and shelter for many species of marine animals.
This group of organisms exhibits morphological differences and high diversity in relation to pigment content, storage products, and components of the cell wall, among others (van den Hoek et al. 1995). Several algal metabolites have been extensively used in the food and beverage, biotechnology, agriculture, medical, cosmetic, bioenergy, and pharmaceutical industries (Cardozo et al. 2006; Gressler et al. 2009, 2011a, b; Martins et al. 2011, 2012; Simas-Rodrigues et al. 2015).
Seaweeds with high content of fatty acids, in particular ω-3 and ω-6, can be used as nutraceuticals (Gressler et al. 2011a; Moraes and Colla 2006). After oil extraction, the remaining seaweed biomass, which is rich in protein, pigments, and carbohydrates, can be used as food, feed, fertilizer, and pigments, adding economic value to the species (van Iersel and Flammini 2010).
Linoleic (ω-6) and linolenic (ω-3) acids are essential nutrients not synthesized by mammals (Patarra 2008). These fatty acids are precursors of arachidonic acid (AA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA), important for maintaining the integrity and fluidity of membranes, as well as the synthesis of eicosanoids (Moreira et al. 2002), which have an important function in the regulation of inflammation (Wall et al. 2010). Since eicosanoids derived from AA promote a proinflammatory physiological condition, while those derived from EPA have anti-inflammatory properties, due to their capacity to inhibit the formation of eicosanoids derived from ω-6 polyunsaturated fatty acids (Wall et al. 2010), human food should contain a ω-6:ω-3 ratio of around 5:1. Despite this, the ratio of ω-6:ω-3 from the ingestion of fatty acids by the population of several countries ranges from 10:1 to 20:1 (Patarra 2008). This is because the ω-6 is present in vegetable oils, milk, eggs, and meat, while algae and fish are the main sources of ω-3. In addition, the ω-3 fatty acids also act in the prevention and modulation of coronary heart diseases, inflammatory diseases, and hypertension, among others (Patarra 2008). Thus, the increase of ω-3 consumption in the human diet and the search for organisms that produce high amounts of this compound are very important.
The quantity and quality of fatty acids vary among different groups and species of seaweeds (Gosch et al. 2012). This diversity provides an opportunity to identify new species with high oil content and with a profile suitable for use as nutraceuticals. Moreover, if a given species shows a poor profile for nutraceuticals, it can be utilized for other purposes, such as biodiesel production.
Light, temperature, salinity, nutrient, and CO2 availability in seawater have effects on photosynthesis and on other metabolic pathways, directly influencing the biosynthesis of algal metabolites. Photosynthesis corresponds to the first stage of converting light to chemical energy. NADPH and ATP, synthesized in the photochemical phase of photosynthesis, are used in the Calvin cycle and other metabolic routes to produce biomolecules (Schenk et al. 2008). The Calvin cycle is part of a chemical phase of photosynthesis, where Ci is assimilated by ribulose 1,5-bisphosphate carboxylase oxygenase (RuBisCo), the main enzyme responsible for the conversion of inorganic to organic carbon, requiring CO2 as substrate (Taiz and Zeiger 2004).
Seaweeds exploit the abundance of bicarbonate in seawater in two ways: (1) active uptake of bicarbonate into the cell by a specific carrier, allowing CO2 conversion by the action of carbonic anhydrase enzyme; and (2) conversion of bicarbonate to CO2 on the surface of the thallus by the action of extracellular carbonic anhydrase (Lobban and Harrison 1994). Fixed carbon provides the necessary carbon skeleton for synthesis of proteins, lipids, and carbohydrates.
The change of some environmental factors allows manipulating metabolic pathways, thus redirecting cellular function for the synthesis of products of interest (Rosenberg et al. 2008). For example, with the increase in CO2 concentration, some algae showed an increase in carbohydrate content, a decrease in the content of protein (Mercado et al. 1999), and an increase in the total amount of fatty acid content and oleic acid (Tsuzuki et al. 1990).
Another natural mechanism by which the algae can alter lipid metabolism is the response to stress from low nitrogen availability. Although this nutrient deficiency inhibits cell cycle and the production of almost all cellular components, the rate of lipid synthesis remains high, which leads to oil accumulation in nitrogen-starved cells (Rosenberg et al. 2008).
In this paper, Dictyota menstrualis (Hoyt) Schnetter, Hörning & Weber-Peukert (Dictyotales, Phaeophyceae) was studied for the production of oil-based bioproducts and co-products. The effects of CO2 availability under different nitrogen conditions were evaluated. Our hypothesis holds that the increase of CO2 in the culture medium will increase photosynthesis which, in turn, will result in the biosynthesis of macromolecules. On the other hand, depletion of N in the medium would divert metabolism for the biosynthesis of lipids.
Materials and Methods
Algal Material and Experimental Setup
The study was conducted with Dictyota menstrualis (Hoyt) Schnetter, Hörning & Weber-Peukert (Dictyotales, Phaeophyceae) collected from Rio do Fogo, Rio Grande do Norte State, Brazil (5°27′79.9″S and 35°37′56′W). After collection, the specimens were transported to the laboratory where they were isolated and propagated. Voucher specimen was deposited in the herbarium of the Institute of Botany under the number SP 427967.
After isolation, unialgal cultures were maintained in medium consisting of sterile seawater enriched with half strength of von Stosch’s solution (VSES/2), as described by Oliveira et al. (1995), and modified with reduction of 50 % in vitamin concentrations (Yokoya 2000). The medium was renewed weekly.
