Abstract
A hyaluronate lyase (BniHL) was purified to homogeneity from a culture of a deep-sea Bacillus niacin strain JAM F8. The molecular mass of purified BniHL was approximately 120 kDa. The purified enzyme degraded hyaluronan as well as chondroitin sulfates A and C by a β-elimination mechanism. The optimal pH and temperature were around pH 6 and 45 °C for hyaluronan degradation. The enzyme required optimally 2, 50, and 100 mM calcium ions for degradation of hyaluronan, chondroitin sulfate C, and chondroitin sulfate A, respectively. Calcium ions slightly increased the thermal stability of the enzyme. In a genome analysis of strain JAM F8, a BniHL coding gene was identified on the bases of the molecular mass and N-terminal and internal amino acid sequences. The gene consisted of 3411 nucleotides and coded 1136 amino acids. The deduced amino acid sequence showed the highest similarity to the hyaluronate lyase of a Bacillus sp. A50 with 89 % identity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hyaluronan (HA) is a linear polysaccharide comprised of repeating disaccharide units of [(1–› 3) β-d-N-acetyl-glucosamine-(1–› 4) β-d-glucuronic acid], and classified as a glycosaminoglycan neither sulfated nor covalently linked to a protein to form a proteoglycan (Senni et al. 2011). HA is abundantly present in nearly all vertebrate tissues, especially extracellular matrix, and in marine invertebrates, only mollusk bivalves produce HA (Yasuda et al. 2011). HA is also produced by some bacteria, for instance, the genus Streptococcus (Jedrzejas 2000; Volpi and Maccari 2003; Senni et al. 2011). HA is degraded by hyaluronidases (EC 3.2.1.35, EC 3.2.1.36) and hyaluronate lyases (EC 4.2.2.1) (HL). Gram-positive and gram-negative bacteria produce HLs, which degrade HA through a β-elimination mechanism, and the final degradation product is unsaturated hyaluro-disaccharide. Many HLs can degrade not only HA but also chondroitin sulfate (ChS) at lower degradation rates, and chondroitin sulfates AC lyases degrade ChS at higher rates than HL does (Hiyama and Okada 1975; Ingham et al. 1979; Linn et al. 1983; Tam and Chan 1985; Hong et al. 2002; Girish and Kemparaju 2007). ChSs are found in shark skin and cartilage, shrimps, and crab (Cássaro and Dietrich 1977; Petit et al. 2006; Senni et al. 2011). Although HA and ChSs are present in only small amounts, HLs may play a pivotal role in a wide range of their recyclings in marine (deep-sea) environments. Actually, chondroitinase (chondroitin sulfates lyase)-producing marine bacteria have been easily isolated (Kitamikado and Lee 1975). On the other hand, there are few reports on HL of marine microorganisms. Recently, we isolated strain JAM F8 using chondroitin sulfate C (ChS-C) as a carbon source and determined it belongs to genus Bacillus. We found that the enzyme produced was more active toward HA than ChS-C, which is the first HL of the deep-sea bacterium. Furthermore, glycosaminoglycan-degrading enzymes from Bacillus strains are rare except for the heparinase and chondroitinase of Bacillus sp. strain BH100 (Bellamy 1990), and the recently reported hyaluronate lyase of Bacillus sp. A50 (Guo et al. 2014). We would like to improve the knowledge about HLs of marine (deep-sea) microorganisms and a HA catabolic pathway of the genus Bacillus. In this report, we describe the purification and enzymatic properties of the first deep-sea HL from Bacillus strain JAM F8 and its nucleotide sequence by genome analysis.
Materials and Methods
Isolation of Hyaluronate Lyase-Producing Bacteria
Deep-sea sediments were collected from the Izu-Ogasawara Trench, Japan (30° 07.05 N, 139° 58.42 E), at a water depth of 2759 m. A portion of sediment suspensions were spread on marine agar 2216 (Difco, Detroit, MI) containing 0.2 % ChS-C (Wako Pure Chemical, Osaka, Japan). After microorganisms had grown at 30 °C for 2 days, a 0.8 % soft agar containing 100 mM MOPS buffer (pH 7) and 0.2 % ChS-C was poured over the agar. After the soft agar solidification, plates were incubated at 30 °C for 2–3 h. Microorganisms showing translucent zones around colonies were detected as ChS-degrading enzyme producers by the method of Smith and Willett (1968) using 1 % bovine serum albumin in 2 N acetic acid. They were picked up and purified on marine agar 2216 several times. The isolates were propagated with shaking in test tubes containing 2 mL of marine broth 2216 and 0.2 % ChS-C at 30 °C for 1 day. ChS lyase activities in the supernatants were measured. One of the isolates among eight candidates, strain JAM F8, showed the highest degrading activity toward both ChS-C and HA.
