Abstract
The aim of this study was monitoring and surveillance in different wards of the PIMS hospital, Islamabad, to understand emerging challenges of antibiotic resistance in particular association with most virulent serotypes of Klebsiella pneumoniae. The study was conducted during March 2015 to September 2015. The study showed that rate of isolation of K. pneumoniae was 37% (103 positives out of a total of 277 clinical samples) and 7.7% (8) were phenotypically and genotypically confirmed to be metallo-β-lactamase resistant (carbapenem resistant) and all of them were multidrug resistant (MDR). These carbapenem-resistant isolates were isolated from blood, endotracheal tubes, and pus. Molecular screening for the presence of integrons indicated that distribution of class I integrons (87.5% of carbapenem-resistant K. pneumoniae isolates) was higher than class II integrons (1.25%) among given isolates. The study indicated that exposure of metallo-beta-lactamase-producing strains through hospitalizations increases the chances of spread of MDR pathogens. There is an urgent need for effective surveillance and monitoring strategies to control the spread of extremely resistant K. pneumoniae implicated in nosocomial infections leading to the increased health burden and enforcement of policy guideline on appropriate antibiotics usage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Beta-lactams are the most recommended antibiotics all over the world because of their assorted qualities, broad spectrum range, and less toxicity. Beta-lactams having beta-lactam ring are distinguished because of the presence of their side chains into various groups as carbapenems, cephalosporins, monobactams, and penicillin’s (Varaldo et al. 1990).
Carbapenems, a class of beta-lactams, gain access to Gram-negative bacteria via proteins of outer membrane generally known as porins, as they cannot easily pass by diffusion through the cell wall of bacteria (Tipper and Strominger 1965). Because of the unavailability of new antibiotics for the treatment of infections by gram-negative bacteria and development of antibacterial resistance, gram-negative bacteria become a serious danger (Podschun and Ullmann 1998). Carbapenemases classes A, B, and D which are beta-lactamases can proficiently hydrolyze beta-lactam inhibitors, cephalosporins, monobactams, and carbapenems (Voulgari et al. 2013). In gram-negative bacteria, commonly beta-lactam resistance is due to the production of beta-lactamases. Beta-lactamases by hydrolyzing amide bond of beta-lactam ring can inactivate the beta-lactam antibiotics (Medeiros 1997). According to Ambler-lactamases classification, class B metallo-β-lactams include imipenem (IMP), Verona integron-encoded metallo-β-lactamase (VIM), German imipenemase (GIM), Seoul imipenemase (SIM), Sao Paulo metallo-β-lactamase (SPM), and New Delhi metallo-β-lactamase (NDM)-1 (Yong et al. 2009).
One of the most clinically important carbapenemases in K. pneumoniae is NDM-1 which was first identified in 2008 (Yong et al. 2009). NDM-positive K. pneumoniae are widely disseminated in the hospitals of major cities of Pakistan as indicated in previous reports (Pesesky et al. 2015; Khan et al. 2016). Integrons are associated with the transfer of resistance genes (Bennett 2008) where gene dissemination from one cell to another involves a DNA element called integrons. Resistance genes are integrated by a mechanism involving site-specific recombination (Recchia and Hall 1997). The presence of integrons in K. pneumoniae isolates has been reported previously (Deng et al. 2015).
In the current study, 103 K. pneumoniae isolates were collected from neonates, geriatrics, and burn patients in the period of March 2015 to September 2015. Investigation on these isolates was done in terms of antibiotic resistance, phenotypic and genotypic detection of metallo-beta-lactamase-producing strains, and detection of integrons (classes I and II).
Methods
Study period and clinical samples
Two hundred seventy-seven samples that include blood, urine, sputum, wound swabs, and pus were obtained from neonates, geriatrics, and burn patients admitted in tertiary care hospital of Islamabad PIMS, between March 2015 and September 2015. Samples were processed as per standard microbiological procedures. Identification was performed on the basis of gram staining, colony morphology on MacConkey’s agar (Oxoid, Basingstoke, UK), and a panel of biochemical tests, i.e., oxidase test, catalase test, indole test, triple sugar iron test, and simmon’s citrate test (Alves et al. 2006).
