Abstract
Morganella morganii is a bacterium belonging to the normal intestinal microbiota and the environment; however, in immunocompromised individuals, this bacterium can become an opportunistic pathogen, causing a series of diseases, both in hospitals and in the community, being urinary tract infections more prevalent. Therefore, the objective of this study was to evaluate the prevalence, virulence profile, and resistance to antimicrobials and the clonal relationship of isolates of urinary tract infections (UTI) caused by M. morganii, both in the hospital environment and in the community of the municipality of Londrina-PR, in southern Brazil, in order to better understand the mechanisms for the establishment of the disease caused by this bacterium. Our study showed that M. morganii presents a variety of virulence factors in the studied isolates. Hospital strains showed a higher prevalence for the virulence genes zapA, iutA, and fimH, while community strains showed a higher prevalence for the ireA and iutA genes. Hospital isolates showed greater resistance compared to community isolates, as well as a higher prevalence of multidrug-resistant (MDR) and extended-spectrum beta lactamase (ESBL)-producing isolates. Several M. morganii isolates from both sources showed high genetic similarity. The most prevalent plasmid incompatibility groups detected were FIB and I1, regardless of the isolation source. Thus, M. morganii isolates can accumulate virulence factors and antimicrobial resistance, making them a neglected opportunistic pathogen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Morganella morganii, previously named Proteus morganii (Chen et al. 2012a, 2012b), is a Gram-negative bacterium belonging to the Morganellaceae family (Adeolu et al. 2016), found in the human intestine and in the environment (Li et al. 2018). Belonging to the tribe Proteeae, together with bacteria of the genus Proteus and Providencia, it can be classified as an opportunistic bacterium that causes nosocomial infections (Chen et al. 2012a, 2012b), mainly involving cases of urinary infections and surgical wounds (Livani and Kabir 2019), sepsis, and other extraintestinal infections (Patil et al. 2012). It has two subspecies, Morganella morganii morganii and Morganella morganii sibonii (Falagas et al. 2006).
The first report of this species causing infections in humans was in 1906 (Morgan 1906). Later, in 1939, this pathogen was reported to cause urinary tract infection (UTI) (Liu et al. 2016), this type of infection being the most prevalent when it comes to M. morganii (Leylabadlo et al. 2016), often associated with long-term urinary catheter usage (Stickler 2008, 2014; Minnullina et al. 2019). Despite not being a prevalent pathogen, this bacterium was reclassified as a rare opportunistic pathogen, due to the increase in the number of cases of infection caused by M. morganii, its ability to accumulate several virulence and resistance factors (Bandy 2020), and its capacity to adapt to different environments (Ghosh and LaPara 2007).
Several virulence-related genes are described in sequences of different strains of M. morganii, deposited as sequenced genomes, in the NCBI database (Chen et al. 2012a, 2012b; Liu et al. 2016; Minnullina et al. 2019). These available described sequences reveal genes associated with fimbriae, adhesins, proteases, lipopolysaccharide (LPS), hemolysins, urease, and siderophores (Liu et al. 2016). The presence of these genes may be associated with the ability of biofilm formation, cytotoxicity, and adhesion and may contribute to the infectious process caused by this bacterium.
Although infections by M. morganii are not as prevalent, this bacterium exhibits a broad intrinsic resistance (CLSI 2021). The emergence of antimicrobial resistance represents a major public health problem, as well as a significant obstacle to the treatment of pathologies associated with microorganisms (Andersson et al. 2020). Several mechanisms can result in the emergence of antimicrobial resistance, whether intrinsic, acquired (through plasmid transfer), or even adaptive (Wilson et al. 2020).
In fact, M. morganii should be considered a clinically significant pathogen, with a broad infectious and potentially emerging spectrum, due to its resistance and virulence mechanisms, which make treatment and the immune system response more complex and difficult (Bandy 2020).
Materials and methods
Bacterial isolation
A total of 138 M. morganii isolates were studied. These microorganisms were isolated from urine of patients treated at the University Hospital of Londrina and from patients in the community (Centrolab Laboratory) in Londrina, PR, Brazil. Of the 138 isolates, 68 isolates belonged to hospitalized patients and 70 from the community, over a period of 5 years (2016 to 2020).
The antibiogram and identification of bacterial isolates were performed using the Vitek® 2 COMPACT system (BioMérieux, MarcyL'Etoile, France). The samples were tested for the following antimicrobials: ertapenem, imipenem, meropenem, amikacin, gentamicin, sulfamethoxazole plus trimethoprim, ciprofloxacin, norfloxacin, nalidixic acid, aztreonam, cefepime, ceftriaxone, ceftazidime, and piperacillin plus tazobactam. Only samples with ≥ 105 colony forming units (CFU) were selected for the study. This research was approved by the Research Ethics Committee (CEP-UEL), opinion 1.590.120.
Phenotypic screening of ESBL by combined disc
To identify possible ESBL-producing isolates, we performed the combined disk assay. For this, in addition to the inoculum carried out in saline solution, respecting the 0.5 McFarland scale, disks of ceftazidime and cefotaxime associated or not with clavulanic acid were used on a Mueller Hinton agar plate. Halos were measured after 24 h. The discs that obtained halos with a size greater than or equal to 5 mm between the disc without and with association with clavulanic acid were considered ESBL (CLSI 2021).
In silico determination of virulence genes and design of oligonucleotide primers
Fourteen complete and detailed sequenced genomes available on the NCBI website were analyzed (NZ_CP027177.1, NZ_CP063843.1, NZ_CP023505.1, NZ_CP048806.1, NZ_CP026046.1, NZ_CP048275.1, NZ_CP014026.2, NZ_LS483498.1, NZ_CP033056.1. NZ_CP025933.1, NC_020418.1, NZ_CP034944.1, NZ_CP064833.1, NZ_CP032295.1). We selected genes that were predominant among the strains and of great significance for bacterial virulence. The genetic sequences of the virulence genes were obtained through analysis combined with the Virulence Factor of Pathogenic Bacteria (VFDB) webserver using the VFanalyzer tool, which is available at http://www.mgc.ac.cn/cgi-bin/VFs/v5/main.cgi?func=VFanalyzer.
After the combined analyses, we performed the construction of the oligonucleotides initiators of the virulence genes (Table 1) selected according to their function and homology. The construction of the primer oligonucleotides was performed using the “PrimerQuest Tool” offered by Integrated DNA Technologies® (IDT), available at (https://www.idtdna.com/Primerquest/Home/Index).
Research of virulence genes
Nine genes associated with virulence of M. morganii obtained from the analysis of genomes and the VFDB webserver, ireA and iutA (siderophores), zapA (protease), hlyA and shA (hemolysins), mrpA and fimH (fimbriae), and invA and tibA (adhesins/invasins), were investigated in our isolates. The DNA used was extracted by the boiling method, followed by cooling. The PCR reaction was performed on the GeneAmp PCR System 9700 thermocycler (Applied Biosystems™) with a final volume of 25 μL. The reaction components included 12.5 μL of the mix (containing 2mM MgCl2 — Invitrogen™, 2.5 μL of 10X-Invitrogen™ buffer, 0.2 mM dNTPs (10mM) — Invitrogen™), 1 μL (20 pMol) of forward and reverse primers (IDT™), 0.2 μL (1U) of Taq DNA polymerase (Invitrogen™), 2.0μL of bacterial lysate, and 8.3μL of ultrapure water. The products of this experiment were evaluated after the electrophoresis using a 1.5% agarose gel stained with SYBR® SAFE (Invitrogen™) and observed by means of an ultraviolet light transilluminator.