Purification of BniHL of Strain JAM F8
A culture supernatant (800 mL) was concentrated to 65 mL with an ultrafiltration module (Microza AIP-0013, Asahi Kasei, Tokyo; 6 kDa Mr cut-off). The concentrate was adequately diluted with distilled water, and the solution was applied to a SuperQ-Toyopearl column (1.5 × 8 cm; Tosoh, Tokyo, Japan) equilibrated with 50 mM Tris–HCl buffer plus 1 mM CaCl2 (pH 7.5). The absorbed proteins were eluted with 160-mL of a linear gradient of 0 to 0.2 M NaCl in the buffer. Ammonium sulfate was added to the combined active fractions at a final concentration of 1 M. The solution was applied to a Butyl-Toyopearl column (1.5 × 6 cm; Tosoh) equilibrated with 1 M ammonium sulfate in 10 mM Tris–HCl buffer plus 1 mM CaCl2 (pH 7.5). Proteins were eluted with 120-mL of a linear gradient of 1 to 0.4 M ammonium sulfate. Fractions showing HL activity were pooled and concentrated by ultrafiltration using an Amicon Ultra-15 (10 kDa Mr cut-off; Millipore, Billerica, MA). The concentrate was sequentially diluted and ultrafiltrated several times with 10 mM Tris–HCl buffer plus 1 mM CaCl2 (pH 7.5) to remove ammonium sulfate.
Determination of 16S rRNA Sequence of Strain JAM F8
The 16S rRNA gene of strain JAM F8 was amplified by PCR using the universal primers 27f (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492r (5′-TACGGTTACCTTGTTACGACTT-3′), and a colony of strain JAM F8 was used as the template in a DNA thermal cycler (model 9700; Applied Biosystems, Foster City, CA) with an LA Taq DNA polymerase (Takara Bio, Shiga, Japan). Nucleotide sequencing was performed using a DNA sequencer (model 377; Applied Biosystems) with an ABI Prism BigDye terminator sequencing kit (Applied Biosystems).
Enzyme Assay and Protein Content
HL activity was measured in 0.5 mL of a reaction mixture composed of 0.2 %(w/v) HA (Wako Pure Chemical), 100 mM acetate buffer (pH 6), 5 mM CaCl2, and enzyme solution. After incubation at 30 °C for 20 min, the reaction was stopped by adding 2 mL of 5 mM HCl, and then the absorbance of the solution was measured at 235 nm. One unit of enzyme activity was defined as the amount of the protein that produced unsaturated oligohyaluronate equivalent to 1 μmol of unsaturated hyaluronate disaccharide using the molecular extinction coefficient value of 5500 M−1 cm−1 at 235 nm (Lin et al. 1994; Hamai et al. 1997). When ChS-A, ChS-C, and heparin (Wako Pure Chemical), ChS-B (Sigma), and chondroitin (Seikagaku Kogyo, Tokyo, Japan) were used as the substrates, all procedures were the same as the case of HA. Alternatively, a viscometric assay was done using an Ostwald viscometer (No. 1; Shibata Scientific Technology, Tokyo, Japan). The reaction mixture included 0.2 % HA, 100 mM acetate buffer (pH 6), 5 mM CaCl2, and a suitably diluted enzyme solution in a total volume of 10 mL. The decrease in viscosity of the mixture was measured at 30 °C at timed intervals. Protein was quantified with a DC Protein Assay Kit (Bio-Rad, Hercules, CA) using bovine serum albumin as the standard.
SDS-PAGE
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) was done by the method of Laemmli (1970) using a 12.5 % polyacrylamide gel (Bio-Rad). Precision-plus protein standards (Bio-Rad) were used as the molecular mass markers for SDS-PAGE.