Antimicrobial susceptibility testing
Antibiotic susceptibility of confirmed K. pneumoniae isolates was determined by Kirby Bauer disc diffusion assay. Results were interpreted according to The Clinical and Laboratory Standard Institute (CLSI) guidelines (2015). Antibiotics used for screening were ampicillin (AMP, 10 μg), ciprofloxacin (CIP, 5 μg), ceftazidime (CAZ, 30 μg), trimethoprim-sulfamethoxazole (SXT, 23.75 μg), ceftriaxone (CTX, 30 μg), and imipenem (IPM, 10 μg).
IMP-ethylenediaminetetra acetic disc diffusion method
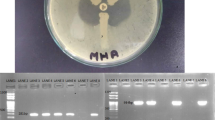
Peptone broth was inoculated with identified colonies and turbidity was set to 0.5 McFarland’s standard. Cotton swabs were soaked in broth containing bacterial colonies and then coated as culture lawn on Muller-Hinton agar (Oxoid, Basingstoke, UK). After drying, two IMP discs (10 μg each) were placed on the bacterial lawn. Four microliters of 0.5 M EDTA was added to one of the IMP discs and incubation was done at 35 °C for 16–18 h. EDTA is a chelating agent and removes zinc ions from the active site of MBL enzyme, thus rendering the enzyme inactive and the bacterium shows sensitivity to carbapenems. Subsequently, measurement and comparison of zone diameters of two IMP discs were done. The difference in the inhibition zones by ≥ 7 mm was regarded as positive (Yong et al. 2002).
Screening of class B metallo-beta-lactamases
Presence of mobile MBL genes (blaGIM, blaIMP, blaSIM, blaSPM, blaVIM) in K. pneumoniae isolates was tested by five pairs of primers (particular for each family of MBLs) multiplex PCR, to amplify fragments of 477 bp (GIM-1), 188 bp (IMP), 570 bp (SIM-1), 271 bp(SPM-1), and 390 bp (VIM) (Ellington et al. 2007). The isolates were further tested for the presence of a new subgroup of MBL, identified as NDM, which originated from New Delhi in India. NDM-1-specific primers singleplex PCR assay was employed for screening the strains for NDM-1. PCR products were analyzed on 2% agarose gel at a voltage of 90 V for 1 h and visualized and recorded in the gel documentation system (Shenoy et al. 2014).
Detection of class I and II integrons among carbapenem-resistant isolates
K. pneumoniae isolates were further subjected to screening for the presence of class I integron, 1009 bps (Int-I) (Brown et al. 2000), and class II integron, 2039 bps (Int-II) (Mathai et al. 2004) structures, by multiplex PCR using primers specific for class I and II integrons.
Results
Isolation frequency of K. pneumoniae and presence of metallo-beta-lactamase resistance
In the present study, the isolation rate of K. pneumoniae was observed to be 37%, i.e., 103 positives out of 277 clinical samples screened.
Antibiotic susceptibility
Overall, K. pneumoniae isolates showed resistance to ampicillin (95.5%), ceftazidime (94.4%), ciprofloxacin (88.2%), sulfamethoxazole (65.7%), ceftriaxone (64.7%), and imipenim (14%) as shown in Fig. 1.
Phenotypic metallo-beta-lactamase detection
Phenotypic metallo-beta-lactamase detection showed that 7% (8/103) K. pneumoniae isolates were found to be MBL positive. All isolates harboring MBL were well distinguished from MBL-negative isolates by the criterion of a ≥ 7-mm increase of inhibition zone with the discs added with EDTA.