The cultures were maintained in a culture room under the following conditions: average temperature of 24 ± 2 °C, salinity of 30–32 psu, pH 8, photoperiod of 14 h, and photon flux density of 80–90 μmol photons m−2 s−1, provided by two fluorescent lamps of 40 W, “daylight” type, arranged horizontally above the culture flasks.
The experiments were performed in a bioreactor system (model TE-BIT-E3; Tecnal, Brazil) composed of six reaction vessels (volume 2.5 l) with stainless steel lids with inputs for temperature (Tecnal), pH (Mettler Toledo), oxygen (Mettler Toledo), and CO2 (Mettler Toledo) sensors. The reaction vessels were connected to a system control and data acquisition (Tecbio-soft; Tecnal) of temperature, pH, as well as variations in dissolved O2 and CO2 in the culture medium.
Macroalgae were grown in sterile seawater enriched with VSES/2 solution, with and without addition of NO3 −, in the proportion of 400 mg of biomass to 1.6 l of culture medium. These treatments (with and without NO3 −) were performed in three different conditions: (1) no aeration addition; (2) aeration addition; (3) aeration addition plus CO2 enrichment. The medium was changed weekly; the experiment lasted 2 weeks and was simultaneously tested with three replicates.
Aeration and CO2 injection were constant throughout the experimental period and were injected by different inputs. Air, from the aeration system module Bio-Tec A (Tecnal), and CO2, from a cylinder, were filtered and then moistened. The input of the air and CO2 to the bioreactor vessel was made by a manifold with six outputs, and the airflow of each exit was adjusted with a rotameter.
The concentration of the forms of dissolved inorganic carbon (DIC), including CO2 and HCO3 − (Table 1), were estimated from the pH and total alkalinity of the medium, using the equation described by Dickson et al. (2007). The calculations were performed with the aid of the seacarb package (Lavigne et al. 2009) for the R programming environment.
At the end of the cultivation period, the macroalgae were frozen in liquid nitrogen and stored at −80 °C until biochemical analysis. The following processes were analyzed: growth rate and photosynthesis; CO2 and nitrogen assimilation, through the activity of the enzymes Nitrate Reductase (NR, the first enzyme of the nitrate assimilation pathway), Carbonic Anhydrase (CA, enzyme responsible for the conversion of HCO3 to CO2), and Ribulose-1,5-bisphosphate Carboxylase Oxygenase (RuBisCo, the first enzyme of the CO2 assimilation pathway). Pigments, total soluble protein, total soluble carbohydrate, total lipid content, fatty acid composition, and content of C and N on tissue were quantified.
Growth Rate
The growth rate (GR) was evaluated weekly by fresh weight measurements and calculated using the following formula:
Determination of Photosynthesis and Irradiance and Dark/light Induction Curves
The photosynthetic rate was assessed by chlorophyll fluorescence analysis following the general procedures described in Necchi (2004) and Yokoya et al. (2007). Chlorophyll fluorescence was measured by a pulse amplitude modulated (PAM) fluorometer (Diving-PAM underwater fluorometer; Walz, Effeltrich, Germany). Apical segments of D. menstrualis were placed directly on the tip of the fiber-optic fluorometer, using the supplied magnet sample holder. Rapid light curves (RLC) consisted of the fluorescence responses to eight increasing irradiance levels, using the “light curve” option of the Diving-PAM. At each irradiance, the exposure time was 15 s. Two main parameters were determined: (1) effective quantum yield of photosystem II (PSII), ΔF/Fm′, which was calculated using the following formula: ΔF = Fm ′ − Ft, where Fm′ is the maximal fluorescence of an illuminated sample, and Ft is the transient fluorescence (Schreiber et al. 1994); (2) relative electron transport rate (rETR) calculated as ΔF/Fm′ × PAR × 0.5, where PAR is the actinic irradiance (μmol photons m−2 s−1) and 0.5 is a multiplication factor to satisfy the transport of a single electron which requires the absorption of two quanta.
Photosynthesis × irradiance curves (PI curves) were generated on the basis of rETR, and the respective parameters were calculated by the equation of Platt et al. (1980): photosynthetic efficiency (αETR), Pmax (rETRmax), and saturation parameter (Ik).
Apart from PI curves, dark/light induction curves (Kautsky curves) were analyzed, followed by a recovery curve. In this analysis, samples acclimated 30 min in the dark were subjected to a pulse saturation to obtain the value of potential quantum yield (PQY). Afterwards, actinic light was turned on (696 μmol photons m−2 s−1), and 13 additional pulses were applied at intervals of 15 s to determine the effective quantum yield (EQY) (Kautsky curve). After shutting down actinic light, six additional saturating pulses were applied at intervals of 10 s, 30 s, 1 min, 2 min, 5 min, and 10 min (recovery curve).
Total Soluble Proteins and Nitrate Reductase (NR) Assay
Cell extraction and NR assay were conducted by modifying the protocol described by Chow et al. (2004). Fresh biomass (200 mg) was macerated in liquid nitrogen and suspended in 1.15 ml of extraction buffer (0.2 M phosphate buffer, pH 8.0, 5 mM EDTA, 1 mM DTT, and protease inhibitor cocktail). The solution was centrifuged at 12,000×g (4 °C for 15 min). The supernatant was desalted by 5 ml commercial columns with Sephadex G-25 superfine matrix that provides a mass cutoff of 5000 Da, ensuring the separation of the protein from nitrate. The concentration of soluble protein of the crude extract was determined by spectrophotometry at 595 nm after addition of Bio-Rad protein test solution by the method of Bradford (1976), and bovine serum albumin was used as standard.