Analysis of N-terminal and Internal Amino Acid Sequences
After SDS-PAGE, the purified enzyme and lysylendopeptidase-digested enzyme fragments were electro-blotted on a polyvinylidene difluoride membrane (Immobilon; Millipore) that had been wetted with methanol. The N-terminal amino acid sequences were determined by a protein sequencer (model 497HT; Applied Biosystems).
Analysis of Degradation Product
The degradation products of HA and ChS-C by the purified enzyme were analyzed by thin layer chromatography (TLC) and electrospray ionisation mass spectrometry (ESI/MS) spectroscopy. The reaction mixture composed of 0.2 % substrate, 2.5 mM CaCl2, 100 mM acetate buffer (pH 6), and a suitably diluted enzyme was incubated at 30 °C up to 18 h. Samples (50 μL) were periodically withdrawn and inactivated at 100 °C for 2 min. The products were analyzed on a silica gel 60 with concentration zone (Merck, Darmstadt, Germany) using a solvent system of n-propanol/ethyl acetate/ammonia (28 % aq. solution)/water = (6/1/1/3, v/v). Spots on the plate were detected by spraying with anisaldehyde-sulfuric acid reagent.
To determine the molecular masses of degradation products of HA and ChS-C, a 0.5-mL reaction mixture containing 100 mM acetate buffer (pH 6.0), 0.1 % substrate, and the enzyme solution was incubated at 30 °C for 16 h. The reaction was terminated by adding 2 mL of acetonitrile. After centrifugation, the supernatant was filtered and diluted with acetnitrile/76 mM ammonium acetate (1:1 v/v). The solution was introduced into a mass spectrometer (Waters Q-TOF Premier, Micromass MS Technologies, Manchester, UK) at a flow rate of 10 μL/min with the capillary voltage of 2.5 V, collision energy of 5.0 V, and source temperature of 100 °C. 2-Acetamido-2-deoxy-3-O-(β-d-gluco-4-enepyranosyluronic acid)-d-glucose (∆Di-HA), 2-acetamido-2-deoxy-3-O-(β-d-gluco-4-enepyranosyluronic acid)-d-galactose (∆Di-0S), 2-acetamido-2-deoxy-3-O-(β-d-gluco-4-enepyranosyluronic acid)-4-sulfo-d-galactose (∆ Di-4S), and 2-acetamido-2-deoxy-3-O-(β-d-gluco-4-enepyranosyluronic acid)-6-sulfo-d-galactose (∆ Di-6S) were used as authentic samples (Seikagaku Kogyo).
Genome Sequencing of Strain JAM F8 and Identification of BniHL Gene
The draft genome sequencing of strain JAM F8 was performed using an Illumina Hiseq platform (Hokkaido System Science, Sapporo, Japan) as described by Kurata et al. (2014). Briefly, the assembly of the short read sequences was performed with the Velvet program (version 1.2.08, http://www.ebi.ac.uk/~zerbino/velvet/) to construct contigs, and the annotation of the contigs was performed by the Microbial Genome Annotation Pipeline (http://www.migap.org). We searched for the BniHL gene in the annotated genome DNA sequence.
Results and Discussion
Phylogenetic Position of Strain JAM F8
A 1225-bp nucleotide sequence of the 16S rRNA gene of strain JAM F8 (GenBank accession no. AB889607) was analyzed and compared with those of other Bacillus. The highest nucleotide sequence match was with those of Bacillus sp. strain BF149 (AM934692.1) and Bacillus sp. strain LMG20241(AJ316313.1) with 99.4 % identity. The next best match was observed on those of Bacillus niacini strain YM1C7 (EU221338.1) and B. niacini IFO15566 (NR_024695.1) with 99.3 % identities. The results suggest that strain JAM F8 is a relative of B. niacini. The strain has been deposited in the Riken Bioresource Center under deposit no. JCM19735.