In the present study, all the eight carbapenem-resistant strains were found to be isolated from ICU (intensive care unit), seven isolates from NICU (neonatal intensive care unit), and one isolate from ICU of burn center, while carbapenem resistance was observed to be absent in the isolates from geriatric patients. Sources of resistant strains were endotracheal tubes, blood, and pus (Table 1).
Metallo-beta-lactamase genes in K. pneumoniae isolates
Molecular screening of MBL indicated the presence of all types of class B beta-lactamases GIM, IMP, NDM, SIM, SPM, and VIM (Fig. 2 and Table 2).
Class I and II integrons in carbapenem-resistant isolates
Molecular screening for the presence of integrons indicated that class I integrons were more frequently observed compared to class II integrons in K. pneumoniae (Fig. 3). Furthermore, class I integron was found to be in 87.5% of carbapenem-resistant K. pneumoniae isolates. Furthermore, NDM was detected in seven strains out of eight (Fig. 4).
Discussion
The carbapenem group of antibiotics is the key therapeutic options for acute infections caused by ESBL-producing gram-negative bacteria. The emergence of carbapenem-resistant strains limits the therapeutic options including aminoglycosides, fosfomycin, polymyxins, temocillin, and tigecycline. Hence, the issue of carbapenem resistance in Enterobacteriaceae needs particular surveillance.
The rationale of the current study was identification and determination of carbapenem resistance in K. pneumoniae isolates from different sources of human infections. K. pneumoniae isolates were mainly collected from neonates, burn patients, and geriatrics. The field of comparative microbial genomics has tremendously grown over the years due to economical high-throughput sequencing technologies. Pan-genome (Tettelin et al. 2005) describing the comprehensive inventory of genes in superbugs will further help in better understanding ecological adaptation, virulence mechanisms, antibiotic resistance, or colonization of a new host. Phylogenetic analysis revealed that the isolated strains showed around 99% similarity among them. Their resistant genes also showed quite a similarity lying in the same evolutionary clad. When compared with reference genome, it revealed some conserved region which can be studied further to analyze regions responsible for the resistance mechanism.
Out of a total of 103 K. pneumoniae isolates of this study, eight (7%) isolates were found to be metallo-beta-lactamase resistant. This frequency is lower as compared to the previous studies, e.g., 14% from Oman (Prakash et al. 2011) and 21% reported from Nepal (Bora et al. 2014). The reason behind this may be that limited data is available on carbapenem resistance among K. pneumoniae isolates in Pakistan as well as perhaps differences in practice for prescribing the drug. Results of our study are supported by a previous study by Deshpande et al. (2010) based on the emerging trend of carbapenem resistance in a tertiary care hospital in North India. Findings from their study identified carbapenem resistance in 6.9% of K. pneumoniae strains (Deshpande et al. 2010). Results from this study depict that burn patients and neonates are at a higher risk of acquiring the infection by K. pneumoniae in comparison to geriatric patients and the major reservoirs for carbapenem-resistant isolates were found to be blood, endotracheal tubes, and pus.
The production of metallo-beta-lactamases in strains limits the therapeutic options. The risk factors for infections associated with MBL-producing bacteria include the provision of β-lactam antibiotics or carbapenems, hospitalization, and/or practices with infected indwelling medical instruments and devices (Okazaki et al. 2016).
Previously, a coproduction of two and three beta-lactamases in a single bacterium has been noticed (Pokhrel et al. 2014; Oduyebo et al. 2015). Alarmingly, 100% of carbapenem-resistant strains were found to be positive for the class B beta-lactamases from our study; additionally, all of them were found to be multidrug resistant (MDR). Another mechanism for carbapenem resistance is integrons mediating integration of resistance gene by site-specific recombination mechanism (Tamura et al. 2013). In this study, integron-I was found to be more responsible for resistance genes acquisition in bacteria.