To determine NR, the crude extract (150 μl) was preincubated in a reaction mixture (0.2 M phosphate buffer, pH 8.0, 6 mM KNO3, and 0.5 mM MgSO4) for 10 min. The mixture was incubated for an additional 30 min after addition of 40 μM NADH to initiate the reaction. The reaction was stopped by adding 1.4 mM ZnSO4 and 43 % ethanol v/v. The solution was centrifuged at 12,000×g (20 °C for 10 min). NO2 concentration was determined by spectrophotometry at 543 nm after addition of 9.6 mM sulfanilamide and 0.7 mM N-(1-naphthyl) ethylenediamine dihydrochloride, respectively. Controls were performed without NADH and NO3.
The content of NO2 produced was transformed into enzymatic activity per protein concentration considering that 1 NR unit (U) corresponds to 1 μmol of NO2 produced per minute (Chapman and Harrison 1988).
Carbonic Anhydrase (CA) Assay
CA activity was analyzed using the method of Haglund et al. (1992). Briefly, the extraction was performed by macerating the samples in liquid nitrogen. The macerate was suspended in an extraction buffer (50 mM Tris (hydroxymethyl) aminomethane, 5 mM EDTA, 25 mM ascorbic acid, pH 8.5) at the proportion of 100 mg of fresh biomass to 2 ml buffer, and samples were maintained on ice until analysis.
CA assay was carried out by adding 1 ml of Milli-Q water saturated with CO2 and measuring the time required for pH to decrease from 7.9 to 7.3. The samples were always kept on ice. After addition of 1 ml of Milli-Q water saturated with CO2, pH decay time was also evaluated in control, consisting of 2 ml of buffer without seaweed extract.
The relative activity of CA, termed relative enzymatic activity (REA), was calculated using the following formula: [(t 0/t c)− 1]/fw, where t 0 is the reduction time of pH within the limit established in control, t c is the reduction time of pH in the sample with seaweed, and fw is the fresh weight (g).
Rubisco
To determine the initial activity of Rubisco, we used the method of Gerard and Driscoll (1996) modified by Wang et al. (2011). Briefly, the extraction was performed by macerating the samples in liquid nitrogen. The macerate was suspended in an extraction buffer (40 mM Tris–HCl, 10 mM MgCl2, 0.25 mM EDTA, and 5 mM reduced glutathione, pH 7.6) in a proportion of 0.2 g fresh weight per 500 μl of buffer. The solution was centrifuged at 2000×g (4 °C for 2 min), and the supernatant was removed and kept on ice for 30 min to activate the enzyme.
The reaction mixture consisted of reaction buffer (0.1 M Tris–HCl, 12 mM MgCl2, and 0.4 mM EDTA, pH 7.8) and 0.01 mM NaHCO3, 0.34 mM NADH, 3.44 mM ATP, 3.44 mM phosphocreatine, 5 U creatine phosphokinase, 5 U glyceraldehyde-3-phosphate dehydrogenase/phosphoglycerate kinase, and 100 μl of crude extract. The reaction was started by adding 2 mM ribulose-1,5-bisphosphate. The oxidation of NADH was monitored by the decrease in absorbance at 340 nm. The spontaneous oxidation of NADH was estimated for a few minutes before the reaction and subtracted from the measured activity of Rubisco. Rubisco activity was related to protein concentration and calculated as described by Wang et al. (2011).
Chlorophyll a
The extraction was performed by macerating the samples in liquid nitrogen. The macerate (100 mg) was suspended in 1 ml of 90 % acetone to obtain homogenization. The solution was centrifuged at 12,000×g (4 °C for 15 min), and the supernatant containing the chlorophyll was transferred to vials, sealed and kept in the dark until analysis by spectrophotometry.
Determination of chlorophyll concentration was carried out using the formulas described by Jeffrey and Humphrey (1975).
Total Soluble Carbohydrates
The lyophilized macerate (20 mg) was suspended in 1 ml of 70 % ethanol to obtain homogenization. The solution was incubated in a water bath for 3 h at 70 °C and then centrifuged at 5000×g for 5 min (Karsten et al. 1999). The total carbohydrate concentration was determined by spectrophotometry at 490 nm after addition of phenol and sulfuric acid (5 %), according to the colorimetric method of phenol sulfuric acid (Dubois et al. 1956).
Analysis of Total Lipids and Fatty Acids
The Bligh and Dyer (1959) method was used for lipid extraction. The macerated lyophilized algae (0.02 g dry mass) were suspended in PBS, followed by the addition of 7.6 μl of C13:0 analytical standard (glyceryl tritridecanoate) solution (5 mg ml−1 in hexane) and 750 μl of chloroform/methanol/water (2:2:1). The mixture was centrifuged, and the chloroform phase was transferred to another flask and dried under N2 (g). The total lipid content was determined gravimetrically, and the dry extract of lipid was methylated to assess fatty acid content.
The methylation reaction of fatty acids to fatty acid methyl esters (FAME) was performed by dissolving the dry extract of lipid in 61 μl of BF3 (7 % in methanol) and 30 μl of toluene and heated to 100 °C for 45 min. After the reaction, water was added at room temperature, and FAME was extracted with hexane. Chromatographic analysis and quantification of the FAMEs were performed as previously described.
The FAMEs were analyzed by gas chromatography coupled with mass spectrometry (QP2010; Shimadzu, Kyoto, Japan) with a 30-m fused silica capillary column (VF-Wax with 0.25 μm film; Agilent). A sample (1 μl) was injected at 220 °C in split mode. Helium was used as the carrier gas at a flow rate of 1 ml min−1 with the following temperature ramp: initial temperature of 60 °C with an increase of 5 °C per minute up to 260 °C, which was maintained for 10 min.