Purification of BniHL
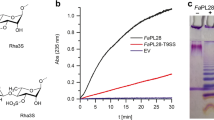
BniHL was purified to homogeneity as judged by SDS-PAGE from a culture broth of strain JAM F8. A typical purification is summarized in Table 1. The purification increased the specific activity 735 times with 6.1 % recovery of the initial activity. The molecular mass was approximately 120 kDa by SDS-PAGE (Fig. 1). The N-terminal amino acid sequence of the purified enzyme was Asn-Glu-Ser-Thr-Leu-Leu-Leu-Asn-Thr-Ser-Phe-Glu-Glu-Thr. Two internal amino acid sequences that were derived from digestion of the purified enzyme by lysylendopeptidase were Thr-Pro-Ala-Asn-Ala-Asp-Ser-Ile-Arg-Val-Gln-Leu and Thr-Phe-Ala-Ser-Met-Asp-Arg-Val-Ile-His-Ser-Lys.
Effects of pH and Temperature on BniHL Activity and Stability
The maximum activity of BniHL was observed at pH 6.0 in 100 mM MOPS buffer for degrading HA at 30 °C for 20 min (Fig. 2a). When the enzyme was incubated at 30 °C for 1 h or at 4 °C for 65 h in 20 mM buffers of various pHs with and without 5 mM CaCl2, the enzyme was stable over the pH range from 6 to 10 in the absence of CaCl2. Ca2+ ions decreased the pH stability of the enzyme, making it stable in the narrower pH range from 7 to 9. The residual activities in the presence of Ca2+ ions were 70 to 80 % or 40 to 60 % of the original activity upon incubation at 30 °C for 1 h or at 4 °C for 65 h, respectively. The result of incubation at 4 °C for 65 h was shown in Fig. 2b as a comprehensive example. When 1 mM CaCl2 was used instead of 5 mM CaCl2, BniHL was stable over the pH range from 6 to 11, regardless if Ca2+ ions were present or not (data not shown). The stability of the enzyme appears to depend on Ca2+ ions concentration. BniHL showed the maximum activity at 45 °C in 100 mM MOPS buffer (pH 6.0). The enzyme activity increased gradually to 45 °C, but it suddenly dropped to 10 % of the maximum activity at 50 °C (Fig. 3a). Thermal stability of BniHL was assessed by incubation at various temperatures for 15 min in 20 mM Tris–HCl buffer (pH 7.5) with and without 5 mM CaCl2. The enzyme was stable during incubation up to 45 °C in the presence of CaCl2, whereas the residual activity incubated at 40 °C without CaCl2 was around 30 % of the initial activity. The residual activity was completely lost at 50 °C both in the presence and absence of CaCl2 (Fig. 3b). Optimal pH and temperature values were similar to those of hyaluronate lyase from Bacillus sp. A50 (Guo et al. 2014).
Effects of pH. a Effect of pH on BniHL activity. HA degrading activity was measured at 30 °C for 20 min in 100 mM following buffers: glycine-HCl (pH 2–4, black square), acetate (pH 4–6, white circle), MOPS (pH 6–8, black circle), Tris–HCl (pH 7–9, white triangle), and glycine-NaOH (pH 8–11, white square) with the purified enzyme (0.047 μg). The highest activity observed in MOPS buffer (pH 6) was taken as 100 %. b Effect of pH on BniHL stability. The enzyme (0.052 μg) was incubated at 4 °C for 65 h in 20 mM buffers with (dotted line) and without (solid line) 5 mM CaCl2, as follows: acetate (pH 4–6, white circle), MOPS (pH 6–8, black circle), glycine-NaOH (pH 8–11, white square), borate (pH 8.9–10.3, multiplication sign), and KCl-NaOH (pH 10.8–12, black triangle). The residual activity was measured under the standard assay conditions using 0.1 mL of the treated enzyme (0.035 μg). The original enzyme activity was taken as 100 %
Effects of temperature. a Effect of temperature on BniHL activity. HL activity was measured under standard assay conditions at the indicated temperature with 0.047 μg purified enzyme. The highest activity observed at 45 °C was taken as 100 %. b Effect of temperature on BniHL stability. The purified enzyme (0.047 μg) was incubated at the indicated temperatures for 15 min in 20 mM buffers with (white circle) and without (black circle) 5 mM CaCl2. The residual activity was measured under the standard assay conditions. The residual activity incubated without CaCl2 at 30 °C for 15 min was taken as 100 %
Substrate Specificity
The most favored substrate for BniHL among those tested was HA. When the degradation rate of HA was taken as 100 %, the enzyme degraded ChS-A, ChS-C, and chondroitin with the relative activities of 44, 30, and 23 %, respectively. ChS-B and heparin were not degraded at all.