Research of resistance genes
In order to carry out research on antibiotic resistance genes, the presence of the following genes was verified through the PCR reaction: CTX-M-1, 2, 8, 9, and 25 (Woodford et al. 2006); TEM and SHV (Arlet 1991); aac'(6)-lb-cr (Chen et al. 2012a, 2012b); sul1, sul2 (Li et al. 2007); qnrA, qnrB, qnrS (Cattoir et al. 2007); qnrD (Wang et al. 2009), and fosA3 (Sato et al. 2013). For this reaction, we used the following: 12.5 μL of the mix (containing 2mM MgCl2 — Invitrogen®, 2.5 μL of 10X-Invitrogen® buffer, 0.2 mM of dNTPs (10mM) — Invitrogen™), 1 μL (20 pMol) of forward and reverse primers (IDT™), 0.2 μL (1U) of Taq DNA polymerase (Invitrogen™), 2.0 μL of bacterial lysate, and 8.3 μL of ultrapure water. The products of this experiment were evaluated after the electrophoresis run, in a 1.5% agarose gel, stained with SYBR® SAFE (Invitrogen™), and observed via an ultraviolet light transilluminator.
ERIC-PCR
Bacterial isolates were tested according to their genetic similarity using the ERIC-PCR technique, following the methodology described by Versalovic et al. (1991). The oligonucleotide primers used were ERIC-1 (5′-ATGTAAGCTCCTGGGGATTCAC-3′) and ERIC-2 (5′-AAGTAAGTGACTGGGGTGAGCG-3′). For this reaction, the following components were used: 12.5 μL of the mix (containing 2 mM MgCl2 — Invitrogen, 2.5 μL of 10X-Invitrogen® buffer, 0.2 mM of dNTPs (10 mM) — Invitrogen™), 1 μL (20pMol) of primers forward and reverse (IDT™), 0.2 μL (1U) of Taq DNA polymerase (Invitrogen™), 2.0 μL of bacterial lysate, and 8.3 μL of ultrapure water. The products of this experiment were evaluated after the electrophoresis run, in agarose gel with a concentration of 2%, followed by ethidium bromide staining. The genetic similarity dendrogram was constructed using the software Gel J 2.0 (HERAS et al. 2015), using the method of unweighted pairs group with arithmetic mean (UPGMA) and the coefficient of similarity of data for the analysis of the clusters (Jaccard), with index tolerance of 1.5. To determine the clusters, we used 85% similarity as a cut-off point.
Biofilm formation
Based on the protocol established by Kwiecinska-Piróg et al. (2014), with modifications, the biofilm formation assay was performed. To evaluate the biofilm formation capacity of the study bacteria, we used 96-well polystyrene plates. The bacteria were previously cultured in trypticasein soy broth (TSB; Difco™). Subsequently, the samples were centrifuged at 4000 rpm for 15 min, their supernatant was discarded and the sedimented material washed in phosphate-buffered saline (PBS). The bacterial suspension was centrifuged at 4000 rpm for 10 min, and the sedimented material was used to prepare a 0.5 McFarland suspension in TSB broth (Difco™). In each well of the plate, we added 20 μL of the bacterial suspension and 180 μL of sterile TSB, in quadruplicate, for each isolate. For the negative controls, we used 200 μL of pure sterile TSB, and for the positive control, we used the E. coli strain (EAEC 042) (Nataro et al. 1995). The plate was incubated at 37 °C for 24 g. After this period, the wells were washed with distilled water and fixed with 200 μL of methanol in each well of the plate. At the end of this process, the methanol was removed, and the plate was dried at 37 °C for 20 min. After this procedure, we added 200 μL of 0.01% crystal violet to each well for 20 min. Subsequently, the plate was washed with distilled water until the wells became colorless. The plates were again taken to drying. For fixation, 200 μL of methanol were added to each well. After the entire process, the plate was taken to the spectrophotometer to read the absorbance at 570 nm. Biofilm was classified as absent, weak, moderate, strong, and very strong based on the value obtained for the arithmetic mean of the negative control and a triple value of its standard deviation (T = Xnc + 3δ).
HeLa cell adhesion protocol
The technique was performed according to the protocol developed by Cravioto et al. (1979). To perform the test, we used HeLa cells in a confluent layer on a round glass coverslip, in 24-well polystyrene plates. Three washes were performed by a PBS solution. Subsequently, 1 mL of Dulbecco’s modified Eagle’s medium (DMEM; Difco™ made with and without 2% D-mannose) was added to each well, along with a 40-μL aliquot of the bacterial culture, into each of the wells of the plate. The plate was incubated for 3 h at 37 °C. After this period, the wells were washed with 1 mL of PBS to remove non-adherent bacteria, and then 1 mL of DMEM (Difco™) was added to the wells. The plate was incubated for an additional 3 h at 37 °C. At the end of these processes, the plate wells were washed again with another 1 mL of PBS. The coverslips were fixed with the aid of 100% methanol and later stained with May-Grünwald. The coverslips were deposited on glass slides, which were observed and photographed with the aid of a photomicroscope. For the negative control, only HeLa cells were used for the test, while for positive controls, we used E. coli strain E2348/69 (Levine et al. 1978) for localized adhesion, E. coli C1845 for diffuse adhesion (Bilge et al. 1989), and E. coli 042 (Nataro et al. 1995) for aggregative-type adhesion.
Cytotoxicity Protocol
The assay was performed based on the protocol of Konowalchuk et al. (1977), with some modifications. For this experiment, we used a confluent layer of Vero cells in a 96-well polystyrene plate. M. morganii isolates were incubated in TSB broth (Difco™) under agitation. After the incubation period, the isolates were submitted to centrifugation at 13,000 g for 10 min. The supernatant obtained was filtered through a syringe filter with a membrane (Durapore™) with pores of 0.22 μm and 47 mm in diameter. The filtered content was transferred to a polystyrene plate at a 1:10 dilution. The plate was incubated for 72 h at 37 °C and 5% CO2. The cytotoxicity of the analyzed samples was quantified through absorbance, from the measurement of the metabolic rate of Vero cells with the MTT test (3-(4,5dimethylthiazol-2-yl)-2,5diphenyltetrazolium bromide), a compound that evaluates the cells viable from MTT conversion, as suggested by Murakami et al. 2000. Wells containing Vero cells with no bacterial supernatant were used as a negative control, and EDL 933 strain (Yu and Kaper 1992) was used as a positive control for the test. Cell death of 50% or more, compared to the negative control, was considered highly toxic.