The standard used to identify the peaks was Supelco 37 (47885-U). The fatty acids were identified by comparison with retention times of standards and/or by comparison of their mass spectra with the spectra library (NIST).
Most FAMEs could be quantified using the line equation of the standard curve of the respective FAME of the Supelco 37 standard. For FAMEs not in the Supelco standard, quantification was performed considering the concentration of the internal standard (C13:0) since peak area was proportional to the concentration of FAME.
Carbon and Nitrogen Content
One milligram of macerated lyophilized sample was introduced into the PerkinElmer 2400 Series II CHNS/O Elemental Analyzer System (Billerica, MA), where combustion occurred at 925 °C in the presence of pure oxygen. The resulting gases (CO2, N2, and H2O) were carried by pure helium gas and subsequently homogenized and separated by specific columns packed with silica. The detection was made by a thermal conductivity detector (Perkin Elmer). The accuracy of the device was ±0.3 % for C, H, and N.
Statistical Analysis
All analyses were performed in triplicate. Data were submitted to variance analysis (ANOVA) of one factor, followed by the Student-Newman-Keuls comparison test, considering confidence level of 95 %.
Results
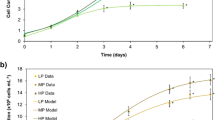
The GR of D. menstrualis varied among treatments, being higher with the addition of NO3 −. Among the treatments with NO3 −, the highest growth occurred when aeration was provided, irrespective of adding CO2 (F 58.22; P < 0.001) (Fig. 1).
Wide variations in Pmax (F 92.95; P < 0.001) and Ik (F 50.00; P < 0.001) were observed among different treatments, and the highest values occurred when D. menstrualis was grown in seawater containing CO2 and NO3 − (Fig. 2, Table 2). EQY (F 37.40; P < 0.001) was greater in specimens cultured in all treatments containing NO3 − and alpha (F 14.49; P < 0.001) was higher in treatment with the addition of NO3 −, but without CO2 (Table 2).
Light/dark induction curves (Kautsky) and PQY recovery showed similar characteristics between samples grown in different treatments (Fig. 3). The highest values of PQY occurred in algae grown with the addition of NO3 − (Fig. 3). The initial and final values of PQY show a high capacity for recovery, except for specimens cultured in culture medium aerated without CO2 and NO3 −, which showed a recovery of approximately 71 % (Table 3).
D. menstrualis grown in the presence of CO2 and NO3 − showed the highest ETR, which gradually increased, becoming constant from about one light-minute. This gradual increase in ETR was also observed for samples grown without aeration, but with NO3 −. Since no significant difference in ETR values could be observed between the first and last periods of light, the same results were not observed for other treatments (Fig. 4).
Electron transport rate (ETR) presented by D. menstrualis cultivated in bioreactors with and without addition of nitrate and CO2 to seawater during dark/light induction curves and recovery period of the quantum yield of algae acclimated to dark. Values represent the mean ± standard deviation (n = 3)
In all treatments, NPQ increased with increasing exposure to light. Specimens grown in culture medium aerated without CO2 and NO3 − showed the highest values of NPQ. A trend of decreasing NPQ values was observed following the termination of light exposure, except for samples grown in treatments without CO2 and NO3 − (Fig. 5).
Non-photochemical quenching (NPQ) presented by D. menstrualis cultivated in bioreactors with and without the addition of nitrate and CO2 to seawater during dark/light induction curves and recovery period of the quantum yield of algae acclimated to dark. Values represent the mean ± standard deviation (n = 3)
The highest values of NR (F 45.48; P < 0.001) and Rubisco (F 8.56; P = 0.017) activities occurred when CO2 and NO3 − were added. It was not possible to detect the enzymatic activity of Rubisco and NR for samples of D. menstrualis cultivated in culture medium without addition of NO3 − by the methods used (Fig. 6a, b). Small differences in CA activity were observed among treatments (F 7.88; P = 0.009), and enzyme activity was higher in specimens cultured with the addition of NO3 − (Fig. 6c).
Nitrate reductase (a), Rubisco (b), and carbonic anhydrase (c) activity of D. menstrualis cultivated in bioreactors with and without the addition of nitrate and CO2 to seawater. Values represent the mean ± standard deviation (n = 3). Treatments with different letters are significantly different according to the multiple comparison Student-Newman-Keuls test. The CA activity was not evaluated in the treatment Ar-N.
Chlorophyll a content was higher in all treatments containing NO3 − and in treatment containing CO2 without NO3 − (F 161.23; P < 0.001) (Fig. 7a). The total lipid content was higher in treatments with CO2 and NO3 − (F 11.83; P < 0.001) (Fig. 7b). The content of soluble carbohydrates was higher in treatments with CO2 and NO3 − (F 19.38; P < 0.001), while the content of total soluble protein (F 32.00; P < 0.001) was higher in all NO3 − treatments (Fig. 7c, d).
Chlorophyll a (a), total lipids (b), total soluble carbohydrates (c), and total soluble protein (d) content of D. menstrualis cultivated in bioreactors with and without the addition of nitrate and CO2 to seawater. Values represent the mean ± standard deviation (n = 3). Treatments with different letters are significantly different according to the multiple comparison Student-Newman-Keuls test
The total nitrogen content of algal tissue was higher (F 125.68; P < 0.001) when treated with NO3 − (Fig. 8a). Total C content also varied (F 48.05; P < 0.001), although this variation was smaller. The largest values of C occurred when the specimens were grown in treatments without NO3 − but either with or without the addition of CO2 (Fig. 8b). Considerable variation was observed in C/N ratio (F 52.93; P < 0.001), and it was higher in treatments without the addition of NO3 − with or without the addition of CO2 (Fig. 8c).