Determination of Degradation Products
A viscometric assay revealed that BniHL degraded HA in an endo fashion, as shown in Fig. 4. The viscosity of HA rapidly decreased to 37 % of the original one after 3-min incubation. After more than 10-min incubation, the relative viscosity decreased to less than 10 %, whereas only 6.5 μmol of the unsaturated oligo-hyaluronates had been produced in the reaction mixture after 1 h incubation. The final products were analyzed by TLC and ESI/MS. As shown in Fig. 5, the final main products of HA and ChS-C were detected on TLC as ∆Di-HA and ∆Di-6S, respectively. ESI-MS spectra revealed the fragment ions m/z = 378 and m/z = 458 as the reaction products of HA and ChS-C, respectively. These values were identical to those of authentic ∆Di-HA and ∆Di-6S. The fragment ions of m/z 175 from HA and m/z 87, 97, 175, 282, 300, and 342 from ChS-C were also detected by the ESI-MS/MS analysis with the collision energy of 18 V. These values were also identical to those of the authentic ∆Di-HA and ∆Di-6S (Supplementary Fig. S1).
Relationship between viscosity reduction and glycoside bond cleavage. Purified BniHl (1.5 μg) was added to a reaction mixture composed of 100 mM MOPS buffer (pH 6) plus 2.5 mM CaCl2 and 0.2 % HA in a total volume of 10 mL. The viscosity of the solution was measured at 30 °C at timed intervals. Initial viscosity was measured by adding distilled water instead of enzyme, which was taken to be 100 %. At the indicated intervals, simultaneously prepared samples were withdrawn and 5 mM HCl added to measure absorbance at 235 nm. The percentage of glycoside bond cleavages was calculated on the basis of total hyaluronate disaccharide units in the substrate. Black circle degree of viscosity reduction, white circle extent of glycoside bond cleavage
TLC of degradation products of HA and ChS-C by BniHL. The reaction mixture was incubated in 100 mM MOPS buffer (pH 6) plus 2.5 mM CaCl2 and 0.2 % HA or 0.4 % ChS-C at 30 °C for 1 and 18 h with 0.07 μg or 0.24 μg enzyme, respectively. The experimental procedures are described in “Materials and methods.” Lane 1 ∆Di-0S, lane 2 ∆ Di-4S, lane 3 ∆ Di-6S, lane 4, ∆Di-HA, and lane 5 products from ChS-C after 1 h incubation; lane 6 those after 18 h incubation; lane 7 products from HA after 1 h incubation; lane 8 those after 18 h incubation; and lane 9 ∆Di-HA
Effects of Metals and Chemicals on BniHL Activity
When metal ions (1 mM each) were added to the reaction mixture, the enzyme was fully activated by Ca2+ ions. The enzyme activity was 20 % or less without metal ions when the enzyme activity with Ca2+ ions was taken as 100 %. The next highest activation was observed by adding Mn2+ and Sr2+ ions, when relative activities were 82.1 and 78.6 %, respectively. Co2+, Ni2+, Fe2+, and Mg2+ ions also activated the enzyme activity by 62.2 to 73.9 %, whereas Zn2+, Hg2+, Cu2+, and Fe3+ ions inhibited the enzyme activity by less than 7 % (Supplementary Table S1). As shown in Fig. 6, BniHL activity toward HA, chondroitin sulfate A (ChS-A), and ChS-C was activated by Ca2+ ions in a concentration-dependent manner. The optimum Ca2+ ion concentration was 5 mM for HA degradation, around 50 mM for ChS-A, and around 100 mM for ChS-C. The effects of Ca2+ ions on enzyme activation may depend on substrate-Ca2+ complexes (Winter and Arnott 1977; Pritchard et al. 2000).