Conclusions
This study is one of the fewer reports on carbapenem-resistant K. pneumoniae that cover information about the origin of isolates, antimicrobial resistance, phenotypic and genotypic detection of MBL-producing strains, and association of these isolates with integrons. Further investigation on carbapenem-resistant K. pneumoniae infections is urgent, with the ultimate aim to adapt the therapeutic approach to the microbiological properties involved. Awareness about the entry of carbapenem-resistant strains into a hospital setting is the first step that clinical microbiologists can take to address this issue. Evaluation of effective antibiotic options and diligent infection control measures will help in the fight against carbapenemase-producing microorganisms.
Abbreviations
- GIM:
-
German imipenemase
- IMP:
-
Imipenem
- NDM:
-
New Delhi metallo-β-lactamase
- SIM:
-
Seoul imipenemase
- SPM:
-
Sao Paulo metallo-β-lactamase
- VIM:
-
Verona integron-encoded metallo-β-lactamase
- EDTA:
-
Ethylene diamine tetra acetate
- MBL:
-
Metallo-beta-lactamase
- MDR:
-
Multidrug resistance
References
Alves MS, Dias RC d S, de Castro ACD, Riley LW, Moreira BM (2006) Identification of clinical isolates of indole-positive and indole-negative Klebsiella spp. J Clin Microbiol 44:3640–3646
Bennett P (2008) Plasmid encoded antibiotic resistance: acquisition and transfer of antibiotic resistance genes in bacteria. Br Aust J Pharm S1:S347–S357
Bora A, Sanjana R, Jha B, Mahaseth S, Pokharel K (2014) Incidence of metallo-beta-lactamase producing clinical isolates of Escherichia coli and Klebsiella pneumoniae in Central Nepal. BMC Res Notes 7:557
Brown A, Rankin S, Platt D (2000) Detection and characterisation of integrons in Salmonella enterica serotype Enteritidis. FEMS Microbiol Lett 191:145–149
Clinical and Laboratory Standards Institute (CLSI) (2015) M100–S25 performance standards for antimicrobial susceptibility testing; Twenty-fifth informational supplement. Clinical and Laboratory Standards Institute, Wayne
Deng Y, Bao X, Ji L, Chen L, Liu J, Miao J, Chen D, Bian H, Li Y, Yu G (2015) Resistance integrons: class 1, 2 and 3 integrons. Ann Clin Microbiol Antimicrob 14:45
Deshpande P, Rodrigues C, Shetty A, Kapadia F, Hedge A, Soman R (2010) New Delhi Metallo-lactamase (NDM-1) in enterobacteriaceae: treatment options with carbapenems compromised. J Assoc Physicians India 58:147–149
Ellington M, Kistler J, Livermore D, Woodford N (2007) Multiplex PCR for rapid detection of genes encoding acquired metallo-β-lactamases. J Antimicrob Chemother 59:321–322
Khan E, Irfan S, Sultan B, Nasir A, Hasan R (2016) Dissemination and spread of New Delhi metallo-beta-lactamase-1 superbugs in hospital settings. JPMA 66:999–1004
Mathai E, Grape M, Kronvall G (2004) Integrons and multidrug resistance among Escherichia coli causing community-acquired urinary tract infection in southern India. APMIS 112:159–164
Medeiros A (1997) Evolution and dissemination of β-lactamases accelerated by generations of β-lactam antibiotics. Clin Infect Dis 24:S19–S45
Oduyebo OO, Falayi OM, Oshun P, Ettu AO (2015) Phenotypic determination of carbapenemase producing Enterobacteriaceae isolates from clinical specimens at a tertiary hospital in Lagos, Nigeria. Nig Postgrad Med J 22:223–227
Okazaki R, Hagiwara S, Kimura T, Tokue Y, Kambe M, Murata M, Aoki M, Kaneko M, Oshima K, Murakami K (2016) Case report Metallo-β-lactamase-producing Klebsiella pneumoniae infection in a non-hospital environment. Acute Med Surg 3:32–35
Pesesky M, Hussain T, Wallace M, Wang B, Andleeb S, Burnham C, Dantas G (2015) KPC and NDM-1 genes in related Enterobacteriaceae strains and plasmids from Pakistan and the United States. Emerg Infect Dis 21:1034–1037