Total nitrogen (a) and total carbon (b) content and C/N ratio (c) in the tissue of D. menstrualis cultivated in bioreactors with and without the addition of nitrate and CO2 to seawater. Values represent the mean ± standard deviation (n = 3). Treatments with different letters are significantly different according to the multiple comparison Student-Newman-Keuls test
The total fatty acid content was higher in all treatments containing NO3 − (ca. 40 %) (F 11.97; P < 0.001). The presence of NO3 − also stimulated the synthesis of polyunsaturated fatty acids by about twofold (F 35.25; P < 0.001). However, no significant differences in the concentration of saturated fatty acids were observed among the different treatments. For monounsaturated fatty acids, the lowest concentration occurred when D. menstrualis was grown without aeration and without NO3 −, with no significant differences among the other treatments (F 5.64; P = 0.007) (Table 4).
Palmitic acid was the major fatty acid found in D. menstrualis biomass for all treatments. However, myristic (C14:0) FA showed variation. While the content of C14:0 was higher than C18:0 in treatments with NO3 − but without CO2, the opposite occurred in treatments without NO3 − and CO2. This difference could be attributed to variation in the content of C14:0 among treatments (F 16.18; P < 0.001), while the concentration of C18:0 and other saturated FAs remained constant (Table 5).
For monounsaturated fatty acids, the concentration of C16:1ω7 was higher in treatments with the addition of NO3 − without CO2 (F 19.37; P < 0.001). On the other hand, the concentration of C18:1ω9 was higher in treatments with aeration and without CO2, but either with or without NO3 −, as well as treatment with CO2 but without NO3 − (F 4.39; P = 0.017) (Table 5).
The major polyunsaturated fatty acid was stearidonic acid (C18:4ω3), followed by arachidonic acid (C20:4ω6). Eicosapentaenoic acid (C20:5ω3) was the only polyunsaturated fatty acid whose concentration did not differ among all treatments. The content of other polyunsaturated fatty acids varied significantly, and, in general, the concentration was higher when NO3 − was added (Table 5).
The content of ω-3 was greater than that of ω-6 in all treatments. The concentration of these fatty acids varied, being higher in treatments with NO3 − (Table 5).
Discussion
The CO2 concentration tested was very high, about 44 times greater than the concentration of treatments without CO2 injection. While GR of D. menstrualis grown under these conditions were not inhibited, it was limited by the absence of NO3 − in the culture medium. In treatments with NO3 − addition, GR ranged from 14 to 16 % day−1. The same was not observed for Ulva rigida C. Agardh (Chlorophyta), Lomentaria articulata (Hudson) Lyngbye (Rhodophyta), and Hizikia fusiforme (Harv.) Okamura (Phaeophyceae) since the increase in CO2 concentration resulted in an increment in their GR (Gordillo et al. 2001; Kubler et al. 1999; Zou 2005). However, for Porphyra leucosticta Thuret et Le Jolis (Rhodophyta), the increase in CO2 concentration in air to only 1 % caused a decrease of GR from 5.8 ± 1.39 (no CO2 treatment) to 0.91 ± 0.11 (treatment with CO2) (Mercado et al. 1999). The growth rate presented by D. menstrualis was high when compared to other seaweeds species. For example, the highest growth rate presented by H. fusiforme was near of 9 % day−1 (Zou 2005). Kuhlenkamp et al. (2001) evaluated the growth of Dictyota dichotoma in different light conditions. The highest growth rates occurred when it was grown with PAR and the values range from about 10 to 15 % day−1.
Cl a content of D. menstrualis was higher in treatments with NO3 −. However, when CO2 was added in the culture medium, Cl a concentration of D. menstrualis cultivated without NO3 − was similar to all treatments with NO3 − added. This result shows that decreased chlorophyll content caused by NO3 − limitation does not occur in high CO2 concentration.
The increase of CO2 did not affect GR. Still, the values of Ik and ETRmax showed a significant increase when D. menstrualis was grown in culture medium with both CO2 and NO3 −. These results were not observed by Zou (2005) who reported no significant difference in the ETRmax values of H. fusiforme grown in treatments with and without CO2 enrichment. However, Ik was lower when this species was grown with CO2. For P. leucosticta, the increase in CO2 concentration resulted in an increase in ETRmax (Mercado et al. 1999).
Differences in ETRmax values of D. menstrualis may be related to the concentration of photosynthetic units and their minimal turnover time. The turnover time of the photosynthetic apparatus, on the other hand, changes as a result of alterations in the electron transfer rate between photosystem I and II (Mercado et al. 1999). Thus, higher values of Ik and ETRmax indicate a higher concentration and better functioning of the photosynthetic apparatus of D. menstrualis cultivated with both CO2 and NO3 −, as evidenced by higher ETR presented in both PI and Kautsky curves. Moreover, in specimens cultured in this treatment, both ETR and EQY increased rapidly during the first minutes of Kautsky curve illumination, indicating activation of Calvin cycle enzymes and increase in CO2 fixation (Heins Walz GmbH 1998), a process that was also evidenced by increased Rubisco activity. However, in treatment with aeration, without the addition of CO2 and NO3 −, the ETR values of Kautsky curve were the lowest, while the NPQ values were the highest. During dark adaptation, the enzymes of the Calvin cycle are partially inactivated. These enzymes are activated by light during the first minutes of illumination. In this period, O2, but not CO2, is the final electron acceptor. The O2-dependent electron flow and cyclic electron flow of photosystem I create a proton gradient used for the synthesis of ATP, which will be consumed when the Calvin cycle is activated. During this period, NPQ increases, but then declines with increasing CO2 fixation and ATP consumption (Heins Walz GmbH 1998). Thus, as shown by the low ETR values and high NPQ values during the period of illumination of the Kautsky curve, the Calvin cycle of D. menstrualis, cultivated with aeration, without CO2 and NO3 − addition, was not fully active, possibly resulting from the lower number of enzymes. However, it was not possible to measure Rubisco in this treatment by the method used.