Effect of calcium ions on BniHL activities. The enzyme (0.047 μg for HA or 0.14 μg for ChS-A and ChS-C) was incubated in 100 mM MOPS buffer (pH 6.0) containing 0.01 to 1000 mM CaCl2, and 0.1 % HA (black circle with solid line), 0.2 % ChS-A (black triangle with coarse dotted line), or 0.2 % ChS-C (white triangle with fine dotted line). The highest specific activity toward HA was taken as 100 %
BniHL was incubated with several chemicals in 10 mM MOPS buffer (pH 6) at 30 °C for 15 min. After diluting the treated solutions 10 times with the same buffer, the residual activity was measured under the standard assay conditions. BniHL was strongly inhibited by 2-hydroxy-5-nitrobenzylbromide (2.5 mM) by 87.4 %. Dithiothreitol (5 mM) and p-chrolomercuribenzoate (0.1 mM) moderately inhibited the enzyme by 30 %. N-ethylmaleimide (5 mM), monoiodo acetate (2.5 mM), and N-bromosuccinimide (1 mM) slightly inhibited the enzyme by 13–21 %. On the other hand, the enzyme was not inhibited at all by 2-mercaptoethanol (5 mM) (Supplementary Table S2).
Kinetics Parameters of BniHL
The kinetic parameters of BniHL toward HA (0.01 to 0.1 %), ChS-A (0.02 to 0.4 %), ChS-C (0.02 to 0.15 %), and chondroitin (0.01 to 0.2 %) were determined using Lineweaver-Burk plots. The Km and kcat/Km values for HA, ChS-A, ChS-C, and chondroitin degradation were 0.011, 0.016, 0.0047, and 0.0037 %, and 33.4, 8.0, 20.1, and 19.9 s−1 %−1, respectively. The kinetic parameters for Ca2+ ions were also determined by Lineweaver-Burk plots from the data of Fig. 6. The Km and kcat/Km values of Ca2+ ions were 0.138, 0.276, and 1.19 mM and 2.5, 0.74, and 0.17 s−1 mM−1 for HA, ChS-A, and ChS-C degradation, respectively.
Nucleotide and Amino Acid Sequences of BniHL
From the genome analysis of strain JAM F8, 170 contigs were obtained, indicating that the genome had been done around 95 % sequenced. The N-terminal amino acid sequence of the purified BniHL was searched for in all sequences, but only one CDS (GenBank accession no. BAWM010020) was completely coincident. Furthermore, the N-terminal amino acid sequences of two lysylendopeptidase digests (amino acid nos. 161–172 and 740–751) were found in the CDS. Based on these results, the gene for BniHL consists of 3411 bp nucleotides starting from an ATG codon and ending at a TAA codon. The deduced amino acid sequence consists of 1136 amino acids containing 30 amino acids of a putative signal sequence; the molecular mass of the mature enzyme is 123,888 Da, and the calculated isoelectric point is 5.0. BniHL is composed of three domains, a CBM_4_9 superfamily (amino acid nos. 44–181), tandem Big_2 domains (bacterial Ig-like domain: amino acid nos. 193–268 and 280–361), and a glycosaminoglycan (GAG)-lyase superfamily (amino acid nos. 366–1068). The GAG-lyase superfamily consists of two domains, which are a PL family 8N-terminal alpha helical domain (amino acid nos. 374–697) and a PL family 8 super-sandwich domain (amino acid nos. 731–1001) as illustrated in Supplementary Fig. S2.
The highest similarity to BniHL (GenBank accession no. AB920375) was the hyaluronate lyase (KC522838.1) of Bacillus sp. A50 (BspHL) with 89 % identity. The next highest matches found were a hypothetical protein (glycosaminoglycan polysaccharide lyase family) (WP_019376859) (VhaHL) of Virgibacillus halodenitrificans, a putative xanthan lyase (AT318176) (PlgXL) of Paenibacillus alginolyticus, a hyaluronate lyase (WP_021257667.1) (PlvHL) of Paenibacillus alvei strain TS-15, and that (WP021255950.1) of P. alvei strain A6-6i-x with 58 to 45 % identities. The highly homologous regions among five enzymes are shown in Fig. 7. All enzymes conserved the catalytic triad (N543, H593, and Y602 numbering in BniHL). Furthermore, several amino acid residues interacting with substrate found in Streptococcal HLs (Li and Jedrzejas 2001; Jedrzejas et al. 2002; Nukui et al. 2003) were conserved in all enzymes (Fig. 7). An aromatic patch (W371, W372, F423 numbering in Streptococcus agalactiae HL) and a negative patch (E468, D478, T480 numbering in S. agalactiae HL) were found in the catalytic cleft of Streptococcal HLs, which anchors the substrate chain into a degradation position (Li and Jedrzejas 2001). Aromatic patches were also conserved in all enzymes (W484, W485, and F521 numbering in BniHL); however, a clearly negative patch was not found. The results suggest that Bacilli group HLs have a unique negative patch consisting of other amino acid residues.