Research of plasmid incompatibility groups (INCs)
To carry out the plasmid incompatibility group gene research assay, the genes described by Carattoli et al. (2005) were searched. For this reaction, we used 12.5 μL of the mix (containing 2 mM MgCl2 — Invitrogen™, 2.5 μL of 10X-Invitrogen™ buffer, 0.2 mM of dNTPs (10 mM) — Invitrogen™), 1 μL (20pMol) of forward and reverse primers (IDT™), 0.2 μL (1 U) of Taq DNA polymerase (Invitrogen™), 2.0 μL of bacterial lysate, and 8.3 μL of ultrapure water. The products of this experiment were evaluated after the electrophoresis run, in a 1.5% agarose gel stained with SYBR® SAFE (Invitrogen™) and observed via an ultraviolet light transilluminator.
Statistics
The evaluation of the relationship between the different factors researched was performed through multivariate logistic regression analysis and odds ratio calculation from the prediction model (Menard 2002). The significance level considered was α = 5%. Statistical analysis was performed using the R statistical software (version 4.1.1).
Results
Virulence
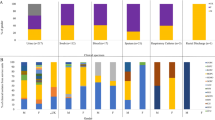
All isolates were tested for the detection of virulence genes, which contribute to pathogenicity in infections caused by M. morganii (Supplementary Figure 1 and Fig. 1). Of the 70 isolates from the community, 66 (94.28%) were positive for the ireA gene, 64 (91.42%) for the iutA gene, 60 (85.71%) for the fimH gene, 56 (80%) for the zapA gene, 55 (78.57%) for the mrpA gene, and 25 (37.14%) for the shlA gene; 12 (17.14%) were positive for the hlyA gene, 3 (4.28%) for tibA, and 1 (1.42%) for invA.
Of the 68 hospital isolates, 65 (95.58%) were positive for the zapA, iutA, and fimH genes; 64 (94.11%) for the ireA gene; 63 (92.64%) for the mrpA gene; 21 (30.88%) for the shlA gene; 11 (16.17%) for the hlyA gene; 6 (8.82%) for the invA gene; and 4 (5.88%) for the tibA gene.
Phenotypic characteristics of virulence
Based on the analyses, we can conclude that M. morganii isolates have the ability to phenotypically express aggregative adhesion (Fig. 2) in HeLa cells. Isolates from the hospital environment are more likely to exhibit adherence compared to community isolates (OR: 2.35; CI: 1.26,4.39), with an adherence rate of 63 (92.64%) isolates, while community isolates expressed adherence in 55 (78.57%) isolates.
Regarding cytotoxicity, 24 (35.29%) of hospital isolates and 24 (34.28%) of community isolates showed cytotoxic effects on Vero cells.
Regarding biofilm formation, the isolates were classified according to the protocol of Kwiecinska-Piróg et al. (2014), in biofilm intensities: absent, weak, moderate, strong, and very strong, according to the result of their absorbance (Fig. 3). The results of biofilm intensity found in hospital isolates were as follows: 20 (29.41%) classified as weak, 32 (47.05%) moderate, 13 (19.12%) strong, and 3 (4.42%) very strong. While in the isolates of community origin, the results obtained were as follows: 17 (24.29%) of low intensity, 41 (58.57%) of moderate intensity, 12 (17.14%) of strong intensity, not obtaining absorbances compatible with the formation of a very strong biofilm for any community isolate. Community isolates are more likely to be producers of moderate intensity biofilm when compared to hospital isolates (OR: 1.79; CI: 0.91; 3.53). Any isolate did not produce biofilm, regardless of its place of origin.
Antimicrobial resistance
Based on the analysis obtained using the Vitek 2 system, the isolates were classified according to their susceptibility and resistance to the tested antimicrobials. The isolates were tested for antimicrobials belonging to different classes (Fig. 4).
Frequency of antimicrobial resistance of hospital and community isolates of UTI caused by M. morganii. PTZ piperacillin associated with tazobactam; CAZ ceftazidime; CRO ceftriaxone; CPM cefepime; ATM aztreonam; NAL nalidixic acid; NOR norfloxacin; CIP ciprofloxacin; SUT sulfamethoxazole associated with trimethoprim; GEN gentamicin; AMI amikacin; ERT ertapenem, IMP imipenem, MER meropenem
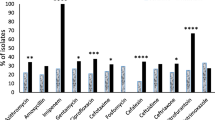
Hospital isolates proved to be more resistant to the antibiotics tested in relation to the community, showing 14.70% resistance to amikacin, 61.00% gentamicin resistance, 79.41% to aztreonam, 69.11% ceftazidime, 64.70% ceftriaxone, 45.58% to cefepime, 5.88% piperacillin associated with tazobactam, 57.35% to ciprofloxacin (p < 0.05); 38.23% to norfloxacin, 35.29% to nalidixic acid, and 63.23% to sulfazotrim.
Community isolates indicate 100% susceptibility to amikacin. They showed 28.75% resistance to gentamicin, 12.5% to aztreonam and ceftazidime, 28.75% ceftriaxone, 15% cefepime, 10% piperacillin associated with tazobactam, 47.14% to ciprofloxacin, 45.71% to norfloxacin, 75.71% nalidixic acid, and 44.28% to sulfazotrim. Despite community isolates being more susceptible to antimicrobials, some antibiotics showed greater resistance when compared to hospital isolates, as in the case of piperacillin associated with tazobactam (OR: 3.57 CI: 1, 12, 67), ciprofloxacin and nalidixic acid (OR: 9, 31 CI: 3.72, 23.31). Regardless of the site of infection, the isolates did not show resistance to any carbapenem tested.
Based on combined disk ESBL phenotypic screening, the isolates were genotypically tested for CTX-M-1, 2, 8, 9, and 25 groups using the PCR technique. Of the 68 hospital isolates, 32 (47.05%) were positive for ESBL, of which 9 were positive for the M-1 group, 22 for the M-2 group, and 2 for the M-9 group. Regarding the isolates of community origin, of the 70 isolates, 9 (12.85%) were positive for ESBL, being these, 7 for the M-1 group, 1 isolated for M-2, and 2 for M-9.
When comparing the antimicrobial resistance of ESBL isolates, we showed that these isolates are predominantly more resistant in relation to non-ESBL (Table 2). In hospital isolates, we showed that resistance to antimicrobials gentamicin (OR: 59.93 CI: 0, > 100), ceftriaxone (OR: 341 CI: 33.66, > 100), cefepime (OR: 400 CI: 0, > 100), acid nalidixic (OR: 7.31 IC: 2.37, 22.51), and sulfazotrim (OR: 30 IC: 6.12, > 100) are remarkably superior in ESBL isolates. In the community, ESBL-producing isolates are > 400 times more likely to be resistant to aztreonam than non-ESBL isolates (OR: 400 IC: 0, > 100).
Regarding the search for resistance genes not related to ESBL, in the isolates from the hospital environment, we evidenced the presence of 21 (30.88%) isolates resistant to qnrD; 10 (24.39%) isolates showed resistance to the sul1 and 30 (73.17%) to the sul2. In the isolates of community origin, we detected the presence of qnrD genes in 23 (32.85%) isolates, sul1 in 11 (32.35%) isolates, and sul2 gene in 19 (55.88%) isolates. No presence of SHV, TEM, fosA3 genes, other variants of the qnr gene, or aac′(6)-lb-cr was found in the studied isolates, regardless of the site of isolation.