Podschun R, Ullmann U (1998) Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev 11:589–603
Pokhrel RH, Thapa B, Kafle R, Shah PK, Tribuddharat C (2014) Co-existence of beta-lactamases in clinical isolates of Escherichia coli from Kathmandu, Nepal. BMC Res Notes 7:694
Prakash K, Arora V, Geethanjali P (2011) Bloodstream bacterial pathogens and their antibiotic resistance pattern in Dhahira region, Oman, Oman. Med J 26:240–279
Recchia G, Hall R (1997) Origins of the mobile gene cassettes found in integrons. Trends Microbiol 5:389–394
Shenoy K, Jyothi E, Ravikumar R (2014) Phenotypic identification & molecular detection of blandm-1 gene in multidrug resistant Gram-negative bacilli in a tertiary care center. Ind J Med Res 139:625–631
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Tettelin H, Masignani V, Cieslewicz MJ, Donati C, Medini D, Ward NL, Angiuoli SV, Crabtree J, Jones AL, Durkin AS, Deboy RT, Davidsen TM, Mora M, Scarselli M, Margarit Y, Ros I, Peterson JD, Hauser CR, Sundaram JP, Nelson WC, Madupu R, Brinkac LM, Dodson RJ, Rosovitz MJ, Sullivan SA, Daugherty SC, Haft DH, Selengut J, Gwinn ML, Zhou L, Zafar N, Khouri H, Radune D, Dimitrov G, Watkins K, O'Connor KJ, Smith S, Utterback TR, White O, Rubens CE, Grandi G, Madoff LC, Kasper DL, Telford JL, Wessels MR, Rappuoli R, Fraser CM (2005) Genome analysis of multiple pathogenic isolates of Streptococcus agalactiae: implications for the microbial “pan-genome”. Proc Natl Acad Sci U S A 102:13950–13955
Tipper D, Strominger J (1965) Mechanism of action of penicillins: a proposal based on their structural similarity to acyl-D-alanyl-D-alanine. PNAS USA 54:1133–1141
Varaldo P, Nicoletti G, Schito G, Maida A, Facinelli B, Stefani S, Gianrossi G, Muresu E (1990) Circulation in Italy of β-lactamase-producing strains within the major groups of bacterial pathogens. Eur J Epidemiol 6:287–292
Voulgari E, Poulou A, Koumaki V, Tsakris A (2013) Carbapenemase-producing Enterobacteriaceae: now that the storm is finally here, how will timely detection help us fight back? Future Microbiol 8:27–39
Yong D, Lee K, Yum J, Shin H, Rossolini G, Chong Y (2002) Imipenem-EDTA disk method for differentiation of metallo-β-lactamase-producing clinical isolates of Pseudomonas spp. and Acinetobacter spp. J Clin Microbiol 40:3798–3801
Yong D, Toleman M, Giske C, Cho H, Sundman K, Lee K, Walsh T (2009) Characterization of a new metallo-β-lactamase gene, blaNDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother 53:5046–5054
Acknowledgments
The authors are thankful to the staff of the neonates intensive care unit, burn center, and ICU for providing help in sample collection.
Funding
HB was financially supported by Higher Education Commission (HEC) Pakistan and Andrea Telatin (BMR Genomics) for sequencing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Clinical samples from neonates, geriatrics, and burn patients were collected after informed and written consent from patients. The project was subjected to evaluation and subsequently approved by the Ethical Review Committee of the Department of Biosciences, COMSATS, during the period January 2015–December 2015.
Consent for publication
Patient’s consent was taken for publication of information about them.
Competing interests
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Humayun, A., Siddiqui, F.M., Akram, N. et al. Incidence of metallo-beta-lactamase-producing Klebsiella pneumoniae isolates from hospital setting in Pakistan. Int Microbiol 21, 73–78 (2018). https://doi.org/10.1007/s10123-018-0006-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10123-018-0006-1