Multiple alignment of deduced amino acid sequence of BniHL with those of similar enzymes. BniHL (AB920375); BspHL hyaluronate lyase of Bacillus sp. A50 (KC522838.1), VhaHL a putative hyaluronate lyase of Virgibacillus halodenitrificans (WP_019376859), PlgXL a putative xanthan lyase of Paenibacillus alginolyticus (AT318176); PlvHL, a hyaluronate lyase of Paenibacillus alvei strain TS-15 (WP_021257667.1). Amino acid residues conserved in all five enzymes and at least four enzymes are shown in bold letters with light- gray shading and bold letters, respectively. The catalytic triads of HLs are shown by filled circles under the amino acid sequences; amino acid residues interacting substrate found in Streptococcal HLs (Li and Jedrzejas 2001; Jedrzejas et al. 2002; Nukui et al. 2003) are identical to all five HLs (number sign) and at least to BniHL and BspHL (double dagger), respectively
BniHL is the first HL of the deep-sea bacterium, and the properties of the enzyme are similar to those of the terrestrial BspHL (Guo et al. 2014). From our results, HLs of gram-positive bacteria appear to be divided into two groups. One is Streptococci group (Girish and Kemparaju 2007) and the other is Bacilli group, although there are few anecdotal reports on the latter enzymes. We are going to isolate other HL-producing deep-sea microorganisms and investigate a HA catabolic pathway of genus Bacillus from the genome analysis data.
References
Bellamy WR (1990) A novel Bacillus sp. capable of degrading sulfated glycosaminoglycans. In: Horikoshi K, Grant WD (eds) Superbugs: microorganisms in extreme environments. Japan Scientific Societies Press, Tokyo, pp 143–157
Cássaro CMF, Dietrich CP (1977) Distribution of sulfated mucopolysaccharides in invertebrates. J Biol Chem 252:2254–2261
Girish KS, Kemparaju K (2007) The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life Sci 80:1921–1943
Guo X, Shi Y, Sheng J, Wang F (2014) A novel hyaluronidase produced by Bacillus sp. A50. PLoS One 9:e94156
Hamai A, Hashimoto N, Mochizuki H, Kato F, Makiguchi Y, Horie K, Suzuki S (1997) Two distinct chondroitin sulfate ABC lyases. J Biol Chem 272:9123–9130
Hiyama K, Okada S (1975) Crystallization and some properties of chondroitinase from Arthrobacter aurescens. J Biol Chem 250:1824–1828
Hong SW, Kim BT, Shin HY, Kim WS, Lee KS, Kim YS, Kim DH (2002) Purification and characterization of novel chondroitin ABC and AC lyases from Bacteroides stercoris HJ-15, a human intestinal anaerobic bacterium. Eur J Biochem 269:2934–2940
Ingham E, Holland KT, Gowland G, Cunliffe WJ (1979) Purification and partial characterization of hyaluronate lyase (EC 4.2.2.1) from Propionibacterium acnes. J Gen Microbiol 115:411–418
Jedrzejas MJ (2000) Structural and functional comparison of polysaccharide-degrading enzymes. Crit Rev Biochem Mol Biol 35:221–251
Jedrzejas MJ, Mello LV, de Groot BL, Li S (2002) Mechanism of hyaluronan degradation by Streptococcus pneumoniae hyaluronate lyase. J Biol Chem 277:28287–28297
Kitamikado M, Lee YZ (1975) Chondroitinase-producing bacteria in natural habitats. Appl Environ Microbiol 29:414–421
Kurata A, Nishimura M, Kishimoto N, Kobayashi T (2014) Draft genome sequence of a deep-sea bacterium, Bacillus niacini strain JAM F8, involved in the degradation of glycosaminoglycans. Genome Announc 2:e00983–14. doi:10.1128/genomeA.00983-14