ERIC-PCR
When analyzing the genetic similarity between hospital and community isolates, we observed the presence of 18 clusters, where 14 of them presented isolates from both sources (C3, C4, C5, C6, C8, C9, C10, C11, C12, C13, C14, C16, C17, and C18), three of them exclusive to hospitals (C1, C2 and C15), and one exclusive of community isolates (C7) (Supplementary Figure 2). The cut-off point for cluster formation was set at 85% similarity.
Plasmid incompatibility groups (INCs)
Based on the results obtained through the PCR technique, we evidenced the presence of incompatibility groups in the studied isolates. The hospital isolates presented the FIB, I1, and A/C groups in 19 (27.94%), 14 (20.58%), and 2 (2.94%), respectively. While in the community, we revealed the presence of incompatibility groups FIB in 19 (27.14%), I1 in 12 (17.14%), and FIA in 3 (4.28%).
Discussion
This study found the presence of several virulence and resistance factors present in isolates of hospital and community origin, which corroborate the pathogenicity and efficacy of the infection. Despite being a poorly isolated microorganism, the presence of M. morganii in both nosocomial and community-acquired infections is emerging and requires attention. In our study, we evaluated the presence and incidence of genes (mrpA, fimH, invA, tibA, zapA, ireA, iutA, hlyA, shlA) related to virulence of M. morganii in hospital and community isolates from the city of Londrina, PR, Brazil.
The zapA gene encodes a metalloprotease commonly present in P. mirabilis, capable of degrading IgA, an important defense component present in mammalian mucosa, by hydrolyzing it (Tolulope et al. 2021; Walker et al. 1999). Studies evaluating the presence of the zapA gene detected the high prevalence of this gene in P. mirabilis (Pathirana et al. 2018). Our study showed a greater presence of this gene in hospital isolates (OR:2.65 CI:1.27, 5.52), which can be justified by the fact that in the hospital environment, this metalloprotease, especially in immunocompromised individuals, favors the infectious process.
In mammals, extracellular iron is associated with the protein transferrin, which delivers iron throughout the body. This association reduces serum iron levels, which is an essential component for bacteria, especially pathogenic ones (Saha et al. 2016). Siderophores are necessary for this acquisition to occur, favoring bacterial survival (Skaar 2010). The iutA gene encodes an aerobactin ferric receptor protein, aiding in iron acquisition (Robinson et al. 2018). In a recent study carried out by Ikeda et al. (2021) with E. coli, the values found for the iutA gene are similar to our study. Like iutA, the ireA gene is also related to iron acquisition (Russo et al. 2001). In a study carried out with P. mirabilis from community-acquired UTIs, all isolates (100%) presented the ireA gene, which is consistent with the high prevalence shown in our study (de Oliveira et al. 2021).
The presence of fimbriae genes such as mrpA is essential for the establishment of the initial phase of infection, favoring adhesion, which is an important factor during the installation of the microorganism and its maintenance in the host (Rocha et al. 2007). In a recent study of P. mirabilis isolates from UTIs, the presence of this gene was detected in all the isolates examined (Sanches et al. 2021), which is consistent with the high prevalence of this gene in our study. Type 1 fimbriae, such as fimH, also support the bacterial adhesion process, establishing an important role in infectious processes, especially in UTI. In a study with uropathogenic E. coli (UPEC), the fimH gene was detected in more than 90% of the strains studied (Haghighatpanah and Mojtahedi 2019), which aligns with the findings of our study.
All M. morganii isolates that showed the ability to adhere to HeLa cells showed the presence of the mrpA gene, regardless of the use or not of D-mannose. However, our results show that the presence of only type 1 fimbriae in the bacterial adhesion test, using D-mannose, inhibited the cell adhesion process, supporting the relationship of type 3 fimbriae with bacterial adhesion capacity, with the aggregative feature. Hospital isolates showed greater adherence capacity when compared to community isolates (OR: 2.35; CI: 1.26, 4.39), which may justify the ability to stay in the hospital environment.
Hemolysins are considered virulence factors that have cytotoxic activity, with the ability to form pores on the cell surface, as is the case with hemolysin HlyA. Previous studies have reported that about 50% of M. morganii isolates have cytotoxic activity (Koronakis et al. 1987). When compared with E. coli hemolysin, it showed similar hemolytic and functional activity (Eberspacher et al. 1990). The shlA gene is the most common virulence factor of Serratia marcescens (González et al. 2020), which encodes a pore-forming toxin, with cytotoxic effects on epithelial cells and fibroblasts (Di Venanzio et al. 2014). Interestingly, our study demonstrates the presence of hlyA or shlA genes in all isolates with cytotoxic activities (p < 0.001).
Biofilms are considered virulence mechanisms of great importance for bacterial pathogenesis (Gellatly and Hancock 2013; Verderosa et al. 2019). It is estimated that about 80% of human bacterial infections result from the formation of biofilms (Davies 2003), especially when associated with the use of long-term catheters (Stickler 2008, 2014). A study with P. mirabilis from community-acquired UTI reports that, like our study, all the isolates studied demonstrated the capacity for biofilm formation (de Oliveira et al. 2021). However, our study showed that the biofilms formed have moderate intensity as the most prevalent, regardless of the isolation site, unlike the results found by de Oliveira et al. (2021), in his study also carried out in the city of Londrina, where 73.2% of the isolates showed very strong intensity biofilm. The capacity for bacterial biofilm formation deserves attention, as these structures can eventually obstruct catheters, aggravating and making treatment difficult.
Normally, hospital-sourced isolates show greater resistance to antimicrobials when compared to community-acquired isolates (Caneiras et al. 2019). This can be explained by the fact that hospitalized patients are frequently exposed to the use of antimicrobials, prolonged hospitalization, and previous infections (Kader and Kumar 2005; Ferjani et al. 2015; Manyahi et al. 2017). In a study with E. coli, they showed that the presence of ESBL isolates is predominant in a hospital environment, and its spread to the community isolates is low (Wollheim et al. 2011), corroborating the results found in our study (p < 0.001).
Previous studies have reported that the most predominant group of ESBL-type enzymes in our country are those of the CTX-M group, with those of groups M-1, 2, 8, 9 being the most detected (Cyoia et al. 2019), in agreement with the findings of our study, where the most prevalent groups detected were M-1 and M-2. Hospital isolates are about 8 times more likely to have the CTX-M-2 gene compared to community M. morganii isolates (p < 0.001). Studies carried out in Japan reported the prevalence of CTX-M-2 and 9 groups in hospital Enterobacteriaceae (Yamanaka et al. 2020), while in Europe, the most predominant CTX-M group was M-1 (Reuland et al. 2016). The identification of antimicrobial resistance, particularly in specific geographic areas, is crucial to ensure effective and appropriate treatment (Ghavidel et al. 2020).