Similar to Rubisco activity observed in D. menstrualis, NR activity was also higher when the algae were cultured with both CO2 and NO3 −. However, little variation was observed in CA activity among different treatments. Similar results were obtained by Zou (2005) who reported that an increase in CO2 concentration in the culture medium led to an increase in NO3 −uptake and NR activity of H. fusiforme, indicating an increase in N assimilation. Despite this, the contents of soluble protein and total N in the tissue of D. menstrualis varied, depending on the presence/absence of NO3 − in the culture medium and not the presence of CO2. For P. leucosticta, an increase of CO2 led to a decrease in total soluble protein content (Mercado et al. 1999). An inverse relationship was also noted between N content of L. articulata tissue and CO2 concentration in the culture medium (Kubler et al. 1999). For Gracilaria lemaneiformis (Bory) Weber-van Bosse (Rhodophyta), no change in protein content could be correlated with CO2 concentration in culture medium (Zou and Gao 2009).
The maximum NR activity of D. menstrualis observed in the present study was 11.5 (±1.4) nmol NO3 − min−1 mg−1 protein, in the treatment with both CO2 and NO3 −. Young et al. (2005) evaluated the NR activity of Dictyota sp. and the value found was 40.6 (±1.6) nmol NO3 − min−1 g−1 FW. These values vary according to the species and growing conditions or the site and time of collection. For example, Young et al. (2005) studying three species of Fucus observed values ranging from 165 to 290 nmol NO3 − min−1 g−1 FW. NR activity was not detected by the method applied in the seaweed grown in the absence of nitrate due to the low protein concentration in the thallus. The results presented in this study are similar with the paper of Young et al. (2009) which observed values of NR activities near to zero for Fucus vesiculosus and Fucus serratus grown for 15 weeks under N deprivation conditions. The same can be said for the activity of Rubisco since the results found in the treatments with nitrate addition were similar to those reported by Wang et al. (2011).
The content of total soluble carbohydrates of D. menstrualis was higher in treatments with both CO2 and NO3 −. Mercado et al. (1999) also noted an increase in the content of total soluble carbohydrates of P. leucosticta with the increase of CO2 in the medium. It has been proposed that N starvation stimulates algae lipid synthesis (Rosenberg et al. 2008). However, D. menstrualis had the highest content of total lipids with NO3 − addition and also there was a CO2 stimulation. For G. lemaneiformis, the response to N starvation depended on CO2 concentration, and in low CO2, lipid synthesis increased (Zou and Gao 2009).
When D. menstrualis was grown in treatments with both CO2 and NO3 −, the content of total lipids was next to 200 mg g−1 dw. This value is higher than those found by Gosch et al. (2012), who studied different species of red, green, and brown seaweeds collected in the field. They could observe that the brown algae have the highest content of total lipids, followed by green algae. Inside the brown algae, the highest values, around 120 mg g−1 dw, occurred in order Dictyotales, particularly in the genus Dictyota and Spatoglossum. The highest value found in the present study may be due to the fact that D. menstrualis samples were derived from the cultivation and not from the field.
The content of total fatty acids of D. menstrualis was lower than that found for other Dictyota species (Gosch et al. 2012, 2015), and greater than that found for other species of red and green algae (Gosch et al. 2012; Gressler et al. 2010, 2011a). There was no significant difference in the content of total saturated fatty acids and total polyunsaturated fatty acids in species of Dictyota studied by Gosch et al. (2012). In the present study, the content of total fatty acids and polyunsaturated fatty acids in D. menstrualis was higher in treatments with NO3 −, irrespective of CO2 concentration. Consequently, in these treatments, the content of total polyunsaturated fatty acids was greater than the total saturated fatty acids. Similar results were found by Hu and Gao (2006) who reported an increase in polyunsaturated fatty acids in Nannochloropsis sp. (Eustigmatophyte), as a function of NO3 − concentration in the culture medium. Unlike the results of this work, Tsuzuki et al. (1990) found that CO2 concentration affected fatty acid composition of the microalgae Chlorella vulgaris Beyerinck (Chlorophyta), and the degree of unsaturation of these fatty acids was highest in cells grown with low CO2 concentration.
The increased in the content of polyunsaturated fatty acids was caused mainly by an increment in the content of C18:4ω3, the major polyunsaturated fatty acid present in Dictyota menstrualis biomass. Although this fatty acid has not been found in biomass of other seaweeds species (Gosch et al. 2012; Gressler et al. 2010, 2011a), it was quantified in large quantities in Dictyota bartayresii, D. dichotoma, Dictyopteris australis, and Spatoglossum macrodontum (Gosch et al. 2012, 2015).
To summarize, with the addition of NO3 − in the culture medium, D. menstrualis showed a higher growth rate, an increase in the assimilation of this nutrient and CO2, which was demonstrated by the increased in NR and Rubisco activity, and an increase in the biosynthesis of Cl a, proteins, lipids, and polyunsaturated fatty acids. The increase in CO2 concentration also stimulated the assimilation of nitrate and CO2 and caused an increase in photosynthesis and biosynthesis of lipids and carbohydrates.