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Li S, Jedrzejas MJ (2001) Hyaluronan binding and degradation by Streptococcus agalactiae hyaluronate lyase. J Biol Chem 276:41407–41416
Lin B, Hollingshead SK, Coligan JE, Egan ML, Baker JR, Pritchard DG (1994) Cloning and expression of the gene for group B streptococcal hyaluronate lyase. J Biol Chem 269:30113–30116
Linn S, Chan T, Lipeski L, Salyers AA (1983) Isolation and characterization of two chondroitin lyases from Bacteroides thetaiotaomicron. J Bateriol 156:859–866
Nukui M, Taylor KB, McPherson DT, Shigenaga MK, Jedrzejas MJ (2003) The function of hydrophobic residues in the catalytic cleft of Streptococcus pneumoniae hyaluronate lyase. J Biol Chem 278:3079–3088
Petit E, Delattre C, Papy-Garcia D, Michaud P (2006) Chondroitin sulfate lyases: applications in analysis and glycobiology. Adv Pharmacol 53:167–186
Pritchard DG, Trent JO, Zhang P, Egan ML, Baker JR (2000) Characterization of the active site of group B streptococcal hyaluronate lyase. Proteins 40:126–134
Senni K, Pereira J, Gueniche F, Delbarre-Ladrat C, Sinquin C, Ratiskol J, Godeau G, Fischer AM, Helley D, Colliec-Jouault S (2011) Marine polysaccharides: a source of bioactive molecules for cell therapy and tissue engineering. Mar Drugs 9:1664–1681
Smith RF, Willett NP (1968) Rapid plate method for screening hyaluronidase and chondroitin sulfatase-producing microorganisms. Appl Environ Microbiol 16:1434–1436
Tam YC, Chan ESC (1985) Purification and characterization of hyaluronidase from oral Peptostreptococcus species. Infect Immun 47:508–513
Volpi N, Maccari F (2003) Purification and characterization of hyaluronic acid from the mollusk bivalve Mytilus galloprovincialis. Biochimie 85:619–625
Winter WT, Arnott S (1977) Hyaluronic acid : the role of divalent cations in conformation and packing. J Mol Biol 117:761–784
Yasuda S, Sugahara K, Özbek S (2011) Evolution of glycosaminoglycans: comparative biochemical study. Commun Integr Biol 4:150–158
Author information
Authors and Affiliations
Corresponding authors
Additional information
Atsushi Kurata and Tohru Kobayashi contributed equally to this work.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Fig. S1
ESI-MS/MS spectrograms of degradation products from HA and ChS-C. A, spectrogram of degradation products from HA; B, that of authentic ∆Di-HA; C, that of degradation products from ChS-C; D that of authentic ∆Di-6S. (PPTX 134 kb)
Fig. S2
Diagram of domain structure of BniHL. Amino acid numbers are shown above diagram. Each domain is shown as follows:, CBM_4_9 superfamily domain; tandem Big_2 domains (bacterial Ig-like domain); glycosaminoglycan (GAG)-lyase superfamily domain; PL family 8N-terminal alpha helical domain; PL family 8 super- sandwich domain (PPTX 49 kb)
ESM 1
CBM_4_9 superfamily domain (GIF 905 bytes)
ESM 2
tandem Big_2 domains (bacterial Ig-like domain) (GIF 1088 bytes)
ESM 3
glycosaminoglycan (GAG)-lyase superfamily domain (GIF 905 bytes)
ESM 4
PL family 8N-terminal alpha helical domain (GIF 1.32 kb)
ESM 5
PL family 8 super- sandwich domain (GIF 997 bytes)
Table S1
(PPTX 51 kb)
Table S2
(PPTX 51 kb)
Rights and permissions
About this article
Cite this article
Kurata, A., Matsumoto, M., Kobayashi, T. et al. Hyaluronate Lyase of a Deep-Sea Bacillus niacini . Mar Biotechnol 17, 277–284 (2015). https://doi.org/10.1007/s10126-015-9618-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-015-9618-z