The diversity of resistance evidenced in our study reports significant data when we consider the great intrinsic resistance of M. morganii (CLSI 2021), which, when accompanied by acquired resistance, reduces the availability of treatment, making it difficult for the patient to recover. Our study also highlights the presence of multidrug-resistant strains (MDR) in the hospital and in the community. Hospital isolates are more likely to be MDR (OR: 1.74 CI: 1.14, 2.66) compared to community ones, showing that the hospital environment is a factor that contributes to the selection of multidrug-resistant strains. A study carried out in the same period and in the same region as ours shows that community strains of P. mirabilis that cause UTI (de Oliveira et al. 2021) show low resistance to the tested antimicrobials, unlike our community isolates that showed a greater potential of M. morganii to resistance.
In our study, all antimicrobials except nalidixic acid, norfloxacin, and piperacillin-tazobactam showed higher resistance rates in hospital isolates (p < 0.05). We also noticed that carbapenems proved to be efficient alternatives for the treatment of infections caused by M. morganii, followed by amikacin and piperacillin-tazobactan, regardless of the place of origin, similar to the results found by Mostafavi et al. (2021) in a study also carried out with UTI.
Quinolone and fluoroquinolone antimicrobials are widely prescribed for the treatment of UTIs caused by enterobacteria (Stuck et al. 2012), justifying the high rate of resistance found for these antimicrobials both in hospitals and in the community. In Brazil, quinolones are widely prescribed, making the scenario even more worrisome (Wirtz et al. 2010; Vieira et al. 2020). A previous study with the Proteeae tribe reported the high prevalence of qnrD genes in hospital isolates in China (Chen et al. 2018). In our study, it was possible to observe that the presence of qnrD may contribute to quinolone resistance and has been commonly found in M. morganii (Mazzariol et al. 2012; Yaiche et al. 2014; Chen et al. 2018). Another class of antimicrobial frequently associated with the treatment of UTI is sulfonamides (Arredondo-García et al. 2004; Naquin et al. 2021). Our study showed high resistance to these antimicrobials, especially in the hospital environment, where the rate was higher compared to the community (OR: 2.28 CI: 1.05, 4.96). Together with this data, we noted the high prevalence of sulfonamide resistance genes, especially the sul2 gene, especially in hospital settings compared to the community (OR: 2.12 CI: 1.2, 3.74), suggesting that the main resistance gene for sulfonamides in our region is the sul2 gene.
The ERIC-PCR technique is indicated for the evaluation of the genetic similarity of enterobacteria, highlighting its high discriminatory power (Versalovic et al. 1991; Michelim et al. 2008). To the best of our knowledge, this is the first study evaluating the clonal relationship of hospital and community M. morganii urinary isolates through the ERIC-PCR technique, also relating the phenotypic and genotypic profile of virulence and antimicrobial resistance. ERIC-PCR technique has already been used to assess the clonal relationship of other members of the Proteeae tribe (Sanches et al. 2021; de Oliveira et al. 2021). These studies reveal the presence of circulating clones with 100% identity, corroborating the results of our study, which show the circulation of possible clones of M. morganii within the hospital and also in the community of Londrina, PR. Interestingly, most of our clusters comprise isolates from both hospital and community sources (C3, C4, C5, C6, C8, C9, C10, C11, C12, C13, C14, C16, C17, and C18). Furthermore, we can see the presence of clone formation with 100% identity between hospital and community isolates, with the same genotypic and phenotypic virulence profile in some clusters (C3, C4, C5, C9, C11, and C12), highlighting the possibility of a circulation of clones between hospital and community of Londrina, PR, being necessary more studies to better evaluate the genetic similarity of these isolates.
Although our isolates showed plasmid incompatibility groups, regardless of the isolation site, the detected replicons were differentiated between community and hospital. Notably, the most present replicons in our study were FIB and I1, being the most prevalent, both in the hospital and in the community. In a recent study carried out in the USA with UPEC isolates from CAUTI, Tarlton et al. (2019) detected a higher prevalence of plasmid incompatibility groups FIB, FIA, and B/O in their isolates. In our study, the plasmid incompatibility group of the highest prevalence detected was also FIB; however, we did not detect B/O in our study, and FIA had a low prevalence among our community isolates. In a study carried out with E. coli isolates from CAUTI in Colombia, the FIB replicon was also the most prevalent detected (De La Cadena et al. 2020), similar to our study. However, further studies involving plasmid incompatibility groups in the hospital setting with UTI need to be performed.
Another important information that should be considered in our study is the presence of the plasmid incompatibility group I1 associated with the CTX-M1 group, both in the hospital (OR: 3.48 CI: 0.98, 12.39) and in the community (OR: 5.75 CI: 1.74, 19.06). These results are similar to those evidenced by Irrgang et al. (2018) and Valcek et al. (2019), suggesting that the main plasmid element carrying the CTX-M-1 group in our region is plasmid I1. In addition, we can also note the relationship of the FIB plasmid incompatibility group often associated with the sul2 gene, both in the hospital environment and in the community (p < 0.05); however, more studies relating these genes are needed.
Conclusion
Our results indicate that M. morganii isolates, regardless of the isolation site, have several virulence genes such as invasins, hemolysins, fimbriae, proteases, and iron acquisition systems, which favor the infectious process provided by this bacterium. The presence of resistance to several antimicrobials and the dissemination of important resistance genes in these isolates make treatment difficult, especially through the circulation of MDR isolates, both in the hospital and in the community. Another relevant factor that must be taken into account is the neglected emerging potential of M. morganii, because in addition to being able to cause different types of opportunistic diseases, they can also accumulate several virulence and antimicrobial resistance factors, necessitating enhanced surveillance measures in healthcare and infection control.