The fatty acid profile of D. menstrualis cultivated in bioreactors was characterized by a high content of polyunsaturated fatty acids, especially ω-3, which is in accordance with literature data (Gosch et al. 2012, 2015). It should be noted that ω-3 and ω-6 are precursors of important metabolites and hormones related to human health. Therefore, they do have a very high market value. For ω-3, an annual market has been estimated at $35 billion for 2016.
Thus, D. menstrualis has to be considered an important source of omega family fatty acid, and its biomass from bioreactor cultivation is suitable for use as a nutraceutical. In addition, the GR, photosynthetic rate, and fatty acid content of D. menstrualis were higher than other species of macroalgae, highlighting its economic importance and the possibility of different phytomedicinal and industrial applications.
References
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cardozo KHM, Carvalho VM, Pinto E, Colepicolo P (2006) Fragmentation of mycosporine-like amino acids by hydrogen/deuterium exchange and electrospray ionisation tandem mass spectrometry. Rapid Commun Mass Spectrom 20(2):253–258
Chapman DJ, Harrison PJ (1988) Nitrogen metabolism and measurement of nitrate reductase activity. In: Lobban CS, Chapman DJ, Kremer B (eds) Experimental phycology. A laboratory manual. Cambridge University Press, Cambridge
Chow F, Oliveira MC, Pedersén M (2004) In vitro assay and light regulation of nitrate reductase in red alga Gracilaria chilensis. J Plant Physiol 161:769–776
Dickson AG, Sabine CL, Christian JR (2007) Guide to best practices for ocean CO2 measurements. PICES Special Publication. Available in ftp://cdiac.ornl.gov/pub/oceans/Handbook_2007/Guide_all_in_one.pdf. Accessed 9 June 2011
Dubois M, Gilles FA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for the determination of sugars and related substances. Anal Chem 28:350–356
Gerard VA, Driscoll T (1996) A spectrophotometric assay for Rubisco activity: application to the kelp Laminaria saccharina and implications for radiometric assays. J Phycol 32:880–884
Gordillo FJ, Niell FX, Figueroa FL (2001) Non-photosynthetic enhancement of growth by high CO2 level in the nitrophilic seaweed Ulva rigida C. Agardh (Chlorophyta). Planta 213:64–70
Gosch BJ, Magnusson M, Paul NA, Nys R (2012) Total lipid and fatty acid composition of seaweeds for the selection of species for oil-based biofuel and bioproducts. GCB Bioenergy 4:919–930
Gosch BJ, Paul NA, Nys R, Magnusson M (2015) Spatial, seasonal, and within-plant variation in total fatty acid content and composition in the brown seaweeds Dictyota bartayresii and Dictyopteris australis (Dictyotales, Phaeophyceae). J Appl Phycol 27:1607–1622
Gressler V, Colepicolo P, Pinto E (2009) Useful strategies for algal volatile analysis. Curr Anal Chem 5:271–292
Gressler V, Yokoya NS, Fujii MT, Colepicolo P, Mancini-Filho J, Torres RP, Pinto E (2010) Lipid, fatty acid, protein, amino acid and ash contents in four Brazilian red algae species. Food Chem 120:585–590
Gressler V, Fujii MT, Martins AP, Colepicolo P, Mancini-Filho J, Pinto E (2011a) Biochemical composition of two red seaweed species grown on the Brazilian coast. J Sci Food Agric 91:1687–1692
Gressler V, Stein EM, Dörr F, Fujii MT, Colepicolo P, Pinto E (2011b) Sesquiterpenes from the essential oil of Laurencia dendroidea (Ceramiales, Rhodophyta): isolation, biological activities and distribution among seaweeds. Rev Bras Farmacogn 21:248–254
Haglund K, Bjork M, Ramazanov Z, Garcia-Reina G, Pedersén M (1992) Role of carbonic anhydrase in photosynthesis and inorganic-carbon assimilation in the red alga Gracilaria tenuistipitata. Planta 187:275–281
Hanisak MD (1983) The nitrogen relationships of marine macroalgae. In: Carpenter EJ, Capone DG (eds) Nitrogen in the marine environment. Academic Press Inc, New York
Heins Walz GmbH (1998) Underwater fluorometer diving-PAM. Submersible photosynthesis yield analyzer. Handbook of operation. Heins Walz GmbH, Germany
Hu H, Gao K (2006) Response of growth and fatty acid compositions of Nannochloropsis sp. to environmental factors under elevated CO2 concentration. Biotechnol Lett 28:987–992
Jeffrey SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, b, c1 e c2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pflanz 167:191–194
Karsten U, West JA, Zuccarello GC, Nixdorf O, Barrow KD, King RJ (1999) Low molecular weight carbohydrate patterns in the Bangiophyceae (Rhodophyta). J Phycol 35:967–976
Kubler JE, Johnston AM, Raven JA (1999) The effects of reduced and elevated CO2 and O2 on the seaweed Lomentaria articulata. Plant Cell Environ 22:1303–1310
Kuhlenkamp R, Franklin LA, Lüning K (2001) Effect of solar ultraviolet radiation on growth in the marine macroalga Dictyota dichotoma (Phaeophyceae) at Helgoland and its ecological consequences. Helgol Mar Res 55:77–86
Lavigne H, Proye A, Gattuso J-P (2009) Calculates parameters of the seawater carbonate system. Package seacarb. http://epic.awi.de/28989/1/Lav2009a.pdf. Accessed 12 Jul 2011
Lobban CS, Harrison PJ (1994) Seaweed ecology and physiology. Cambridge University Press, Cambridge