References
Adeolu M, Alnajar S, Naushad S, S Gupta R (2016) Genome-based phylogeny and taxonomy of the “Enterobacteriales”: proposal for Enterobacterales ord. nov. divided into the families Enterobacteriaceae, Erwiniaceae fam. nov., Pectobacteriaceae fam. nov., Yersiniaceae fam. nov., Hafniaceae fam. nov., Morganellaceae fam. nov., and Budviciaceae fam. nov. Int J Syst Evol Microbiol 66:5575–5599. https://doi.org/10.1099/ijsem.0.001485
Andersson DI, Balaban NQ, Baquero F, Courvalin P, Glaser P, Gophna U, Kishony R, Molin S, Tønjum T (2020) Antibiotic resistance: turning evolutionary principles into clinical reality. FEMS Microbiol Rev 44:171–18810 1093/femsre/fuaa001
Arlet AP (1991) Construction by polymerase chain reaction and use of intragenic DNA probes for three main types of transferable β-lactamase (TEM, SHV, CARB). FEMS Microbiol Lett. https://doi.org/10.1111/j.1574-6968.1991.tb04833.x
Arredondo-García JL, Figueroa-Damián R, Rosas A, Jáuregui A, Corral M, Costa A, Cardeñosa-Guerra O (2004) Comparison of short-term treatment regimen of ciprofloxacin versus long-term treatment regimens of trimethoprim/sulfamethoxazole or norfloxacin for uncomplicated lower urinary tract infections: a randomized, multicentre, open-label, prospective study. J Antimicrob Chemother. https://doi.org/10.1093/jac/dkh414
Bandy A (2020) Ringing bells: Morganella morganii fights for recognition. Public Health 182:45–50. https://doi.org/10.1016/j.puhe.2020.01.016
Bilge SS, Clausen CR, Lau W, Moseley SL (1989) Molecular characterization of a fimbrial adhesin, F1845, mediating diffuse adherence of diarrhea-associated Escherichia coli to HEp-2 cells. J Bacteriol. https://doi.org/10.1128/jb.171.8.4281-4289.1989
Caneiras C, Lito L, Melo-Cristino J, Duarte A (2019) Community-and hospital-acquired Klebsiella pneumoniae urinary tract infections in Portugal: virulence and antibiotic resistance. Microorg. https://doi.org/10.3390/microorganisms7050138
Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ (2005) Identification of plasmids by PCR-based replicon typing. J Microbiol Methods. https://doi.org/10.1016/j.mimet.2005.03.018
Cattoir V, Poirel L, Rotimi V, Soussy CJ, Nordmann P (2007) Multiplex PCR for detection of plasmid-mediated quinolone resistance qnr genes in ESBL-producing enterobacterial isolates. J Antimicrob Chemother. https://doi.org/10.1093/jac/dkm204
Chen X, Zhang W, Pan W, Yin J, Pan Z, Gao S, Jiao X (2012a) Prevalence of qnr, aac (6′)-Ib-cr, qepA, and oqxAB in Escherichia coli isolates from humans, animals, and the environment. Antimicrob Agents Chemother. https://doi.org/10.1128/AAC.06191-11
Chen YP, Lei CW, Kong LH, Zeng JX, Zhang XZ, Liu BH, Wang HN (2018) Tn 6450, a novel multidrug resistance transposon characterized in a Proteus mirabilis isolate from chicken in China. Antimicrob Agents Chemother. https://doi.org/10.1128/AAC.02192-17
Chen Y-T, Peng H-L, Shia W-C, Hsu F-R, Ken C-F, Tsao Y-M, Chen C-H, Liu C-E, Hsieh M-F, Chen H-C, Tang C-Y, Ku T-H (2012b) Whole-genome sequencing and identification of Morganella morganii KT pathogenicity-related genes. BMC Genomics 13(Suppl 7):S4. https://doi.org/10.1186/1471-2164-13-S7-S4
Clinical and Laboratory Standards Instuitte (CLSI) (2021) Performance standards for antimicrobial susceptibility testing, 28th edn. CLSI supplement M100. Wayne, p 32
Cravioto A, Gross RJ, Scotland SM, Rowe B (1979) An adhesive factor found in strains of Escherichia coli belonging to the traditional infantile enteropathogenic serotypes. Current Microbiol. https://doi.org/10.1007/BF02602439
Cyoia PS, Koga VL, Nishio EK, Houle S, Dozois CM, De Brito KCT, Kobayashi RKT (2019) Distribution of ExPEC virulence factors, blaCTX-M, fosA3, and mcr-1 in Escherichia coli isolated from commercialized chicken carcasses. Front Microbiol. https://doi.org/10.3389/fmicb.2018.03254
Davies D (2003) Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov. https://doi.org/10.1038/nrd1008
De La Cadena E, Mojica MF, Castillo N, Correa A, Appel TM, García-Betancur JC, Villegas MV (2020) Genomic analysis of CTX-M-group-1-producing extraintestinal pathogenic E. coli (ExPEc) from patients with urinary tract infections (UTI) from Colombia. Antibiot. https://doi.org/10.3390/antibiotics9120899
de Oliveira WD, Barboza MGL, Faustino G, Inagaki WTY, Sanches MS, Kobayashi RKT, Rocha SPD (2021) Virulence, resistance and clonality of Proteus mirabilis isolated from patients with community-acquired urinary tract infection (CA-UTI) in Brazil. Microb Pathog. https://doi.org/10.1016/j.micpath.2020.104642
Di Venanzio G, Stepanenko TM, García VE (2014) Serratia marcescens ShlA pore-forming toxin is responsible for early induction of autophagy in host cells and is transcriptionally regulated by RcsB. Infect Immun. https://doi.org/10.1128/IAI.01682-14
Eberspacher B, Hugo F, Pohl M, Bhakdi S (1990) Functional similarity between the haemolysins of Escherichia coli and Morganella morganii. J Med Microbiol. https://doi.org/10.1099/00222615-33-3-165
Falagas ME, Kavvadia PK, Mantadakis E, Kofteridis DP, Bliziotis IA, Saloustros E, Maraki S, Samonis G (2006) Morganella morganii infections in a general tertiary hospital. Infection 34:315–321. https://doi.org/10.1007/s15010-006-6682-3
Ferjani S, Saidani M, Amine FS, Boutiba-Bem IB (2015) Prevalence and characterization of plasmid-mediated quinolone resistance genes in extended-spectrum β-lactamase-producing Enterobacteriaceae in a Tunisian hospital. Microb Drug Resist. https://doi.org/10.1089/mdr.2014.0053
Gellatly SL, Hancock RE (2013) Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog Dis. https://doi.org/10.1111/2049-632X.12033
Ghavidel M, Gholamhosseini-Moghadam T, Nourian K, Ghazvini K (2020) Virulence factors analysis and antibiotic resistance of uropathogenic Escherichia coli isolated from patients in northeast of Iran. Iran J Microb 12(3):223
Ghosh S, LaPara TM (2007) The effects of subtherapeutic antibiotic use in farm animals on the proliferation and persistence of antibiotic resistance among soil bacteria. ISME J 1:191–203. https://doi.org/10.1038/ismej.2007.31
González GM, Treviño-Rangel RDJ, Campos CL, Villanueva-Lozano H, Bonifaz A, Franco-Cendejas R, Andrade A (2020) Surveillance of antimicrobial resistance in Serratia marcescens in Mexico. New Microbiol 43(1):34–37
Haghighatpanah M, Mojtahedi A (2019) Characterization of antibiotic resistance and virulence factors of Escherichia coli strains isolated from Iranian inpatients with urinary tract infections. Infect Drug Resist. https://doi.org/10.2147/IDR.S219696
Heras J, Domínguez C, Mata E, Pascual V, Lozano C, Torres C, Zarazaga M (2015) GelJ–a tool for analyzing DNA fingerprint gel images. BMC Bioinform. https://doi.org/10.1186/s12859-015-0703-0
Ikeda M, Kobayashi T, Fujimoto F, Okada Y, Higurashi Y, Tatsuno K, Moriya K (2021) The prevalence of the iutA and ibeA genes in Escherichia coli isolates from severe and non-severe patients with bacteremic acute biliary tract infection is significantly different. Gut Pathogens. https://doi.org/10.1186/s13099-021-00429-1
Irrgang A, Hammerl JA, Falgenhauer L, Guiral E, Schmoger S, Imirzalioglu C, Käsbohrer A (2018) Diversity of CTX-M-1-producing E. coli from German food samples and genetic diversity of the blaCTX-M-1 region on IncI1 ST3 plasmids. Vet Microbiol. https://doi.org/10.1016/j.vetmic.2018.06.003
Kader AA, Kumar A (2005) Prevalence and antimicrobial susceptibility of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in a general hospital. Annals Saudi Med. https://doi.org/10.5144/0256-4947.2005.239
Konowalchuk J, Speirs JI, Stavric S (1977) Vero response to a cytotoxin of Escherichia coli. Infect Immun. https://doi.org/10.1128/iai.18.3.775-779.1977
Koronakis V, Cross M, Senior B, Koronakis E, Hughes C (1987) The secreted hemolysins of Proteus mirabilis, Proteus vulgaris, and Morganella morganii are genetically related to each other and to the alpha-hemolysin of Escherichia coli. J Bacteriol. https://doi.org/10.1128/jb.169.4.1509-1515.1987
Kwiecinska-Piróg J, Bogiel T, Skowron K, Wieckowska E, Gospodarek E (2014) Proteus mirabilis biofilm-qualitative and quantitative colorimetric methods-based evaluation. Braz J Microbiol. https://doi.org/10.1590/S1517-83822014000400037
Levine M, Nalin D, Hornick R, Bergquist E, Waterman D, Young C, Rowe B (1978) Escherichia coli strains that cause diarrhoea but do not produce heat-labile or heat-stable enterotoxins and are non-invasive. Lancet. https://doi.org/10.1016/S0140-6736(78)90299-4
Leylabadlo HE, Kafil HS, Yousefi M, Aghazadeh M, Asgharzadeh M (2016) Persistent infection with metallo-beta-lactamase and extended spectrum β-lactamase producer Morganella morganii in a patient with urinary tract infection after kidney transplantation. J Nat Sci Biol Med 7:179–181. https://doi.org/10.4103/0976-9668.184707
Li G, Niu X, Yuan S, Liang L, Liu Y, Hu L, Liu J, Cheng Z (2018) Emergence of Morganella morganii subsp. morganii in dairy calves, China. Emerg Microbes Infect 7:172. https://doi.org/10.1038/s41426-018-0173-3
Li Q, Sherwood JS, Logue CM (2007) Characterization of antimicrobial resistant Escherichia coli isolated from processed bison carcasses. J Appl Microbiol. https://doi.org/10.1111/j.1365-2672.2007.03470.x
Liu H, Zhu J, Hu Q, Rao X (2016) Morganella morganii, a non-negligent opportunistic pathogen. Int J Infect Dis 50:10–17. https://doi.org/10.1016/j.ijid.2016.07.006
Livani F, Kabir S (2019) Gram-negative folliculitis caused by Morganella morganii. JAAD Case Rep 5:558–559. https://doi.org/10.1016/j.jdcr.2019.04.021
Manyahi J, Moyo SJ, Tellevik MG, Ndugulile F, Urassa W, Blomberg B, Langeland N (2017) Detection of CTX-M-15 beta-lactamases in Enterobacteriaceae causing hospital-and community-acquired urinary tract infections as early as 2004, in Dar es Salaam, Tanzania. BMC Infect Dis. https://doi.org/10.1186/s12879-017-2395-8
Mazzariol A, Kocsis B, Koncan R, Kocsis E, Lanzafame P, Cornaglia G (2012) Description and plasmid characterization of qnrD determinants in Proteus mirabilis and Morganella morganii. Clin Microbiol Infect. https://doi.org/10.1128/AAC.02192-17
Menard S (2002) Applied logistic regression analysis, 2nd edn. SAGE Publications, Inc, United States of America. https://doi.org/10.4135/9781412983433
Michelim L, Muller G, Zacaria J, Delamare APL, Costa SOPD, Echeverrigaray S (2008) Comparison of PCR-based molecular markers for the characterization of Proteus mirabilis clinical isolates. Braz J Infect Dis. https://doi.org/10.1590/s1413-86702008000500014
Minnullina L, Pudova D, Shagimardanova E, Shigapova L, Sharipova M, Mardanova A (2019) Comparative genome analysis of uropathogenic Morganella morganii strains. Front Cell Infect Microbiol 9:167. https://doi.org/10.3389/fcimb.2019.00167
Morgan H d R (1906) Report XCV. Upon the bacteriology of the summer diarrhoea of infants. Br Med J 1:908–912. https://doi.org/10.1136/bmj.1.2364.908
Mostafavi S, Rostami S, Rezaee Nejad Y, Ataei B, Mobasherizadeh S, Cheraghi A, Shokri D (2021) Antimicrobial resistance in hospitalized patients with community acquired urinary tract infection in Isfahan, Iran. Arch Iran Med. https://doi.org/10.34172/aim.2021.29
Murakami A, Nakamura Y, Torikai K, Tanaka T, Koshiba T, Koshimizu K, Ohigashi H (2000) Inhibitory effect of citrus nobiletin on phorbol ester-induced skin inflammation, oxidative stress, and tumor promotion in mice. Cancer Res 60(18):5059–5066
Naquin E, Soorya H, Oubre C, Rogers S, Boopathy R (2021) Effect of sulfonamide class of antibiotics on a bacterial consortium isolated from an anaerobic digester of a sewage treatment plant. Env Qual Manag. https://doi.org/10.1002/tqem.21709
Nataro JP, Yikang D, Cookson S, Cravioto A, Savarino SJ, Guers LD, Tacket CO (1995) Heterogeneity of enteroaggregative Escherichia coli virulence demonstrated. J Infect Dis. https://doi.org/10.1093/infdis/171.2.465
Pathirana HNKS, De Silva BCJ, Wimalasena SHMP, Hossain S, Heo GJ (2018) Comparison of virulence genes in Proteus species isolated from human and pet turtle. Iran J Vet Res 19(1):48
Patil AB, Nadagir SD, Lakshminarayana S, Syeda FM (2012) Morganella morganii, subspecies morganii, biogroup A: an unusual causative pathogen of brain abscess. J Neurosci Rural Pract 3:370–372. https://doi.org/10.4103/0976-3147.102631
Reuland EA, Al Naiemi N, Kaiser AM, Heck M, Kluytmans JAJW, Savelkoul PHM, Vandenbroucke-Grauls CMJE (2016) Prevalence and risk factors for carriage of ESBL-producing Enterobacteriaceae in Amsterdam. J Antimicrob Chemother. https://doi.org/10.1093/jac/dkv441
Robinson AE, Heffernan JR, Henderson JP (2018) The iron hand of uropathogenic Escherichia coli: the role of transition metal control in virulence. Fut microbiol. https://doi.org/10.2217/fmb-2017-0295
Rocha SP, Pelayo JS, Elias WP (2007) Fimbriae of uropathogenic Proteus mirabilis. FEMS Immunol Med Microbiol. https://doi.org/10.1111/j.1574-695X.2007.00284.x