Martins AP, Jr Necchi O, Colepicolo P, Yokoya NS (2011) Effects of nitrate and phosphate availabilities on growth, photosynthesis and pigment and protein contents in colour strains of Hypnea musciformis (Wulfen in Jacqu.) J.V. Lamour. (Gigartinales, Rhodophyta). Rev Bras Farmacogn 21:340–348
Martins AP, Colepicolo P, Yokoya NS (2012) Comparison of extraction and transesterification methods on the determination of the fatty acid contents of three Brazilian seaweed species. Rev Bras Farmacogn 22:854–860
Mercado JM, Javier F, Gordillo L, Niell FX, Figueroa FL (1999) Effects of different levels of CO2 on photosynthesis and cell components of the red alga Porphyra leucosticta. J Appl Phycol 11:455–461
Moraes FP, Colla LM (2006) Alimentos funcionais e nutracêuticos: definições, legislação e benefícios à saúde. Rev Eletrônica Farm 3:109–122
Moreira NX, Curi R, Mancini-Filho J (2002) Ácidos graxos: uma revisão. Rev Nutrire 24:105–123
Necchi O (2004) Light-related photosynthetic characteristics of lotic macroalgae. Hydrobiologia 525:139–155
Oliveira EC, Paula EJ, Plastino EM, Petti R (1995) Metodologias para cultivo no axenico de macroalgas marinhas in vitro. In: Alveal K, Ferrario ME, Oliveira EC, Sar E (eds) Manual de métodos ficológicos. Universidad de Concepción, Concepcion
Patarra ARF (2008) Pesquisa de ácidos gordos em macroalgas marinhas do litoral dos açores. Master dissertation. Universidade do Porto
Platt T, Gallegos CL, Harrison WG (1980) Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J Mar Res 38:687–701
Rosenberg JN, Oyler GA, Wilkinson L, Betenbaugh MJ (2008) A green light for engineered algae: redirecting metabolism to fuel a biotechnology revolution. Curr Opin Biotechnol 19:430–436
Schenk PM, Thomas-Hall SR, Stephens E, Marx UC, Mussgnug JH, Posten C, Kruse O, Hankamer B (2008) Second generation biofuels: high-efficiency microalgae for biodiesel production. BioEnerg Res 1:20–43
Schreiber U, Bilger W, Neubauer C (1994) Chlorophyll fluorescence as a non-intrusive indicator for rapid assessment of in vivo photosynthesis. In: Schulze E-D, Caldwell MM (eds) Ecophysiology of photosynthesis. Springer, Berlin, p 49–70
Simas-Rodrigues C, Villela HDM, Martins AP, Marques LG, Colepicolo P, Tonon AP (2015) Microalgae for economic applications: advantages and perspectives for bioethanol. J Exp Bot 66:4097–4108
Taiz L, Zeiger E (2004) Fisiologia vegetal. Artmed, Porto Alegre
Tsuzuki M, Ohnuma E, Sato N, Takaku T, Kawaguchi A (1990) Effects of CO2 concentration during growth on fatty acid composition in microalgae. Plant Physiol 93:851–856
van den Hoek C, Mann DG, Jahns HM (1995) Algae: an introduction to phycology. Cambridge University Press, Cambridge
van Iersel S, Flammini A (2010) Algae-based biofuels: applications and co-products. FAO environmental and natural resources management working paper. Food and Agriculture Organization of the United Nations, Rome
Wall R, Ross RP, Fitzgerald GF, Stanton C (2010) Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev 68:280–289
Wang C, Fan X, Wang G, Niu J, Zhou B (2011) Differential expression of rubisco in sporophytes and gametophytes of some marine macroalgae. PLoS One. doi:10.1371/journal.pone.0016351
Yokoya NS (2000) Apical callus formation and plant regeneration controlled by plant growth regulators on axenic culture of the red alga Gracilariopsis tenuifrons (Gracilariales, Rhodophyta). Phycol Res 48:133–142
Yokoya NS, Necchi O Jr, Martins AP, Gonzalez SF, Plastino EM (2007) Growth responses and photosynthetic characteristics of wild and phycoerythrin-deficient strains of Hypnea musciformis (Rhodophyta). J Appl Phycol 19:197–205
Young EB, Lavery PS, van Elven B, Dring MJ, Berges JA (2005) Nitrate reductase activity in macroalgae and its vertical distribution in macroalgal epiphytes of seagrasses. Mar Ecol Prog Ser 288:103–114
Young EB, Berges JA, Dring MJ (2009) Physiological responses of intertidal marine brown algae to nitrogen. Physiol Plant 135:400–411
Zou D (2005) Effects of elevated atmospheric CO2 on growth, photosynthesis and nitrogen metabolism in the economic brown seaweed, Hizikia fusiforme (Sargassaceae, Phaeophyta). Aquaculture 250:726–735
Zou D, Gao K (2009) Effects of elevated CO2 on the red seaweed Gracilaria lemaneiformis (Gigartinales, Rhodophyta) grown at different irradiance levels. Phycologia 48:510–517
Acknowledgments
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) [projects 10/50193-1 and 12/19844-1], Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) [by scholarships provided to the first author], Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil), and the Ministério da Saúde, Ministério de Ciência e Tecnologia and CNPq-INCT-Redoxoma.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martins, A.P., Yokoya, N.S. & Colepicolo, P. Biochemical Modulation by Carbon and Nitrogen Addition in Cultures of Dictyota menstrualis (Dictyotales, Phaeophyceae) to Generate Oil-based Bioproducts. Mar Biotechnol 18, 314–326 (2016). https://doi.org/10.1007/s10126-016-9693-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-016-9693-9