Russo TA, Carlino UB, Johnson JR (2001) Identification of a new iron-regulated virulence gene, ireA, in an extraintestinal pathogenic isolate of Escherichia coli. Infect Immun. https://doi.org/10.1128/IAI.69.10.6209-6216.2001
Saha M, Sarkar S, Sarkar B, Sharma BK, Bhattacharjee S, Tribedi P (2016) Microbial siderophores and their potential applications: a review. Envirol Sci Pollut. https://doi.org/10.1007/s11356-015-4294-0
Sanches MS, da Silva CR, Silva LC, Montini VH, Barboza MGL, Guidone GHM, de Oliva BHD, Nishio KN, Galhardi LCF, Vespero EC, Nogueira MCL, Rocha SPD (2021) Proteus mirabilis from community-acquired urinary tract infections (UTI-CA) shares genetic similarity and virulence factors with isolates from chicken, beef and pork meat. Microb Pathog. https://doi.org/10.1016/j.micpath.2021.105098
Sato N, Kawamura K, Nakane K, Wachino JI, Arakawa Y (2013) First detection of fosfomycin resistance gene fosA3 in CTX-M-producing Escherichia coli isolates from healthy individuals in Japan. Microbial Drug Resist. https://doi.org/10.1089/mdr.2013.0061
Skaar EP (2010) The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathog. https://doi.org/10.1371/journal.ppat.1000949
Stickler DJ (2008) Bacterial biofilms in patients with indwelling urinary catheters. Nat Clin Pract Urol 5:598–608. https://doi.org/10.1038/ncpuro1231
Stickler DJ (2014) Clinical complications of urinary catheters caused by crystalline biofilms: something needs to be done. J Intern Med 276:120–129. https://doi.org/10.1111/joim.12220
Stuck AK, Täuber MG, Schabel M, Lehmann T, Suter H, Mühlemann K (2012) Determinants of quinolone versus trimethoprim-sulfamethoxazole use for outpatient urinary tract infection. Antimicrob Agents Chemother. https://doi.org/10.1128/AAC.05321-11
Tarlton NJ, Moritz C, Adams-Sapper S, Riley LW (2019) Genotypic analysis of uropathogenic Escherichia coli to understand factors that impact the prevalence of β-lactam-resistant urinary tract infections in a community. J Global Antimicrob Resist. https://doi.org/10.1016/j.jgar.2019.03.002
Tolulope A, Ewaoche IS, Ibemologi A (2021) UreC and ZapA virulence genes amplification in clinical specimen of Proteus mirabilis in Bayelsa state, Nigeria. J Microbiol Exp. https://doi.org/10.15406/jmen.2021.09.00317
Valcek A, Roer L, Overballe-Petersen S, Hansen F, Bortolaia V, Leekitcharoenphon P, Hammerum AM (2019) IncI1 ST3 and IncI1 ST7 plasmids from CTX-M-1-producing Escherichia coli obtained from patients with bloodstream infections are closely related to plasmids from E. coli of animal origin. J Antimicrob Chemother. https://doi.org/10.1093/jac/dkz199
Verderosa AD, Totsika M, Fairfull-Smith KE (2019) Bacterial biofilm eradication agents: a current review. Front Chem. https://doi.org/10.3389/fchem.2019.00824
Versalovic J, Koeuth T, Lupski R (1991) Distribution of repetitive DNA sequences in eubacteria and application to finerpriting of bacterial enomes. Nucleic Acids Res. https://doi.org/10.1093/nar/19.24.6823
Vieira DC, Lima WG, de Paiva MC (2020) Plasmid-mediated quinolone resistance (PMQR) among Enterobacteriales in Latin America: a systematic review. Mol Biol Rep. https://doi.org/10.1007/s11033-019-05220-9
Walker KE, Moghaddame-Jafari S, Lockatell CV, Johnson D, Belas R (1999) ZapA, the IgA-degrading metalloprotease of Proteus mirabilis, is a virulence factor expressed specifically in swarmer cells. Mol Microbiol. https://doi.org/10.1046/j.1365-2958.1999.01401.x
Wang M, Guo Q, Xu X, Wang X, Ye X, Wu S, Wang M (2009) New plasmid-mediated quinolone resistance gene, qnrC, found in a clinical isolate of Proteus mirabilis. Antimicrob Agents Chemother. https://doi.org/10.1128/AAC.01400-08
Wilson DN, Hauryliuk V, Atkinson GC, O’Neill AJ (2020) Target protection as a key antibiotic resistance mechanism. Nat Rev Microbiol 18:637–648. https://doi.org/10.1038/s41579-020-0386-z
Wirtz VJ, Dreser A, Gonzales R (2010) Trends in antibiotic utilization in eight Latin American countries, 1997-2007. Rev Panam Salud Publ. https://doi.org/10.1590/s1020-49892010000300009
Wollheim C, Guerra IMF, Conte VD, Hoffman SP, Schreiner FJ, Delamare APL, da Costa SOP (2011) Nosocomial and community infections due to class A extended-spectrum β-lactamase (ESBLA)-producing Escherichia coli and Klebsiella spp. in southern Brazil. Braz J Infect Dis 15(2):138–143. https://doi.org/10.1016/S1413-8670(11)70159-3
Woodford N, Fagan EJ, Ellington MJ (2006) Multiplex PCR for rapid detection of genes encoding CTX-M extended-spectrum (beta)-lactamases. J Antimicrob Chemother 57:154–155. https://doi.org/10.1093/jac/dki412
Yaiche M, Rafraf I, Guo Q, Mastouri M, Aouni M, Wang M (2014) Type II and type IV topoisomerase mutations in clinical isolates of Morganella morganii harbouring the qnrD gene. Annals Clinl Microb Antimicrob. https://doi.org/10.1186/s12941-014-0034-4
Yamanaka T, Funakoshi H, Kinoshita K, Iwashita C, Horikoshi Y (2020) CTX-M group gene distribution of extended spectrum beta-lactamase-producing Enterobacteriaceae at a Japanese Children’s hospital. J Infect Chemother. https://doi.org/10.1016/j.jiac.2020.05.017
Yu J, Kaper JB (1992) Cloning and characterization of the eae gene of enterohaemorrhagic Escherichia coli O157: H7. Mol Microbiol. https://doi.org/10.1111/j.1365-2958.1992.tb01484.x
Acknowledgements
We thank the State University of Londrina and to the Laboratory of Virology of the State University of Londrina for the supply of the cell lines used in this study.
Availability of data and materials
Not applicable.
Funding
This study was financed by the Coordination for the Improvement of Higher Education Personnel - Brazil (CAPES) Finance Code 001.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Luana Carvalho Silva, Matheus Silva Sanches, Gustavo Henrique Migliorini Guidone, Victor Hugo Montini, Bruno Henrique Dias de Oliva, Arthur Bossi do Nascimento, and Lígia Carla Faccin Galhardi. The first draft of the manuscript was written by Renata Katsuko, Takayama Kobayashi, Eliana Carolina Vespero, and Sergio Paulo Dejato Rocha, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of University State of Londrina (CEP-UEL 1.590.120).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Silva, L.C., Sanches, M.S., Guidone, G.H.M. et al. Clonal relationship, virulence genes, and antimicrobial resistance of Morganella morganii isolated from community-acquired infections and hospitalized patients: a neglected opportunistic pathogen. Int Microbiol 27, 411–422 (2024). https://doi.org/10.1007/s10123-023-00400-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10123-023-00400-x